Forecasting Hepatitis C liver disease burden on real-life data. Does the hidden iceberg matter to reach the elimination goals?
Funding information
The PITER platform has been supported by the Italian Ministry of Health: “Research Project PITER2010” RF-2010-2315839 to SV. This study was funded by the Polaris Observatory Through Grants from the John C. Martin Foundation and Center for Disease Analysis.
Abstract
Background & Aims
Advances in direct-acting antiviral treatment of HCV have reinvigorated public health initiatives aimed at identifying affected individuals. We evaluated the possible impact of only diagnosed and linked-to-care individuals on overall HCV burden estimates and identified a possible strategy to achieve the WHO targets by 2030.
Methods
Using a modelling approach grounded in Italian real-life data of diagnosed and treated patients, different linkage-to-care scenarios were built to evaluate potential strategies in achieving the HCV elimination goals.
Results
Under the 40% linked-to-care scenario, viraemic burden would decline (60%); however, eligible patients to treat will be depleted by 2025. Increased case finding through a targeted screening strategy in 1948-1978 birth cohorts could supplement the pool of diagnosed patients by finding 75% of F0-F3 cases. Under the 60% linked-to-care scenario, viraemic infections would decline by 70% by 2030 but the patients eligible for treatment will run out by 2028. If treatment is to be maintained, a screening strategy focusing on 1958-1978 birth cohorts could capture 55% of F0-F3 individuals. Under the 80% linked-to-care scenario, screening limited in 1968-1978 birth cohorts could sustain treatment at levels required to achieve the HCV elimination goals.
Conclusion
In Italy, which is an HCV endemic country, the eligible pool of patients to treat will run out between 2025 and 2028. To maintain the treatment rate and achieve the HCV elimination goals, increased case finding in targeted, high prevalence groups is required.
Abbreviations
-
- AIFA
-
- Italian Medicines Agency
-
- CHC
-
- chronic hepatitis C
-
- DAA
-
- direct-acting antiviral
-
- DCC
-
- decompensated cirrhosis
-
- GHSS
-
- Global Health Sector Strategy Goals for Viral Hepatitis
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- Hepatitis C virus
-
- PITER
-
- Italian Platform for the study of Viral hepatitis Therapies
-
- SVR
-
- sustained virological response
-
- WHO
-
- World Health Organization
Key Points
- In Italy, the eligible pool of chronic HCV infected patients to treat will run out between 2025-2028, leaving a significant proportion of infected individuals undiagnosed and without access to care.
- Increased case finding in high prevalent birth cohorts of the general population through targeted screening strategies are necessary to achieve the WHO goals for elimination of viral hepatitis.
- If the treatment rate decreases before 2025, increased case finding in individuals born between 1948-1978 is necessary to achieve the WHO elimination goals. If the treatment rate is sustained until 2028, screening strategies focusing on individuals born between 1958-1978 are warranted.
1 INTRODUCTION
Hepatitis C virus (HCV) is a leading cause of liver-related morbidity and mortality worldwide. An estimated 71 million people are affected by chronic hepatitis C (CHC) infection1 and a significant number of those chronically infected progress to cirrhosis or liver cancer if left untreated.2, 3 However, the development of direct-acting antiviral (DAA) therapy has revolutionized the approach to treatment and reinvigorated public health initiatives aimed at identifying patients with CHC. Galvanized by these results, the World Health Organization (WHO) foresees the elimination of HCV infection by 2030 through achieving the Global Health Sector Strategy Goals (GHSS) for Hepatitis.4 While targeted screening programmes for high-risk populations such as injection drug users are necessary for elimination of Hepatitis C,5, 6 little has been done to understand what increases in diagnosis and treatment are necessary in the general population of high endemic countries for achieving these goals. Given that the use of DAAs regardless of fibrosis stage is cost-effective,7 it is crucial that health policies expand treatment access for all HCV-infected individuals. The goal of this study was to use a new modelling approach, grounded in real-life cohort data of diagnosed and treated patients, to compare different linkage to care scenarios to the overall HCV-infected population in Italy. We aimed to evaluate the possible impact of only linked-to-care individuals on overall HCV burden and to identify a possible strategy to achieve the WHO targets by 2030.
