Hepatitis E virus in South America: The current scenario
Abstract
Hepatitis E virus (HEV) is one of the most frequent causes of acute viral hepatitis of enteric transmission worldwide. In South America the overall epidemiology has been little studied, and the burden of the disease remains largely unknown. A research of all scientific articles about HEV circulation in South America until November 2017 was carried out. Human seroprevalences of HEV varied according to the studied population: blood donors presented prevalence rates ranging from 1.8% to 9.8%, while reports from HIV-infected individuals, transplant recipients and patients on hemodialysis showed higher prevalence rates. Only 2 cases of chronic hepatitis in solid-organ transplant patients from Argentina and Brazil have been described. Detection of HEV in the swine population is widely prevalent in the region. Anti-HEV antibodies have also been recently documented in wild boars from Uruguay. Although scarce, studies focused on environmental and food HEV detection have shown viral presence in these kind of samples, highlighting possible transmission sources of HEV in the continent. HEV genotype 3 was the most frequently detected in the region, with HEV genotype 1 detected only in Venezuela and Uruguay. HEV is widely distributed throughout South America, producing sporadic cases of acute hepatitis, but as a possible agent of chronic hepatitis. Finding the virus in humans, animals, environmental samples and food, show that it can be transmitted through many sources, alerting local governments and health systems to improve diagnosis and for the implementation of preventive measures.
Abbreviations
-
- ELISA
-
- Enzyme-Linked Immunosorbent Assay
-
- HBV
-
- hepatitis B virus
-
- HCV
-
- hepatitis C virus
-
- HEV
-
- hepatitis E virus
-
- HIV
-
- human immunodeficiency virus
-
- IgG
-
- immunoglobulin G
-
- IgM
-
- immunoglobulin M
-
- ORF
-
- open reading frame
-
- RNA
-
- ribonucleic acid
Key points
- HEV has been detected in humans, pigs, wild boars, environmental samples (water, sewage) and food in South America.
- Seroprevalences vary according to the region and the population studied, ranging from 0.1% to 38%.
- HIV positive patients, hemodialysis patients and solid-organ transplant patients present higher HEV seroprevalences.
- HEV genotype 3 has been the most widely found in the continent.
- Only 2 cases of chronic hepatitis E in transplant patients (liver and kidney) have been described; one from Brazil and the other from Argentina.
1 INTRODUCTION
The hepatitis E virus (HEV) is an emergent causative agent of enteric acute hepatitis worldwide. The World Health Organization estimates 20 million HEV infections occur every year all over the world, leading to an estimated 3.3 million symptomatic cases of viral hepatitis because of the virus.1 HEV (family Hepeviridae, genus Orthohepevirus, specie Orthohepevirus A) is a non-enveloped virus with a positive stranded RNA genome of 7.2 kb that encodes 3 partially overlapped open reading frames (ORFs): ORF1 encodes viral replication proteins, while ORF2 encodes the capsid protein and ORF3 a protein involved in particle secretion.2 Eight genotypes (1-8) with multiple subtypes have been described for this virus; only genotypes 1, 2, 3, 4 and 7 are known to infect humans.3, 4 Genotypes 1 (HEV-1) and 2 (HEV-2) produce epidemic outbreaks via faecal-oral transmission route in endemic developing countries in Asia and Africa, and have been detected sporadically in America.5 Genotypes 3 (HEV-3) and 4 (HEV-4) are zoonotic viruses that infect humans by ingestion of contaminated food or direct contact with infected animals. Importantly, infection by these genotypes has been shown to cause persistent infection in immunocompromised individuals.2, 5 HEV-3 has a worldwide distribution, while HEV-4 circulates in Asia and Europe.5 Genotype 7 (HEV-7) was detected in dromedaries and humans fed with camel milk in the Middle East and very little is known about it.6
Since HEV was first described in South America in 19947 many studies have reported its circulation in the continent, including molecular detection in a variety of sources (human sera, human and animal stool, water, sewage) and serological surveys in many populations (general population, blood donors, HIV+ individuals, paediatric population, dialyzed patients, solid organ transplant recipients, etc.). However, there are still several important questions that have not been answered or that have been only partially answered, such as the role of pigs in human-zoonotic transmission, food consumption as a risk factor, the importance of HEV in the progression to chronic hepatitis in certain regions, and the overall burden of chronic hepatitis E in the South American continent.
