Influenza A(H1N1)pdm09 vaccine effectiveness and other characteristics associated with hospitalization in chronic liver disease patients
Abstract
Background & Aims
To date, few studies have investigated the clinical effectiveness of influenza vaccine in chronic liver disease patients. The aim of this study was to examine the effectiveness of monovalent inactivated influenza A(H1N1)pdm09 vaccine and other characteristics associated with hospitalization in patients with chronic hepatitis C.
Methods
We conducted a hospital-based cohort study during influenza A(H1N1)pdm09 pandemic. A total of 408 patients (132 vaccinated, 276 unvaccinated) with detectable HCV-RNA were followed up with respect to any hospitalization using a weekly postal questionnaire. Reported hospitalizations were verified by medical records.
Results
During the epidemic period, 28 hospitalizations (6 vaccinated, 22 unvaccinated) were observed. After adjustment for potential confounders, vaccination decreased the odds ratio (OR) for hospitalization with marginal significance (OR = 0.43, 95%CI = 0.16–1.17). Besides, positive association with hospitalization was observed in patients with albumin levels <3.5 g/dl (OR = 8.40, 3.66–19.3) and steroid users (OR = 5.58, 0.98–31.7).
Conclusions
Among patients with chronic hepatitis C, A(H1N1)pdm09 vaccine appeared to have a protective effect against hospitalization. Those patients with a higher risk for hospitalization should be carefully followed during the influenza season, even when vaccinated.
Patients with chronic liver disease are classified as a high-risk group for influenza-related complications 1, 2. Influenza infection can cause hepatic decompensation and hospitalization in patients with advanced liver disease 3, 4. Thus, preventing severe influenza that requires hospitalization has been an important issue in patients with chronic liver disease.
As influenza vaccination is the most effective method for preventing influenza and its complications, the Advisory Committee on Immunization Practices in the USA has recommended annual influenza vaccination for patients with chronic liver disease since 2007 5. In Japan, however, no recommendations about influenza vaccination for these patients had been proposed prior to the 2009 influenza A (H1N1) pandemic. One of the reasons for this lack of recommendations might have been little scientific evidence regarding the clinical effectiveness of influenza vaccine among patients with chronic liver disease. To the best of our knowledge, there has been only one study on this topic until now. The Korean study indicated that seasonal influenza vaccine decreased the incidence of laboratory-confirmed influenza and associated symptoms in cirrhotic patients 6. However, no studies so far have demonstrated the effectiveness of influenza vaccine to prevent hospitalization in these patients.
Thus, the primary objective of this study was to examine the effectiveness of influenza A(H1N1)pdm09 vaccine in preventing hospitalization among patients with chronic liver disease. Using these data, the other characteristics associated with hospitalization were also assessed as a secondary objective.
Material and methods
Study subjects
In Japan, monovalent inactivated unadjuvanted split-virus influenza A(H1N1)pdm09 vaccine became available for tiered use in October 16, 2009. Vaccination was scheduled first for healthcare workers, pregnant women and then provided to patients with underlying illnesses (including the present study subjects) from November 2009, according to the order of priority of the groups. The present hospital-based cohort study was performed under the constraint of this national vaccination strategy.
Between November 2009 and January 2010 (i.e. recruitment), patients with chronic hepatitis C who had been under clinical follow-up at three medical institutions in Osaka, Japan, were invited to participate in this study. Eligible patients were those with detectable HCV-RNA levels at the time of recruitment, whereas those with a prior episode of influenza A(H1N1)pdm09 virus infection were excluded. A total of 416 subjects who agreed to participate were enrolled. All study subjects provided their written, informed consent after the nature and possible consequences of this study had been explained.
The study protocol was approved by the Ethics Committees at the Osaka City University Faculty of Medicine, Osaka City Juso Hospital and Osaka City General Hospital, and was performed in accordance with the Declaration of Helsinki.