2 METHODS
2.1 Study design
Two Markov-disease burden models were developed to assess the current and future HCV disease burden in Italy. The “Italy Polaris” model is grounded in the natural history of HCV progression and forecasts the HCV impact on the general population. A similar HCV disease burden model, grounded in the current distribution of linked-to-care patients of the PITER (Italian Platform for the Study of Viral Hepatitis Therapies) cohort was also developed.8
2.1.1 PITER cohort
PITER is an ongoing cohort of 9145 (at time of study) consecutively enroled patients from 90 public, general hospitals and university medical centres distributed across Italy. The PITER cohort is considered a representative sample of linked-to-care patients with no treatment access restrictions on the basis of healthcare system reimbursement criteria.8 PITER aims to evaluate the expected impact of DAAs on the natural course of hepatitis infection and on long-term morbidity and mortality in a real-life setting in Italy. The PITER inclusion criteria are: all HCV-infected patients (any stage, any genotype, including HBV, HDV, or HIV co-infection) at least 18 years of age consecutively referred to outpatient clinics of the participating clinical centres during enrolment phases, who are untreated at the time of enrolment. The mean age of enrolled patients is 61 (range 18-94) years of age and the ratio of males to female is 1/1.2 (55% male).8 The older age of patients enrolled in PITER represents the cohort effect of HCV infection in Italy. Of patients enrolled in the PITER cohort in 2016, 52% were F0-F3, 38% F4 and 10% had decompensated cirrhosis or HCC.8 Treatment initiations occurring among enrolled patients cover the full evolution of DAA access in Italy from 2014 on.
2.1.2 AIFA treatment data
Real-life reported treatment data were provided from January 2015 through August 2017 by the Italian Medicines Agency (AIFA).9 AIFA reimbursement criteria included fibrosis stage ≥F3 patients, patients with extrahepatic manifestations in any stage of fibrosis and liver transplant recipients, until the end of 2016. Beginning in 2017, treatment was expanded to all patients independent of fibrosis score.9
2.1.3 Italy Polaris and PITER adjusted models
For this analysis, two separate models were constructed. First, a Markov HCV disease progression model (the “Italy Polaris model”) was built using previously described methodology10 to forecast the annual prevalence of chronic HCV infection in Italy by liver disease stage, sex and age. In this model, the number of annual historical HCV incident cases, starting in 1950, and their sex and age group distribution was back-calculated to match the modelled prevalence by sex and age group in 2015 to reported estimates11 (Section 1 in Appendix S1). The reported number of annual treated patients as tracked in the AIFA Monitoring Registry for DAAs9 was allocated to the age and liver disease stage of the eligible HCV-infected population by the relative size of population in each treatment-eligible disease stage (Table 1). The number of annual treatments initiated within each disease stage was uniformly distributed across treatment-eligible ages.
| Italy specific parameters in model | Year | Value (Range) | Source |
|---|---|---|---|
| Total viraemic population | 2015 | 849 000 (371 000-1 240 000) | 13 |
| Viraemic prevalence | 2015 | 1.39% (0.6%-2.00%) | 13 |
| Viraemic diagnosed population | 2015 | 357 000 (255 000-510 000) | Expert input |
| Annual newly linked to care for treatmenta | 2013 | 30 400 | Expert input |
| Annual number treated | 2015 | 31 000 | 9 |
- a Annual Newly Linked to Care for Treatment encompasses those newly diagnosed each year.
Afterwards, the Italy Polaris model was adapted to initiate the model in 2015 (the “PITER adjusted model”) with the disease stage, sex and age group distribution of linked-to-care prevalent cases as reported in PITER (Section 2 in Appendix S1). Background mortality by 5-year age and sex cohort, standard mortality ratios and the future incident cases were applied as in the Italy Polaris model. The number of annual treatments initiated in this model was the real-life number of treatments with DAAs from 2015 to August 2017 by disease stage and age as provided by the AIFA Monitoring Registry.9
2.2 Statistical analysis
Two general population scenarios describe the forecasted disease burden through 2030 and three scenarios based on PITER data evaluate the impact of linkage to care on viraemic prevalence (Table 2).