The aim of this review is to provide and discuss updated information about HEV in South America, which may lead to a better understanding of the dynamics of HEV, as well as to promote preventive measures in this part of the world. For that purpose, we searched published scientific articles regarding HEV in South America by assessment of Pubmed/NLM using the following keywords: HEV, South America, seroprevalence, genotypes, circulation. The search yielded 81 articles published until November 2017, and these were evaluated to address the seroprevalence of HEV in human and animal samples, acute and chronic cases, associated risk factors and viral environmental circulation in the region.
2 DETECTION OF HEV IN HUMANS IN SOUTH AMERICA
2.1 Seroprevalence of HEV in immunocompetent individuals
The updated prevalence of IgG anti-HEV antibodies among general populations and risk groups of South America is shown in Table 1.
| Country | Population | No of cases | IgG anti-HEV % of positivity | Test utilized | Reference |
|---|---|---|---|---|---|
| Argentina | Paediatrics | 1304 | 0.15 | Abbot GmbH Diagnostika, Germany | 10 |
| Previous surgery | 1735 | 3.1 | Abbot GmbH Diagnostika, Germany | 10 | |
| Blood donors | 2157 | 1.8 | Abbot GmbH Diagnostika, Germany | 18 | |
| HIV + | 484 | 6.6 | Abbot GmbH Diagnostika, Germany | 18 | |
| General population | 433 | 4.4 | Diapro, Italy | 37 | |
| Volunteers | 95 | 9.5 | Diapro, Italy | 38 | |
| Volunteers | 28 | 14.3 | Wantai, China | 38 | |
| Blood donors | 24 | 16.7 | Wantai, China | 38 | |
| Healthcare workers | 27 | 14.8 | Wantai, China | 38 | |
| HIV + | 28 | 35.7 | Wantai, China | 38 | |
| HIV + | 204 | 7.3 | Diapro, Italy | 40 | |
| Solid organ transplant recipients | 120 | 5.8 | Diapro, Italy | 45 | |
| Dialysis patients | 88 | 10.2 | Diapro, Italy | 45 | |
| Brazil | Gold miners | 97 | 6.1 | Genelabs Inc., Redwood City, CA | 9 |
| Blood donors | 200 | 2.0 | Abbott Lab., Chicago | 12 | |
| Acute NANBNC hepatitis relatives | 66 | 10.6 | Abbott Lab., Chicago | 24 | |
| Acute NANBNC hepatitis | 16 | 12.2 | Abbott Lab., Chicago | 24 | |
| Schistosomiasis carriers | 30 | 10.0 | Abbott Lab., Chicago | 12 | |
| Acute Hepatitis C | 12 | 0.0 | Abbott Lab., Chicago | 12 | |
| Dialysis patients | 392 | 0.0 | Abbott Lab., Chicago | 12 | |
| General population | 1059 | 1.7 | NA | 15 | |
| General population | 97 | 6.1 | NA | 16 | |
| Acute NANBNC hepatitis | 17 | 29.0 | NA | 23 | |
| Cleaning service workers | 53 | 13.2 | Abbot GmbH Diagnostika, Germany | 19 | |
| Women at risk of HIV infection | 214 | 17.7 | Abbot GmbH Diagnostika, Germany | 19 | |
| Healthcare workers | 170 | 2.6 | Abbot GmbH Diagnostika, Germany | 19 | |
| Blood donors (ALT>2UNL) | 40 | 7.5 | Abbot GmbH Diagnostika, Germany. | 19 | |
| Pregnant women | 304 | 1.0 | Abbott Lab., Chicago | 20 | |
| Individuals from rural area | 145 | 2.1 | Abbott Lab., Chicago | 20 | |
