Reproductive organs and spermatogenesis of the peculiar spermatozoa of the genus Kryptodasys (Gastrotricha, Macrodasyida), with an appraisal of the occurrence and origin of the tail-less spermatozoa in Gastrotricha
Contributing authors: Maria Balsamo ([email protected]), Marco Ferraguti ([email protected]), M. Antonio Todaro ([email protected])
Abstract
enThe presence of aflagellate spermatozoa in two species of Kryptodasys (Fam. Macrodasyidae), a gastrotrich genus recently described, is reported. Their ultrastructure shows common features, which appear peculiar to the genus and different from those of male gametes of the phylogenetically unrelated Dolichodasys (Fam. Cephalodasyidae), the only other macrodasyidan genus known to have aflagellate sperm. Additional information on the ultrastructure of the genital apparatus of the two Kryptodasys species is given, which confirm the data of the originals description and the systematic delimitation of this new genus from Macrodasys, which was mainly based on the structure of the reproductive system until now. The functional and phylogenetic significance of the aflagellate condition of spermatozoa in the two taxa under study and in other species is discussed also in the light of the generally flagellate condition of functional sperm in Gastrotricha.
Astratta
itÉ descritta la presenza di spermatozoi aflagellati in due specie di Kryptodasys (Fam. Macrodasyidae), genere recentemente descritto nell’ambito del phylum Gastrotricha. La loro ultrastruttura mostra caratteri comuni che appaiono peculiari del genere e diversi da quelli degli spermatozoi del genere Dolichodasys (Fam. Cephalodasyidae), filogeneticamente non correlato a Kryptodasys, ma che rappresenta l’unico altro genere noto di Macrodasyida con spermatozoi aflagellati. Inoltre, sono fornite anche informazioni ultrastrutturali dell’apparato genitale delle due specie di Kryptodasys, le quali confermano i dati della descrizione originale, basata principalmente sulla struttura del sistema riproduttivo, e la posizione sistematica del genere come clado distinto dal genere Macrodasys, Il significato funzionale e filogenetico della condizione aflagellata degli spermatozoi, nelle specie qui studiate e in altre specie, viene discusso alla luce della generale condizione flagellata degli spermatozoi nei Gastrotricha.
1 INTRODUCTION
Gastrotricha compose a phylum of minute, acoelomate, and aquatic worms, which recent studies have found to be phylogenetically allied with the Platyhelminthes, with which it forms a clade named Rouphozoa (e.g., Egger et al., 2015; Struck et al., 2014; see also Balsamo et al., 2020). Gastrotrichs, similarly to their likely sister taxon, are primarily hermaphroditic, although parthenogenesis is known to occur in several marine lineages and is the rule in the numerous freshwater species encompassing a wide taxonomic spectrum (e.g., Kånneby et al., 2013; Leasi & Todaro, 2009; Weiss, 2001). Uniparental species reproduce by apomictic parthenogenesis while reproduction in hermaphroditic species is biparental through internal cross fertilization. In the latter species, the spermatozoon usually is a filiform cell including an anterior region, containing acrosome and nucleus, and a posterior flagellar portion with a typical 9 × 2 + 2 axoneme. The anterior region appears different when compared with the spermatozoa of the representatives of the two orders, Chaetonotida and Macrodasyida, into which the phylum is currently subdivided (e.g., Balsamo et al., 2013; Hummon & Todaro, 2010). In the Chaetonotida, it is rectilinear, while in the Macrodasyida, it is spiral in virtue of a corkscrew-shaped acrosome and a spring-shaped nucleus often coiled around an internal large mitochondrion. However, exceptions to these general schemes have been unveiled over the years (e.g., Balsamo et al., 2007; Ferraguti et al., 1995; Fischer, 1994; Guidi et al., 2017; Marotta et al., 2005).
The most striking example of male gametes diverging from the usual appearance and ultrastructure of the Gastrotricha spermatozoa is offered by the small, stout sperm cells reported for several taxa and described by the authors as commaform, rod-like, nail sperm, and others due to their appearance under light microscopy (e.g., Kisielewska, 1981; Remane, 1936; Ruppert, 1991; Ruppert & Shaw, 1977; Weiss, 2001). A shared feature of most of these spermatozoa is the alleged absence of a flagellum, although other traits of their general organization may be very different, for example, presence/absence of mitochondria and/or acrosome (e.g., Balsamo, 1992; Hummon, 1984).