| A | 2015 | 2016 | 2017 | 2018 | 2019 | 2020+ |
|---|---|---|---|---|---|---|
| Annually treated | ||||||
| Base 2016 | 31 000 | 33 700 | 29 500 | 25 300 | 21 100 | 16 900 |
| PITER (40%, 60%, 80%) | — | 33 700 | 33 700 | 33 700 | 33 700 | 33 700 |
| Tx-eligible stages | ||||||
| Base 2016 | ≥F3 | ≥F3 | ≥F3 | ≥F3 | ≥F3 | ≥F3 |
| PITER (40%, 60%, 80%) | ≥F3 | ≥F3 | ≥F0 | ≥F0 | ≥F0 | ≥F0 |
| Tx-eligible ages | ||||||
| Base 2016 | 15-64 | 15-85+ | 15-85+ | 15-85+ | 15-85+ | 15-85+ |
| PITER (40%, 60%, 80%) | 15-85+ | 15-85+ | 15-85+ | 15-85+ | 15-85+ | 15-85+ |
| SVR | ||||||
| Base 2016 | 93% | 93% | 93% | 93% | 93% | 93% |
| PITER (40%, 60%, 80%) | — | 93% | 95% | 98% | 98% | 98% |
| B | 2015 | 2016 | 2017 | 2020 | 2022 | 2025+ |
|---|---|---|---|---|---|---|
| Annually treated | ||||||
| WHO targets | 31 000 | 33 700 | 33 700 | 35 700 | 36 700 | 38 000 |
| Newly linked to carea | ||||||
| WHO targets | 30 400 | 30 400 | 30 400 | 33 400 | 35 400 | 36 400 |
| Tx-eligible stages | ||||||
| WHO targets | ≥F3 | ≥F3 | ≥F0 | ≥F0 | ≥F0 | ≥F0 |
| Tx-eligible ages | ||||||
| WHO targets | 15-64 | 15-85+ | 15-85+ | 15-85+ | 15-85+ | 15-85+ |
| SVR | ||||||
| WHO targets | 93% | 93% | 95% | 95% | 98% | 98% |
- SVR, sustained virological response; Tx, treatment; WHO, World Health Organization.
- a Annual Newly Linked to Care for Treatment encompasses those newly diagnosed each year.
2.2.1 Scenarios—Italy Polaris Model
Base 2016
Represents the 2016 standard of care in Italy (treatment of patients with fibrosis stage ≥F3) maintaining the same fibrosis stage, treatment age and SVR rate assumptions through 2030. In 2016, 30 400 patients were considered to be newly linked-to-care for treatment annually and 33 700 patients were treated that year. As no screening strategy is in place in Italy, the number of treated patients was expected to decrease by half by 2020 due to the depleting pool of eligible patients to treat.12
WHO Targets
The WHO Targets scenario identifies the expansion of diagnosis and treatment necessary to achieve the WHO's 2030 targets for incidence, mortality and diagnosis coverage for HCV defined in the GHSS on Viral Hepatitis.4
2.2.2 Scenarios—PITER adjusted model
Utilizing the PITER cohort data, three scenarios were created representing different assumptions regarding proportions of the prevalent population in 2015 being diagnosed and under care (40%, 60%, and 80% linked to care). The annual number of patients treated, fibrosis restrictions, ages eligible for treatment and SVR were the same between the three scenarios (Table 2). In each PITER scenario, the number of patients to be treated annually following the year 2017 was kept constant. Under the 40% linkage-to-care scenario, it was assumed (given expert feedback), that 40% of the prevalent population (357 000 patients) in Italy in 2015 was diagnosed and under care.13 Since the exact number of patients linked-to-care remains unknown, the same scenario was then run under the assumption of 60% (510 000 patients) and 80% (680 000 patients) linkage-to-care. The 80% scenario was derived from from a recent study in the Italian general population13 and the 60% scenario was chosen as the midpoint.
2.3 Sensitivity analysis
To assess the effect of uncertainties in model inputs, we used Crystal Ball, a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) add-in by Oracle (Oracle Corporation, Redwood City, CA, USA) to generate 95% uncertainty intervals on modelled outcomes through Monte Carlo simulation (1000 simulations per analysis). We used Beta-PERT distributions for all uncertain inputs to estimate the impact on total viraemic infections in 2030. The key drivers for prevalence uncertainties used in the sensitivity analysis are reported in the Results and Section 1 in Appendix S1. In addition, we considered how the variance in prevalence affects the suggested targeted screening strategies.