| Individuals from urban area | 260 | 0.0 | Abbott Lab., Chicago | 20 | |
| IDVU | 102 | 11.8 | Abbott Lab., Chicago, USA | 20 | |
| Individuals living in low socioeconomic community | 260 | 2.4 | Abbott Lab., Chicago, USA | 21 | |
| Blood donors (ALT<2UNL) | 165 | 3.0 | Abbott Lab., Chicago, USA | 22 | |
| Pediatrics | 487 | 4.5 | Abbott Lab., Chicago, USA | 22 | |
| Acute NANBNC hepatitis | 12 | 17.0 | NA | 25 | |
| Acute hepatitis A | 50 | 38.0 | NA | 25 | |
| Acute hepatitis B | 42 | 10.0 | NA | 25 | |
| Blood donors | 996 | 2.3 | Abbott Lab., IL, USA | 97 | |
| Individuals from rural área | 310 | 8.4 | MP Biomedicals Asia Pacific Pte Ltd, Singapore | 31 | |
| Blood donors | 110 | 4.0 | MP Biomedicals Asia Pacific Pte Ltd, Singapore | 31 | |
| Individuals from rural área | 388 | 12.9 | Biokit, Spain | 35 | |
| Recyclable waste pickers | 431 | 5.1 | Mikrogen GmBH, Germany | 36 | |
| Renal transplant patients | 192 | 15.0 | Mikrogen, Neuried, Germany | 33 | |
| Acute NANBNC hepatitis | 379 | 5.3 | Mikrogen GmBH, Germany | 41 | |
| Schitosomiasis carriers | 80 | 18.8 | Wantai, China | 42 | |
| Blood donors | 300 | 10.0 | Wantai, China | 47 | |
| Blood donors | 500 | 9.8 | Wantai, China | 43 | |
| Blood donors | 780 | 30.3 | In house | 44 | |
| HIV+ | 354 | 10.7 | Mikrogen GmBH, Germany | 46 | |
| Bolivia | Individuals from rural area | 490 | 7.3 | Mikrogen GmBH, Germany | 17 |
| General population | 236 | 6.3 | Diapro, Italy | 30 | |
| Chile | Blood donors | 1360 | 8.0 | Abbott Lab | 11 |
| Healthcare workers | 72 | 12.5 | Abbott Lab | 11 | |
| Prisoners | 241 | 7.5 | Abbott Lab. | 11 | |
| Indigenous community | 100 | 17.0 | Abbott Lab | 11 | |
| Acute NANBNC hepatitis | 59 | 7.0 | Abbott Lab | 26 | |
| Pedriatics living in low socieconomic community | 168 | 1.2 | Abbott Lab | 29 | |
| Colombia | Individuals from rural area with occupational exposure to pigs | 98 | 11.2 | Diapro, Italy | 32 |
| Acute NANBNC hepatitis | 344 | 8.7 | Diapro, Italy | 34 | |
| Individuals from rural area with occupational exposure to pigs | 159 | 15.7 | Diapro, Italy | 39 | |
| Individuals from rural area without occupational exposure to pigs | 34 | 5.9 | Diapro, Italy | 39 | |
| Individuals from urban area | 983 | 7.2 | Diapro, Italy | 39 | |
| French Guiana | General population | 996 | 6.4 | NA | 14 |
| Peru | Healthy sewage workers | 191 | 10.5 | Abbot GmbH Diagnostika, Germany | 27 |
| Uruguay | General population | 214 | 2.8 | Abbot GmbH Diagnostika, Germany | 13 |
| Blood donors | 252 | 1.2 | Abbot GmbH Diagnostika, Germany | 13 | |
| Venezuela | Pregnant women | 184 | 1.6 | NA | 7 |
| Prisoners | 204 | 3.9 | NA | 7 | |
| Indigenous community | 223 | 5.4 | NA | 7 | |
| Pregnant women from low income population | 106 | 1.9 | NA | 8 | |
| Pregnant women from medium-high economic class population | 105 | 1.3 | NA | 8 | |
| Indigenous community | 463 | 9.7 | NA | 28 |
- NA, not available.