The sperm of macrodasyidan gastrotrichs are frequently observed to move inside the mature animals in the gonads or, more often, in the partner's accessory sexual organ known as the frontal organ, which is female in function (i.e., seminal receptacle). The spermatozoa stored in the frontal organ are acquired from a partner by internal fertilization generally via an external pore. Details regarding different sperm transfer modalities may be found in the studies of Ruppert (1978a) and Kieneke et al. (2008), Kieneke et al. (2009), while specifics on the function of the frontal organ are reported by Todaro, Dal Zotto, Kånneby, et al. (2019). In several instances, allospermatozoa have been seen actively exiting the frontal organ through an internal pore and reaching a nearby oocyte, thus allowing fertilization (e.g., Kieneke & Shmidt-Rhaesa, 2015; Ruppert, 1991). Consequently, sperm movement may be considered a proxy to attest their capability to fertilize the egg. On the other hand, the tail-less spermatozoa appear to be always static, casting doubts about their real functionality, but yet the growing oocytes inside the animals suggest that fertilization has occurred. The absence of a flagellum can raise the question of how this cell can move, allowing it to exit the frontal organ, to head for the oocyte, and to finally fertilize.
The genus Kryptodasys includes vermiform, marine, interstitial species, up to 1000 μm in total length. The general morphology of these gastrotrichs, including the bare cuticle, recalls that of Macrodasys species (Figures 1a and 2a). However, the layout of the reproductive system and the structure of the accessory reproductive organs clearly differentiate species of the two genera. Phylogenetic analyses based on the 18S rRNA gene showed the genus Kryptodasys as part of the Macrodasyidae but on a clade distinct from the genus Macrodasys (Todaro, Dal Zotto, Kånneby, et al., 2019).
Recently, we have described the pod-like, tail-less spermatozoa of Dolichodasys sp. (Macrodasyida, Cephalodasyidae) from the Pacific coast of Panama, for which a hypothesis about the way it could move and thereby allowing fertilization has been put forward (Guidi et al., 2017). The main goal of this paper falls within this line and aims at describing the structure and ultrastructure of the tiny spermatozoa of two species belonging to Kryptodasys, a recently described macrodasyidan genus of the family Macrodasyidae whose spermatozoa appear, under light microscopy, lacking a tail and motionless (Todaro, Dal Zotto, Kånneby, et al., 2019). Aspects of the gametogenesis and of the structure of the reproductive organs in one species of the genus are also clarified.
2 MATERIAL AND METHODS
Sand containing specimens of Kryptodasys carlosrochai was collected by skin diving in April 2002, from the sublittoral (2–4 m water depth) of Praia de Castelhanos, a beach on the East coast of Ilhabela, São Paulo, Brazil (see also Todaro, Dal Zotto, Kånneby, et al., 2019). Specimens were extracted by the narcotization–decantation technique (using a 7% magnesium chloride solution as anesthetic), identified, and photographed under a Zeiss Axioscop 2 Plus microscope equipped with DIC lenses and an attached Nikon Coolpix 995 camera. Subsequently, animals were recovered from the slides, fixed overnight in a 0.1-M phosphate buffered (pH 7.3) solution of paraformaldehyde, glutaraldehyde, and picric acid (Ermak & Eakin, 1976), and stored for future analysis (Todaro & Rocha, 2004).
Sand containing specimens of Kryptodasys sp. was collected by scuba diving in 2001 at about 10 m water depth, off Pomonte, Elba Island, Italy. Extraction, fixation, and storage until further processing were similar to those described previously; however, identification and documentation were carried out with a Leitz Dialux microscope.