3 RESULTS
3.1 Base 2016 (Italy Polaris Model)
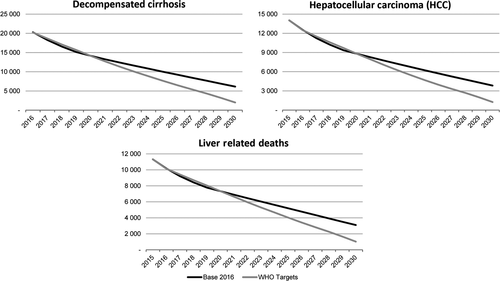
There were an estimated 849 000 (95% UI: 371 000-1 240 000) infected individuals in 2015. The forecasted impact of each scenario on total number of viraemic infections, HCV liver-related morbidity and mortality were compared through 2030 (Figures 1 and 2). Given the relatively large number of patients treated in Italy, total infections are expected to decline to 288 000 (95% UI: 71 880-424 640), or 65%, by 2030. DC cases are forecasted to decrease 75% from 22 900 (95% UI: 4300-46 700) in 2015 to 6100 (95% UI: 100-15 300) in 2030 (Figure 2). HCC cases are also expected to decline from 14 000 (95% UI: 3300-35 100) to 3800 (95% UI: 60-12 500) by the same year. HCV liver-related mortality is expected to decline by 75% from 11 300 (95% UI: 2600-19 600) to 3100 (95% UI: 50-7000) deaths by 2030.
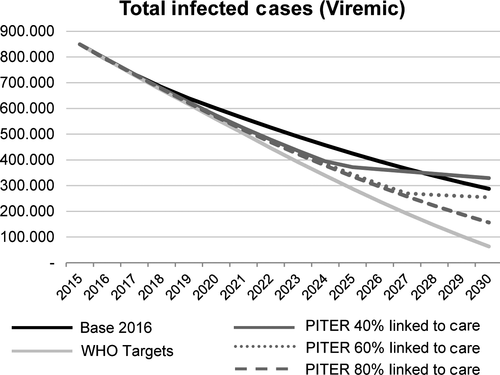
3.2 WHO targets (Italy Polaris Model)
In order to achieve the WHO GHSS targets, treatment was expanded to 38 000 patients annually by 2025; restrictions by fibrosis stage were lifted, and SVR was increased incrementally over the next 10 years to represent the higher efficacy of treatments in coming years (Table 2b). Total HCV viraemic infections and HCV liver-related morbidity and mortality are expected to decline substantially, by 95%, 90% and 90%, respectively, by 2030 (Figure 2).
3.3 PITER adjusted model, 40%, 60% and 80% linked-to-care patients
Given the 40% linkage-to-care scenario, total viraemic infections would decline by 60%, to 329 000 (95% UI: 199 960-365 960) patients, by 2030. However, the eligible patients to treat would be depleted by 2025. Under the 60% linkage-to-care scenario, the patients eligible for treatment would run out in 2028. Total infections were expected to decline to less than 260 000 (95% UI: 127 900-298 100) by the same year. Under the 80% linkage-to-care scenario, total viraemic infections are forecasted to decline by 80%, from 849 000 infections to 157 000 (95% UI: 99 380-196 980) by 2030. The pool of eligible patients to treat is expected to be depleted by 2031 (Figure 1).
In order to understand the age distribution of the eligible infected individuals for treatment and to suggest strategies to increase case finding for different linkage to care scenarios, the model estimates the age cohorts with the highest prevalence of asymptomatic individuals, as shown in Table 3. Because 30% of advanced stage liver disease (fibrosis stage ≥F3) patients are considered on treatment; by 2020, approximately 70% of all infected, asymptomatic (F0-F3) individuals would be found in those born in the years 1948-1978. According to the 40% linked-to-care scenario, targeted screening strategies in the 1948-1978 birth cohorts could be implemented to sustain the current treatment rate. If 60% of the infected population are linked-to-care, then screening fewer, younger birth cohorts, compared to the 40% linked to-care scenario, specifically those born in the years 1958-1978 (Table 3) could be useful in finding at least 30% more of eligible infected F0-F3 individuals.
| Birth year | Proportion of F0-F3 infected cases in PITER Model (%)a | Proportion of F0-F3 infected cases in Italy Polaris Model (%)a |
|---|---|---|
| 1938-1948 | 28 | 32 |
| 1948-1958 | 35 | 42 |
| 1958-1968 | 41 | 26 |
| 1968-1978 | 23 | 17 |
| 1978-1988 | 10 | 10 |
| 1988+ | 5 | 8 |
- a Does not sum to 100% due to overlapping birth cohorts.