The first serological studies of HEV infection in the continent date from the 1990s and the beginnings of 2000s, when several reports were published with prevalence rates ranging between 0.1% to 8% among either rural or urban populations,7-22 and between 3% and 29% within groups with risk factors or with hepatitis of unknown source.10, 12, 18-21, 23-29
A 10-year gap in knowledge followed, until 2011, when studies from Argentina, Bolivia, Brazil and Colombia, reported respective HEV prevalences.30-47 This wave of studies followed the global reports of chronic hepatitis E virus infection by HEV-3 in immunosuppressed individuals, especially those with solid organ transplantation as well as the reports of extra-hepatic manifestations of HEV. Studies performed in blood donors show current prevalence rates between 1.8% and 9.8%, showing moderate circulation. The only longitudinal study found, reports a significant linear trend of IgG anti-HEV positivity with years in Brazilian blood donors, showing that the prevalence of IgG anti-HEV antibodies varied between 0% to 4.3% from 1997 to 2006, with the highest frequencies observed from 2011 to 2013: 5.9% (2/34), 8.6% (12/139) and 6.1% (9/148) respectively.48 In general, HEV positivity trended to increase with age.15, 17, 31, 35, 37, 43, 47, 48 There is only 1 study that reported new HEV infections in a 7 years period, carried out in Brazil, although it did not involve the follow-up of a group of patients. Five hundred and fifty-two IgM tests were performed from 2006 to 2013, and the IgM anti-HEV positivity rate ranged from 0.0% to 8.8% annually, showing a linear trend over time.48 Despite these studies, the data from the region is still limited and incomplete.
Some of the main barriers to a proper understanding about HEV include: (i) discrepancy in the sampling criteria of the studies (sample sizes, study periods, study populations); (ii) use of different commercial diagnostic kits, which can yield different results in the same population; (iii) different diagnostic criteria, as some authors report confirmation based on western blot or immunoblot after a positive ELISA result, which make comparisons difficult. Regarding the use of different commercial diagnostic kits, studies performed using Wantai ELISA kits show higher prevalence rates than those in which other ELISA kits are used (Table 1). In this sense, a study that compared 8 different commercial kits coated with HEV genotypes 1 and 2 antigens, demonstrated that the Mikrogen and the Wantai assays had greater sensitivity and specificity than the rest, although all current commercial HEV ELISAs can be used to diagnose HEV infection adequately.49 A recent study with a new “in house” immunoassay developed in Brazil using a recombinant HEV-3 capsid protein revealed a high prevalence of antibodies (30%),44 suggesting, for the first time, that the Southern region of Brazil might be endemic to HEV-3. This finding raises new challenges in the evaluation of the circulation of the virus in the region, which should be explored in a consensual way in the near future, extending the range of study with this new configuration of immunoassays.