Information collection
Three kinds of data were collected for each subject. Two kinds of data, physical and environmental characteristics, as well as clinical characteristics, were collected for use as baseline data, whereas data regarding subsequent hospitalization were collected weekly in the follow-up survey. Information on the following physical and environmental characteristics was collected using a self-administered questionnaire: status of influenza A(H1N1)pdm09 vaccination and date of vaccination (if vaccinated); sex, age (years), height (cm) and weight (kg); steroid treatment for two or more consecutive weeks within the last 6 months; underlying illnesses other than liver disease (hereinafter referred to as ‘other chronic diseases’) including diabetes mellitus, chronic heart disease, chronic renal disease, neuromuscular disease, asthma and chronic respiratory disease; smoking and alcohol habits; number of family members; and total room space in the patient's house (m2).
In addition, information about clinical characteristics was collected using a structured questionnaire that was completed by the physician-in-charge at the time of recruitment. The questionnaire gathered information about: current treatment with interferon; hepatocellular carcinoma; ascites; hepatic encephalopathy; and laboratory data such as platelet count (×104/mm3), albumin (g/dl) and prothrombin activity (%). Using these data, Child–Pugh Scores were calculated according to the conventional method 7. Child–Pugh Scores of 5 or more were considered to indicate cirrhosis.
With respect to the follow-up survey, the subjects were requested to fill out a weekly postal questionnaire about the following episodes during the preceding week: physician-diagnosed influenza, results of rapid antigen testing, if applicable, and hospitalization. The postal questionnaire was to be returned to the Department of Public Health, Osaka City University Faculty of Medicine each week during the follow-up period, which was between recruitment and the 15th week of 2010 (April 12–18). For subjects who had been vaccinated within 2 weeks before recruitment, to consider the time length required for a sufficient immune response, the follow-up started 2 weeks after vaccination 8. Reported hospitalizations were verified by medical records at three participating hospitals.
Outcome definitions and epidemic
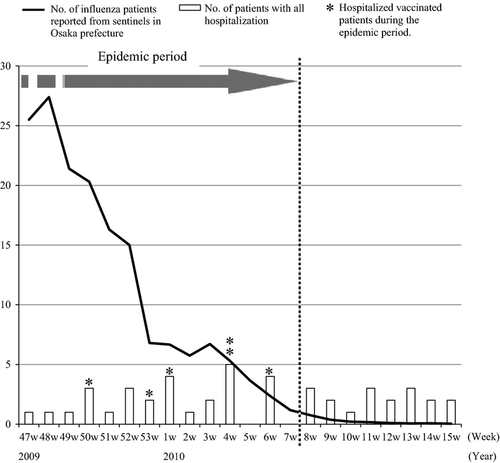
The study outcome was defined as hospitalization that occurred during the epidemic period of influenza A(H1N1)pdm09. The epidemic period was determined using the surveillance data in Osaka Prefecture and was defined as the period in which the weekly number of influenza patients remained at ≥1 per sentinel 9. Based on the epidemic curve (Fig. 1), the epidemic peaked in November (when this study started) and continued to the 7th week of 2010 (February 15–21). All influenza viruses isolated in Osaka Prefecture during this period were influenza A(H1N1)pdm09 virus strains.
Statistical analysis
Baseline characteristics were compared between vaccinated and unvaccinated subjects using the χ2 test and the Wilcoxon rank sum test. To evaluate the association between baseline characteristics and outcome, univariate and multivariate logistic regression models were employed to obtain crude and adjusted odds ratios (OR) and their 95% confidence intervals (CI).
In constructing a multivariate model, nine variables were selected for inclusion in the initial model, as three variables were distributed differently between vaccinated and unvaccinated subjects (P < 0.1) and the remaining variables were considered medically significant in relation to outcomes. Then, the reduced model was constructed, as the initial model included too many variables for the number of outcome events. In this process, variables that had no association with hospitalization in the results of initial models were excluded. Eventually, the final model included the following four variables: vaccination; other chronic diseases; steroid treatment within the last 6 months; and albumin level.
The results were also verified in the subgroup who was not receiving interferon therapy, as subjects receiving interferon therapy were likely to develop influenza-like symptoms because of the side effects of interferon, which might affect the results.