3.4 Sensitivity analysis
The model inputs that had the largest contribution to the uncertainty in the Italy Polaris and PITER adjusted models are shown in Figure 3A,B. For the Italy Polaris model, the anti-HCV prevalence in 2015 had the largest effect on the 2030 forecast of infections (Figure 3A). There would be approximately 535 000 remaining viraemic cases in 2030, as compared to 288 000, if there were 1.24 million infections in 2015. More than 89% of the variability in the 2030 forecasted viraemic infections could be explained by the estimated number of treated patients. If the eligible linked to care patients were to diminish and only 10 000 patients would be eligible for treatment moving forward (rather than the base case of 33, 700), there would be an estimated 260 000 viraemic cases in 2030, almost 100 000 more than under the base assumption. The top five factors explained more than 98% of the variability in both models (Figure 3A,B).

In addition, we assessed how prevalence may impact the different linkage-to-care scenarios and the related case finding strategies. Given the “low” prevalence rate, the eligible patients to treat would be depleted sooner than under the base case (4). If prevalence was 370 000 infections rather than the 849 000, we would expect the eligible patients to treat to be depleted by 2022 under the 40% scenario; and in 2025 under both the 60% and 80% linkage to care scenarios. However, if prevalence was more than a million patients, the eligible patient pool would be reduced by 2027 under the 40% linked-to-care scenario. Under the 60% and 80% scenarios, the number of treated patients (approximately 35 000) could be maintained annually through 2030. The prevalence did not have a significant impact on the identified targeted screening strategies. Assuming a prevalence of approximately 370 000 cases, we estimate a less than 5% change in the distribution of F0-F3 cases by birth cohort.
| Linkage to care scenario | Prevalence | ||
|---|---|---|---|
| Low (n) 371 000 | Base (n) 849 000 | High (n) 1 240 000 | |
| 40% | 2022 | 2025 | 2027 |
| 60% | 2025 | 2028 | — |
| 80% | 2025 | 2031 | — |
- a To assess how the uncertainty in the prevalence estimate impacts the estimated number of eligible patients to treat, the linkage to care scenarios were run on the range of prevalence values (low: 371 000, base: 849 000, high: 1 240 000) to assess when the treated patients may exceed eligible patients (“be depleted”). —Signifies that given the “high” prevalence estimate, the treated patients will not exceed eligible patients and treatment levels can be maintained through 2030.
4 DISCUSSION
Italy has been considered the country with the highest HCV prevalence in Western Europe, with the peak prevalence in older ages (>70 years).14-16 However, many studies estimating HCV prevalence in the Italian general population were conducted more than 20 years ago and have shown regional variances.17 The highest prevalence rates have been reported in Southern Italy, though many of these earlier studies were conducted in smaller, more rural areas. Recent studies have also reported decreasing rates of HCV prevalence in the country.11, 18, 19
The limitation of HCV therapy is no longer treatment efficacy or adherence, but the identification of available patients to treat.20 As with prevalence, the availability of treatment and linkage to care varies across the country. Though in Italy a National Hepatitis Plan exists, decentralized models of HCV care persist and there are no uniform strategies across regional networks. Only 2 (Sicily and Veneto) of 20 regions throughout Italy have developed adequate organizational and operational politics regarding HCV elimination.21-23 Linkage to care is limited in that no enhanced HCV screening and diagnosis is implemented in the country. The number of prescribers is restricted only to gastrointestinal and infectious disease specialists whom are limited per region. It was recently estimated that there are 1500 residents per general practitioner in Italy, often curbing the availability of referral and linkage to care to a specialist.13 In addition, no specific strategies for marginalized patients and at-risk groups are implemented at the national level.