2.2 Seroprevalence of HEV in immunosuppressed individuals
Multiple studies from our group and others worldwide have reported that immunosuppressed individuals could present higher HEV prevalences, compared to general population.45, 50, 51
Brazil and Argentina are the only countries in South America which have reported studies about HEV circulation in immunocompromised populations (Table 1).33, 38, 40, 45, 46 In Argentina higher circulation of HEV was registered in HIV+ individuals, compared to blood donors (6.6% vs 1.8%),18 volunteers (35.7% vs 14.3% or 9.5%, depending on the kit used)38 and general population (7.3% vs 4.4%).40 A study from our group found that HIV+ individuals with severe immunosuppression (CD4 cell count < 200/mm3) presented significantly higher prevalence for HEV (16%) than those with CD4 cell count > 200/mm3 (4.5%), suggesting a correlation between T-cell immunity and risk for HEV infection.40 However, during this study patients were not divided by age, place of living or socio-economic groups, so it was not possible to elucidate if these variables influenced the prevalence results. The only study conducted in Brazil in this population (prevalence of 10.7% for IgG anti-HEV) found no correlation between serological status with sex, age, CD4 T cell count, HIV viral load, antiretroviral therapy, liver enzyme levels or co-infection with hepatitis B virus (HBV) and/or hepatitis C virus (HCV).46
Among transplant recipients, prevalences found are between 5.8% and 15% (Table 1). Most studies performed in Brazil were conducted among renal transplant patients compared to blood donors, while those in Argentina were among kidney and liver transplant recipients compared to the general population.33, 45
In patients undergoing dialysis, the only South American reported study was conducted in the central region of Argentina, showing a significantly higher prevalence than the registered for general population (10.2% vs 4.4%).45
The higher values of prevalence reported in immunosuppressed populations in South America would be owing to several factors not fully elucidated. The low CD4 cell count (in HIV+ patients),40 the exposure to parenteral route, which increases the chances of infection (particularly during the hemodialysis period in dialysis and renal transplant patients), and blood transfusions33 seem to be some of them. In the case of hemodialysis patients, a positive correlation between IgG anti-HEV prevalence and fish consumption was observed.45
As we described above, most reports come from a limited number of studies from few countries in South America. It is critical to expand the knowledge of the epidemiological distribution of HEV among immune-compromised individuals.
2.3 Acute hepatitis E
Despite high documented seroprevalences of HEV in South America, reports of clinically significant cases are low or infrequent, similar to the observed in developed countries.52 This fact likely reflects a high rate of asymptomatic cases associated with HEV-3 (the most frequent genotype in South America), and unrecognized symptomatic cases secondary to limited access to diagnostic tests and awareness of the virus.
Table 2 summarizes the acute cases of HEV infection reported in the last 17 years in South America, diagnosed by the presence of serological or molecular specific markers (anti-HEV IgM and/or HEV RNA). HEV-3 was the most frequent: subgenotype 3a was detected in Argentina and subgenotypes 3b and 3i in Argentina and Brazil.48, 53 Recently in Colombia, symptomatic acute hepatitis E was reported for the first time in 22% (9/40) of the cases analyzed.54
| Country | Studied patients | IgM anti-HEV | HEV RNA | HEV genotype (N) | References |
|---|---|---|---|---|---|
| Argentina | Not given | Nd | 2 | 3i (2) | 91 |
| 35 | 3 | 3 | 3i (3) | 92 | |
| 231 | 6 | 9 | 1a (1)a | 53 | |
| 3a (5) | |||||
| 3b (1) | |||||
| 143 | 4 | 9 | 3a (7) | 38 | |
| 3i (2) | |||||
| Brazil | 17 | 5 | Nd | Nd | 98 |
| 64 | 1 | 1 | 3b (1) | 76 | |
| 96b | 0 | 3 | 3 (2) | 96 | |
| 3i (1) | |||||
| 552 | 6c | 6 | 3b (1) | 48 | |
| Chile | 59 | 1 | Nd | Nd | 26 |
| 35 | 12 | Nd | Nd | 99 | |
| Colombia | 40 | Nd | 9 stool | 3 | 54 |
| Peru | 747 | 4 | Nd | Nd | 100 |
| 2 | Nd | 2 | Nd | 101 | |
| Uruguay | Not given | 9 | 9 | 3 (9) | 93 |
| 1 | 1 | 1 | 1 | 56 | |
| Venezuela | 74 | 22 | 3 | 1 (2) | 55 |
| 3 (1) |
- Nd, not determined.
- a Imported case from India.
- b Renal transplant recipients.
- c Two liver transplant recipients.