Furthermore, to consider the vaccine effectiveness according to liver function, stratified analysis by platelet counts or albumin levels was also conducted. All tests were two-sided. All analyses were performed using SAS version 9.1.3 software (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics of study subjects
Of the 416 patients with chronic hepatitis C, eight unvaccinated patients (2%) were excluded because of incomplete data in the follow-up surveys. Eventually, data from a total of 408 patients (132 vaccinated, 276 unvaccinated) were analysed.
Table 1 compares baseline characteristics between vaccinated and unvaccinated patients. The vaccinees included a smaller proportion of males (23% vs. 41%, P < 0.001), and had less habit of smoking (never smokers: 78% vs. 64%, P = 0.015) and alcohol drinking (never-drinkers: 77% vs. 66%, P = 0.038). Variables that were thought to be potentially associated with influenza, such as age, body mass index, steroid treatment, other chronic diseases, room space per person, interferon treatment, hepatocellular carcinoma and laboratory data suggesting cirrhosis, were distributed similarly between the vaccinated and unvaccinated patients.
| Characteristics | Category | Vaccinated (n = 132) | Unvaccinated (n = 276) | P a |
|---|---|---|---|---|
| Sex | Male | 31 (23) | 112 (41) | <0.01 |
| Age (years) | 65.0+ | 85 (64) | 176 (64) | 0.90 |
| Body mass index (kg/m2) | 25.0+ | 20 (15) | 49 (18) | 0.49 |
| Data missing | 2 | |||
| Steroid treatment within the last 6 months | Received | 5 (4) | 5 (2) | 0.22 |
| Data missing | 1 | |||
| Underlying illness other than liver disease | 57 (43) | 106 (39) | 0.37 | |
| Diabetes mellitus | 14 (11) | 33 (12) | 0.68 | |
| Chronic heart disease | 11 (8) | 15 (5) | 0.27 | |
| Chronic renal disease | 7 (5) | 13 (5) | 0.80 | |
| Neuromuscular disease | 7 (5) | 10 (4) | 0.43 | |
| Malignant neoplasm | 3 (2) | 14 (5) | 0.29 | |
| Asthma | 6 (5) | 8 (3) | 0.40 | |
| Blood dyscrasia | 3 (2) | 11 (4) | 0.37 | |
| Othersb | 8 (6) | 20 (7) | 0.65 | |
| Data missing | 1 | |||
| Smoking habit | Never | 103 (78) | 176 (64) | 0.02 |
| Ever | 16 (12) | 53 (19) | ||
| Current | 13 (10) | 47 (17) | ||
| Alcohol drinking habit | Never | 102 (77) | 181 (66) | 0.04 |
| Ever | 21 (16) | 57 (21) | ||
| Current | 9 (7) | 38 (14) | ||
| Room space per person (m2) | Mean (SD) | 43.2 (25.9) | 40.8 (26.8) | 0.37 |
| Unknown | 3 | 6 | ||
| Clinical characteristics at the time of recruitment | ||||
| Interferon treatment | Receiving | 40 (30) | 105 (38) | 0.12 |
| Data missing | 1 | |||
| Hepatocellular carcinoma | Present | 26 (20) | 56 (21) | 0.82 |
| Data missing | 5 | |||
| Laboratory data | ||||
| Platelet count (×104/mm3) | <10.0 | 42 (32) | 89 (32) | 0.89 |
| Data missing | 2 | |||
| Albumin level (g/dl) | <3.5 | 22 (17) | 41 (15) | 0.61 |
| Data missing | 1 | |||
| Prothrombin activity (%) | <80 | 19 (18) | 23 (13) | 0.35 |
| Data missing | 24 | 105 | ||
| Child–Pugh score | 5+ | 43 (40) | 72 (42) | 0.68 |
| A (5–6) | 37 (34) | 62 (36) | 0.80 | |
| B (7–9) | 5 (5) | 10 (6) | ||
| C (10+) | 1 (1) | 0 (0) | ||
| Data missing | 24 | 106 | ||
- SD, standard deviation. Data expressed as n (%) unless otherwise indicated.
- a The χ2 test or Wilcoxon rank sum test was employed where appropriate.
- b Others included 11 atopic disease, 7 pregnancy, 5 collagen disease, 4 cerebrovascular disease, 3 chronic respiratory disease and 1 immunosuppressive disease.