A true cascade of care for HCV infection is lacking in Italy as the number of patients under care remains uncertain. Recent studies reported between 20% and 80% of HCV+ individuals are aware of their status.11, 23 This uncertainty has clear implications for treatment, as the population first needs to be identified in order to be placed in care. While the real number of linked-to-care patients in Italy remains unknown, the PITER cohort is considered a representative sample of linked-to-care patients in Italy.8 The PITER linkage-to-care scenarios are based on the characteristics of patients enrolled in PITER and estimates different possible proportions of the “tip of the iceberg.” Extrapolating the age and fibrosis stage distribution of current linked-to-care patients (PITER model) to the general population in Italy (Polaris model) is useful for understanding what may occur in the overall infected population if current HCV trends are to remain at current levels. As seen in the Base 2016 Scenario, the WHO goal of reducing HCV liver-related deaths by 65% by 2030 is achievable in Italy if the treatment rate is maintained at current levels. However, in the 40% and 60% linkage-to-care scenarios, given the same number of treated patients through 2030, the eligible pool of patients to be treated would run out between 2025 and 2028, leaving a significant proportion of infected individuals undiagnosed and without access to care, as is shown in Figure 1.
Considering the cohort effect of HCV infection in Italy and low rates of injection drug use, the younger cohorts (1988+) are those with the lowest prevalence of HCV infection in Italy. We did not consider possible screening strategies for individuals born in 1935-1948 as the natural depletion of the virus in those individuals was assumed. This modelling estimates that more than 70% of the infected (F0-F3) individuals, those that are most often asymptomatic and undiagnosed (the underwater portion), were within the 1948 to 1978 birth cohorts in 2020 (Table 3). This signifies a potential need to increase case finding in these individuals if the treatment rate starts to decrease prior to the year 2025 (40% linked to care scenario). Similarly, though approximately 75% of individuals with chronic HCV in the United States are within the 1945-1965 birth cohort, screening in this population is not systematically done and a large portion of infected individuals fall outside of this cohort.24
The PITER adjusted model refers to a population with a mean age of 59 years, which in part reflects that of populations in other parts of the world that have similar epidemiological characteristics (ie individuals infected previously through blood transfusion or nosocomial transmission with historical trends of high incidence of infection).25 As seen in this modelling study, the rate of treatment uptake will decline unless screening and linking diagnosed patients to care is improved. In the country of Georgia, one of the nine countries on track to achieve the WHO Targets by 2030, the number of newly diagnosed patients entering the national treatment programme has fallen in the past year, suggesting that identification and linkage-to-care of HCV-infected patients in the country might be slowing.26, 27 The potential targeted screening strategies that were produced in this analysis are useful tools that can be used in countries with comparable HCV epidemiology. A similar approach can also be used for countries with different HCV epidemiology, in that it addresses the improvement of diagnosis and the linkage to care—key factors for achieving the elimination goals.28, 29
Several limitations of the analysis exist. This analysis was not focused on treatment as prevention. While neither disease burden model dynamically considers new infections nor reinfections in the population, the high treatment rate in Italy coupled with the reduced treatment restrictions exceeds the proportion required for treatment when compared to other dynamic models.30-32 In addition, although the PITER cohort is considered reasonably representative of those receiving care across the country, the PITER model uses a disease stage distribution based on a small proportion (9145 vs 357 000) of diagnosed and linked-to-care patients. The true proportion of the linked-to-care population in Italy is unknown. While rates of up to 80% have been reported,11 experts involved in the analysis have suggested that 40% of the total infected population is linked-to-care. To address this uncertainty, we presented the PITER analysis under three scenarios of 40%, 60% and 80% linkage-to-care. In addition, we evaluated the impact of this uncertainty and found the percent change in the linked-to-care population had a smaller influence on viraemic prevalence in 2030 than other factors (Figure 2A,B). Lastly, the variance in prevalence had limited influence on the proportion of F0-F3 patients identified and would not impact the screening strategies discussed.
This analysis highlights that Italy is on track to meeting the WHO target of 65% reduction in liver-related mortality by 2030. However, given the same number of annually treated patients through 2030, the eligible pool of patients to be treated would run out between 2025 and 2028, leaving a significant proportion of infected individuals undiagnosed and without access to care. Increased case finding through potential targeted screening strategies are necessary to achieve the WHO goals. This modelling analysis is a useful tool that can be used by different countries to develop screening strategies for HCV elimination.
ACKNOWLEDGEMENTS
The authors thank the PITER collaborating group available at www.progettopiter.it; Center for Disease Analysis Foundation's Polaris Observatory, which collaborated on this project on a voluntary basis; Antonietta Coratrella and Italian Drug Agency for providing detailed DAA treatment data used in this study.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.