In the last 5 years, 2 cases of autochthonous hepatitis E by HEV-1 were reported in Venezuela and one in Uruguay.55, 56 An imported case by this genotype was registered in Argentina in an international traveller.53
2.4 Hepatitis E associated to extra hepatic manifestations and chronicity: Case reports
Autochthonous hepatitis E has a wide spectrum of reported complications, including “acute-on-chronic” liver failure, neurological disorders, pancreatitis and development of chronic hepatitis.57
Chronic infections by HEV-3 and HEV-4 have been identified among immunocompromised persons worldwide, including organ transplant recipients, patients receiving cancer chemotherapy and HIV-infected persons.50, 51, 58
In South America, there are few reported cases of chronic hepatitis E or HEV infection with extra hepatic complications. Argentina is the only South American country where 2 cases of acute HEV associated to extra hepatic manifestations had been reported, both in immunocompetent adult patients with confirmed HEV-3a infection. One of the cases corresponded to a 45 year old male health worker who developed subacute thyroiditis,59 and the other, to a 59–year-old alcoholic female patient who developed aplastic anaemia.60
One case of “acute-on-chronic” liver failure was reported in an individual from Peru who developed autoimmune hepatitis and severe hepatic decompensation associated to HEV infection.61
Two cases of chronic hepatitis E have been reported in transplant patients: an adult kidney transplant recipient in Argentina and a paediatric liver transplant recipient in Brazil.62, 63 These cases highlight the need to increase the search of this virus in susceptible populations and to increase awareness to clinicians to develop a higher sense of suspicion of HEV when addressing complex clinical cases.
3 DETECTION OF HEV IN DOMESTIC AND WILD ANIMALS IN SOUTH AMERICA
Hepatitis E virus is the only known hepatitis virus with an animal reservoir,57 and accumulating lines of evidence has suggested that HEV is a zoonosis.64 Pigs are the main reservoirs and other animals have also been shown to be susceptible to HEV. These include wild boars, deers, cows, sheeps, goats, camels, horses, dogs, cats, rats and mongooses worldwide.65-67
Data regarding HEV prevalence and molecular epidemiology in animal reservoirs in South America is still scant. Most of the serological surveys reported from Brazil, show similar average seroprevalence rates ranging from 88.4% to 97.3% in animals between 22 and 25 weeks respectively,68, 69 suggesting that HEV is widely distributed among swine herds. By contrast, low antibody rates have been described in swine herds from the Brazilian Amazonia.70
Domestic pigs are also highly exposed to HEV in Colombia, with seroprevalence rates of 100% in slaughter-aged pigs from the Antioquia region.71
In Chile, Argentina and Uruguay, by contrast, antibody prevalences among swine herds seem to be lower and exhibit a significant variability among different geographical regions, ranging from 0.6% to 58%, depending on the study.72-74
Molecular epidemiology of swine HEV has been studied in many South American countries. In 2006, Munné et al73 reported for the first time in the region, the detection and molecular characterization of swine strains of HEV isolated from faecal samples in a herd located in Buenos Aires province. In Brazil, Paiva et al75 (2007) described the first HEV strain isolated from pigs in 2007, even when no autochthonous cases of HEV infection in humans had yet been reported in this country. Later, subtype 3b strains of HEV-3 isolated from swine and effluent samples from slaughterhouses, were found to be closely related to the sample obtained from the first reported autochthonous human case in Brazil.76-79 Furthermore, co-circulation of subtypes 3c and 3f has been reported.70
A study from Bolivia also reported the circulation of HEV-3 among domestic pigs, but a different genetic variant was found from that recovered from humans in the same rural community, suggesting no zoonotic transmission in that region.79, 80
As in the rest of the South American countries, HEV seems to be widely spread among swine herds from Colombia. Very recent studies have reported high infection rates of HEV subtype 3a in pigs from Antioquia.81 Interestingly, HEV RNA had been previously detected in 25% of pig livers from grocery stores in the same geographical region.82
In Uruguay, Mirazo et al74 (2018) have recently documented the detection of HEV-3 RNA in liver samples from slaughter-age pigs (16.6% of positivity), indicating a wide circulation of the virus and a potential risk for zoonotic transmission.