Association of influenza A(H1N1)pdm09 vaccine with hospitalization
Figure 1 shows the distribution of outcome events from the follow-up surveys of study subjects (bars). During the epidemic period (from the 47th week of 2009 to the 7th week of 2010), there were 28 hospitalizations (7%), including 6 vaccinated patients.
Table 2 shows the crude and adjusted ORs of influenza vaccine for hospitalization during the epidemic period. Compared with unvaccinated patients, vaccinated lowered the OR for hospitalization to about half in the crude analysis (OR = 0.55, 95%CI = 0.22–1.39). After adjustment for potential confounders, the decreased OR of vaccination reached the marginally significant level (OR = 0.43, 95%CI = 0.16–1.17). Even in the subjects who were not receiving interferon therapy, both the proportion of outcome events and the ORs of vaccination were almost the same as for the entire study subjects. However, ORs of vaccination somewhat fluctuated according to their liver function, and subjects with better liver disease status (i.e. platelet count ≥10.0 × 104/mm3 or albumin level ≥3.5 g/dl) seemed to be more likely to manifest vaccine effectiveness. Especially in subjects with platelet count ≥10.0 × 104/mm3, vaccination was associated with a decreased OR for hospitalization with marginal significance (OR = 0.19, 95%CI = 0.03–1.22).
| Stratified category | Vaccination status | N | n (%) | Crude OR (95%CI) | Adjusteda OR (95%CI) |
|---|---|---|---|---|---|
| Entire study subjects | Unvaccinated | 276 | 22 (8) | 1.00 | 1.00 |
| Vaccinated | 132 | 6 (5) | 0.55 (0.22–1.39) | 0.43 (0.16–1.17) | |
| Interferon therapy | |||||
| Not receiving | Unvaccinated | 170 | 15 (9) | 1.00 | 1.00 |
| Vaccinated | 92 | 5 (5) | 0.59 (0.21–1.69) | 0.43 (0.14–1.35) | |
| Receiving | Unvaccinated | 105 | 7 (7) | 1.00 | 1.00 |
| Vaccinated | 40 | 1 (3) | 0.36 (0.04–3.01) | 0.40 (0.04–3.87) | |
| Platelet count (×104/mm3) | |||||
| ≥ 10.0 | Unvaccinated | 185 | 12 (6) | 1.00 | 1.00 |
| Vaccinated | 90 | 2 (2) | 0.33 (0.07–1.50) | 0.19 (0.03–1.22) | |
| <10.0 | Unvaccinated | 89 | 10 (11) | 1.00 | 1.00 |
| Vaccinated | 42 | 4 (10) | 0.83 (0.25–2.82) | 0.75 (0.21–2.66) | |
| Albumin level (g/dl) | |||||
| ≥3.5 | Unvaccinated | 235 | 13 (6) | NA | NA |
| Vaccinated | 109 | 0 (0) | |||
| <3.5 | Unvaccinated | 41 | 9 (22) | 1.00 | 1.00 |
| Vaccinated | 22 | 6 (27) | 1.33 (0.40–4.40) | 1.13 (0.32–4.00) | |
- OR, odds ratio; CI, confidence interval; NA, not applicable.
- a Model includes underlying illnesses other than liver disease, steroid treatment within the last 6 months and albumin level, other than the stratified variable.
Association of other clinical variables with hospitalization
Table 3 shows the association of other baseline characteristics with hospitalization during the epidemic period. Patients with other chronic diseases had about a two-fold increased OR for hospitalization with marginal significance in the crude analysis (crude OR = 2.10, 95%CI = 0.97–4.57). After adjustment for potential confounders, however, the increased OR was not significant. Instead, OR of steroid use showed a marginal association with hospitalization (adjusted OR = 5.58, 95%CI = 0.98–31.7). In addition, patients with a lower albumin level had significantly increased ORs for hospitalization both in the crude and adjusted analyses (adjusted OR = 8.40, 95%CI = 3.66–19.3).