All these data strongly suggest that HEV is highly disseminated in the swine population of South America. In fact, both swine and human strains isolated in most regions seem to be phylogenetically related, suggesting that the high prevalence of HEV in domestic pigs might present an important risk for human infection.
In addition to pigs, specific anti-HEV antibodies and HEV RNA have been recently detected, for the first time in South America, in wild boars from Uruguay (22.1% and 9.3% respectively).74 The potential role of these animals as zoonotic sources of infection remains unknown.
4 DETECTION OF HEV IN ENVIRONMENTAL MATRICES AND FOOD IN SOUTH AMERICA
Since HEV is an enteric virus whose main transmission route is faecal-oral, aqueous matrices are postulated to have an important role in the transmission and maintenance of the virus.83, 84
Drinking water as well as irrigation water can be contaminated with HEV via animal stool, with concomitant contamination of vegetables, fruits and fish, leading to food safety risk.77, 83 In addition, the presence of the virus in surface waters (rivers, dams), constitutes a potential risk of infection for susceptible persons who are in contact with them, since some of these watercourses are usually used with recreational purpose (for swimming, sailing, kayaking, surfing and more).
Studies about environmental HEV detection in South America are scarce. Only 4 studies, performed in Argentina, Brazil and Colombia, have searched the virus on environmental samples (Table 3).37, 77, 84-86 In all studies HEV-3 was found and the subtypes described belonged to clade 3abchij. Most of the sequences were closely related to human and/or swine strains, showing that the environment could play an important role in the maintenance of the virus in many regions of South America, possibly being the source of infection for exposed human and animal populations, but also as receptor for viral discharge from these same populations. That is the case of environments close to places where pigs are present, which have very high HEV detection rates (50%-100%, Table 3),77, 87 emphasizing the participation of these animals in the transmission of HEV. Also the presence of virus in sewage, which is assumed to have human origin,37, 84 which could be discharged to recreational waters.
| Country | Source | % of HEV detection (positive/n) | HEV genotype and subtype | Amplified genomic region | Year of detection | Reference |
|---|---|---|---|---|---|---|
| Argentina | River water | 3.2% (1/32) | 3c | ORF2a | 2010 | 37 |
| Dam water | 2.1% (1/48) | 3 | ORF2a | 2013 | 86 | |
| 7.57% (5/66) | 2015 | |||||
| Sewage | 6.3% (3/48) | 3a, 3b, 3c | ORF2a | 2007, 2010, 2011 | 37 | |
| Brazil | Slautherhouses effluent | 50% (3/6) | 3b | ORF1 and ORF2a,b | 2008 | 77 |
| Effluents from slurry lagoons (near pig farms) | 100% (8/8) | 3b | ORF1a | 87 | ||
| River water | 0% (0/250) | - | ORF1a | From 2012 to 2014 | 85 | |
| River sediment | 0% (0/68) | - | ORF1a | 2014 | 85 | |
| Colombia | Water from a drinking water plant or a creek | 23.3% (7/30) | 3 | ORF2/3a | From 2012 to 2014 | 84 |
| Sewage | 16.7% (5/30) | 3 | ORF2/3a | From 2012 to 2014 | 84 |
- a Nested PCR;
- b Real time PCR.
One particularly concerning point is the presence of HEV in water samples for consumption.84 This constitutes a risk for human populations that directly consume these waters or use them for animal consumption or irrigation of crops. There is evidence of HEV outbreaks in other parts of the world where drinking water was pointed to be the source of infection,88 so this possibility should not be discarded in our region.
Foodborne transmission of HEV by consumption of raw or undercooked liver, meat or sausages prepared from infected animals (swine, deer, wild board) has been documented in North America, Europe and Asia, but only few studies have been published on the detection of HEV in pork products so far.89 South America is not the exception, and, although the virus has been described to circulate in swines in this region, there is only 1 study performed in Brazil, in which viral presence in pork products was investigated. During this study, 50 samples of pate and blood sausage marketed in the Sinos River region were tested for the presence of HEV genome, resulting in 36% of positivity; all samples belonged to HEV-3.85 Although studies about HEV in food are scarce, these results show the potential for zoonotic transmission of HEV infection through foods of porcine origin in places where pig infection has been demonstrated in our continent. Besides, they emphasize the need to deepen research of HEV in food matrices, as well as to promote implementation of preventive measures.