| Baseline characteristics | n (%) | Crude OR (95%CI) | Adjusteda OR (95%CI) |
|---|---|---|---|
| Vaccination status | |||
| Unvaccinated | 22 (8) | 1.00 | 1.00 |
| Vaccinated | 6 (5) | 0.55 (0.22–1.39) | 0.43 (0.16–1.17) |
| Underlying illness other than liver disease | |||
| Absent | 12 (5) | 1.00 | 1.00 |
| Present | 16 (10) | 2.10 (0.97–4.57) | 1.82 (0.80–4.14) |
| Steroid treatment within the last 6 months | |||
| Not received | 26 (7) | 1.00 | 1.00 |
| Received | 2 (20) | 3.57 (0.72–17.7) | 5.58 (0.98–31.7) |
| Albumin level (g/dl) | |||
| <3.5 | 15 (24) | 7.96 (3.57–17.7) | 8.40 (3.66–19.3) |
| 3.5+ | 13 (4) | 1.00 | 1.00 |
- OR, odds ratio; CI, confidence interval; NA, not applicable.
- a Model includes all variables in this table.
Other liver function markers were also investigated by incorporating them into the model instead of the albumin level, as the positive association between a lower albumin level and hospitalization seemed to represent an association with advanced liver disease. The adjusted ORs for hospitalization of any liver function markers were also increased: platelet count <10.0 × 104/mm3 (OR = 2.10, 95%CI = 0.96–4.60), prothrombin activity <80% (OR = 4.32, 95%CI = 1.69–11.1), Child–Pugh Score of 5 or more (OR = 3.51, 95%CI = 1.38–8.92) and hepatocellular carcinoma (OR = 3.09, 95%CI = 1.38–6.91).
Discussion
In this study among patients with chronic hepatitis C, there was an indication of vaccine effectiveness for preventing severe outcomes requiring hospitalization during an epidemic. Although the limited number of outcome events made it difficult to detect significant vaccine effectiveness, the present results support the usefulness of influenza vaccine for patients with chronic hepatitis C.
To date, no study has reported the effectiveness of influenza A(H1N1)pdm09 vaccine against hospitalization in patients with specific underlying medical conditions including chronic liver disease. However, based on the reports about vaccine effectiveness among subjects with any high-risk condition, a cohort study in Denmark showed that vaccine conferred protection against influenza-related hospitalization to 44% (−19–73%) among subjects <65 years with underlying illnesses 10. A matched case–control study in the Netherlands indicated that the vaccine effectiveness for influenza-related hospitalization was 19% (−28–49%) among subjects with high-risk conditions 11. Although these studies did not refer to vaccine effectiveness in patients with individual underlying illnesses, the present results among patients with chronic hepatitis C would correspond to those in subjects with any high-risk conditions.
Influenza infection occasionally causes hepatic decompensation without typical influenza symptoms in patients with chronic liver disease 4, 6, which might bring about delayed antiviral therapy and increase influenza-related mortality. Thus, it was an important finding that influenza vaccine had some effect for reducing hospitalization during the epidemic period, although the present results were not significant. According to the previous studies, vaccination for cirrhotic patients lowered the incidence of laboratory-confirmed influenza and atypical influenza symptoms such as myalgia, hepatic decompensation, oliguria and uncontrolled ascites during influenza season 6. Furthermore, some reports have indicated that influenza vaccine was sufficiently immunogenic in patients with cirrhosis 12-15. Taken together, it would be reasonable to advise vaccination for patients with chronic liver disease. In fact, the Advisory Committee on Immunization Practices in the USA has recommended annual influenza vaccination for patients with chronic liver disease since 2007 5, and the WHO position paper has indicated that patients with specific chronic medical conditions continue to be an appropriate target group for annual influenza vaccination 16.
In this study, however, subjects with advanced liver disease (represented by lower albumin level) had a higher risk for hospitalization during the epidemic period, irrespective of their vaccination status (Table 3). These results corresponded to a previous case report in which influenza infection caused hepatic decompensation and hospitalization in patients with advanced liver disease 4. Influenza virus itself could cause hepatitis 17, and influenza infection could induce toxic metabolites and proinflammatory cytokines such as TNF-α, IL-1 and IL-6, which contribute to hepatic damage 18, 19. These seemed to result in disease deterioration, especially in patients with advanced liver disease. Thus, it would be better for subjects with advanced liver disease to be followed with special attention during the season, even when vaccinated.