5 HEV IN BLOOD PRODUCTS IN SOUTH AMERICA
HEV has also been transmitted by blood transfusion from asymptomatic viremic donors, and has been detected in blood components in other parts of the world.90 In South America, there are no studies about molecular detection of HEV in blood products, which constitutes a gap in the knowledge of HEV in the continent. This could be critical, since blood recipients are usually people with some disease or immunosuppressed individuals, and HEV infection could be a serious additional problem. Studies focusing the search of RNA HEV in blood products and/or blood donors are needed to determine the impact of HEV in local blood banks.
6 MOLECULAR EPIDEMIOLOGY OF HEV IN SOUTH AMERICA
Eight HEV genotypes have been described4 from these, HEV-1, HEV-2, HEV-3, HEV-4 and HEV-7 can infect humans and produce disease, and have a particular geographical distribution.3 In South America, only HEV-1 and HEV-3 have been detected. HEV-3 is the most frequent genotype found in this region, isolated from humans, pigs and environmental samples (as described above) in Argentina, Brazil, Bolivia, Colombia, Uruguay and Venezuela (Figure 1). This genotype presents a high heterogeneity, reflected by the large number of subtypes described worldwide,3 which is also evidenced in South America, where several subtypes have been reported: 3a, 3b, 3c, 3h, 3i, 3e, 3f.37, 38, 53, 60, 69, 70, 73-75, 77, 78, 80, 91-96 In some regions of the continent, the same subtypes (or subtypes belonging to the same clade) have been reported in humans, pigs and environmental samples,37, 38, 53, 69, 73, 74, 76 showing that these hosts and matrices could be involved in the HEV life cycle, and confirming the zoonotic transmission of this virus. On the other hand, in Bolivia, the subtype detected in humans and swines differed, suggesting host-specific infection without zoonotic-transmission.80
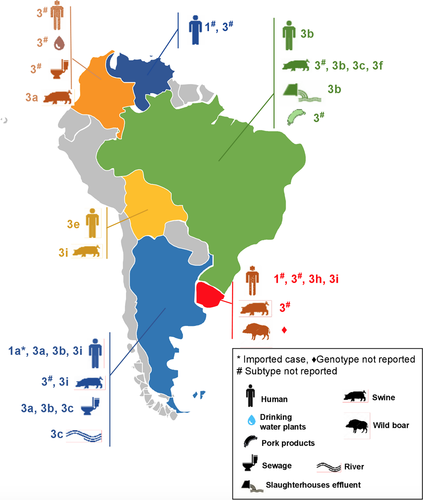
Hepatitis E virus-1 in South America was only detected in Venezuela and Uruguay (Figure 1), isolated from acute human hepatitis cases, although no large outbreaks have been reported in the continent.55, 56
A limitation for the study of molecular epidemiology in South America is the existence of short genomic fragments of different genomic regions (of all genotypes and subtypes), which makes comparisons and molecular analyses difficult to perform. Sequencing of larger genomic regions and/or complete genomes is needed to continue studying the phylogenetic and evolutionary relationships of the strains in the continent.
7 CONCLUSIONS
Hepatitis E is a global infection with an estimated incidence of millions of cases per year worldwide. In South America, there is sufficient evidence of its circulation in humans, animals and environmental matrices, although no large HEV outbreaks have been reported, showing a similar behaviour to other regions considered of low endemicity, such as Europe. The epidemiology of HEV in this continent displays some regional variations, which may impact in the public health in each country. Prospective studies aimed to investigate the clinical impact (presence of acute and chronic infections in populations not studied yet), genetic variations, life cycle and other aspects of this virus in our continent, would be suitable.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.