In addition, steroid treatment and the presence of other chronic diseases were related to hospitalization during the epidemic period, independent of vaccination status or liver function. Steroid treatment and the presence of chronic diseases have been the known high-risk factors for influenza and its complications 5. In the 2009 influenza pandemic, immunosuppressive therapy and chronic diseases (especially asthma) were among the highest comorbid conditions in critically ill patients with influenza A(H1N1)pdm09 infection in the USA 20, Canada 21, Australia 22 and Mexico 23. The present results agreed with these findings. Patients on immunosuppressive therapy have impaired vaccine responses 24, and patients with asthma are expected to have similar vaccine responses, as they often receive steroid treatment. These backgrounds of poor immunological responses might bring about the high sensitivity for influenza infection and severe outcomes owing to influenza.
When interpreting the present results, however, the following limitations should be considered. Firstly, the insufficient statistical power owing to the small sample size and the limited number of outcome events is obviously important. This limitation made it difficult to detect significant vaccine effectiveness. If more subjects could be recruited, more meaningful results would be obtained. However, studies on pandemic influenza vaccine must be conducted under strict time constraints, as pandemic influenza virus had circulated and pandemic influenza vaccines became available during the epidemic. In addition, the epidemic subsided after sufficient distribution of the vaccines. This tight time schedule represented a major obstacle to recruiting a sufficient number of vaccinated and unvaccinated subjects for any observational prospective cohort study.
Secondly, voluntary enrolment in the observational study might lead to selection bias in the vaccination status. In fact, female patients, non-smokers and non-drinkers tended to receive vaccination in this study, which might lead to a healthy vaccinee effect. However, even when additional analyses that adjusted for these variables were conducted, similar results were obtained (ORs of vaccination were 0.45 (95%CI = 0.16–1.26). On the other hand, the determination of vaccination status relied on patients’ self-reports and could not be confirmed by their medical records, as patients usually received any vaccination in their neighbouring clinic. Thus, some non-differential misclassification in the vaccination status might have occurred.
Thirdly, there might be some concern about outcome misclassification, as hospitalization is a less specific outcome for influenza. In this study, however, the methods in which outcomes were confined into the epidemic period would have helped to minimize outcome misclassification and obtain a higher specificity of influenza for hospitalization. Furthermore, hospitalization is essentially considered an objective outcome that can be verified by the medical records, and therefore misclassification owing to non-influenza illness, if any, would be non-differential between vaccinated and unvaccinated patients 25. Such misclassification leads to an underestimation of vaccine effectiveness and does not materially affect the validity of the results.
Finally, previous immunity in unvaccinated patients might affect the underestimation of vaccine effectiveness to some extent. Based on a serological study, about one-third of subjects aged ≥65 years was reported to have pre-existing antibody before the epidemic, as many had been exposed to antigens similar to influenza A(H1N1)pdm09 virus during childhood 26. In this study, however, although about two-thirds of subjects were ≥65 years old, the proportion of subjects with pre-existing antibody was expected to be lower than in previous studies, because the immunogenicity study of influenza A(H1N1)pdm09 vaccine, in which part of this study subjects participated, indicated that only about 5% of subjects had the pre-existing antibody at the beginning of the pandemic 12. Thus, the effect of previous immunity, if any, would be very minimal.
In conclusion, among patients with chronic hepatitis C, influenza A(H1N1)pdm09 vaccine was suggested to have some protective effect against hospitalization during the epidemic period. As patients with advanced liver disease, steroid treatment and other chronic diseases (especially asthma) are considered to be at higher risk for hospitalization during the epidemic period, they should be followed up with special attention during the season, even when vaccinated.
Acknowledgements
Financial support: This study was supported by a research grant for Research on Emerging and Re-emerging Infectious Diseases, Health and Labour Sciences Research Grants from the Ministry of Health, Labor and Welfare, Japan (H20-SHINKO-IPPAN-002).
Conflicts of interest: The authors do not have any disclosures to report.