For transmission electron microscopy, five specimens of K. carlosrochai and four of Kryptodasys sp. were taken from the fixative solution, washed with 0.1 M sodium cacodylate buffer and post-fixed in 1% osmium tetroxide in the same buffer. Then they were repeatedly washed in the buffer, dehydrated in a graded ethanol series, and embedded in Araldite. Semi-thin (2 µm thick) and ultra-thin sections (70 nm thick) from the same specimen were cut with an LKB Ultrotome 2088V. Thick sections were stained with toluidine blue, whereas thin sections were contrasted with uranyl acetate (saturated solution in 50% ethanol) followed by lead citrate solution. The observations of ultra-thin sections were carried out under a Philips CM10 transmission electron microscope. The position of the morphological characters along the body is given in percentage units (U) of the total body length measured from anterior to posterior (Hummon et al., 1992).
3 RESULTS
3.1 Reproductive apparatus: general arrangement
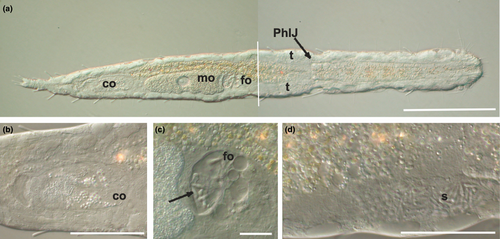
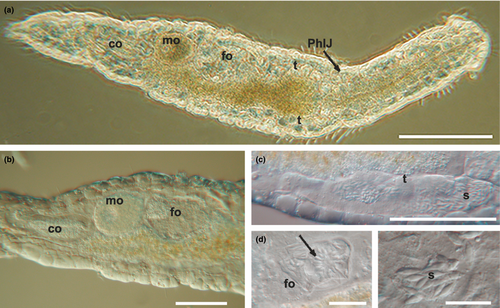
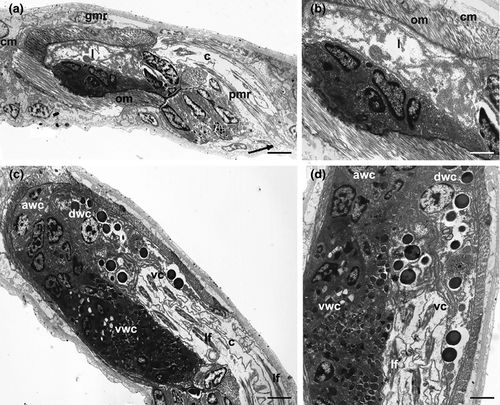
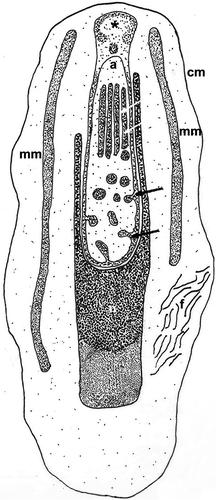
Mature specimens of K. carlosrochai and Kryptodasys sp. are hermaphroditic. Two elongate testes start laterally just anterior to the pharyngo-intestinal junction (PhIJ) and extend posteriorly into short sperm ducts, which presumably open on the ventral surface (Figures 1a,d and 2a,c). A single ovary is located in the second third of the trunk dorsally to the intestine. The mature oocyte is located slightly after the mid body (Figures 1a and 2a,b). A vesicular frontal organ divided into two compartments and containing some spermatozoa is present anteriorly to the largest oocyte (Figures 1a,c and 2a,b,d,e). A glandulo-muscular caudal organ lies in the caudal trunk region: it is bullet shaped and shows an internal canal opening on the ventral surface, anteriorly to the anus (Figures 1a,b and 2a,b).
3.2 Ovary and oogenesis of K. carlosrochai
In K. carlosrochai, a single ovary dorsal to the posterior intestine extends from about half to two thirds of the body posteriorly (from U54 to U76). In mature specimens, at least six to seven oocytes maturing in a caudo-cephalic direction are arranged in a series from the front end of the caudal organ to the posterior margin of the frontal organ. The most anterior oocyte (at U61) is full-grown and reaches the size of 90 μm in length and 30 μm in width (Figure 1a). The cytoplasm of the mature egg is densely filled with yolk granules (Figure 3a) with a variable size (diameter ranging from 0.2 to 2 μm), texture, and electron density. Most granules show an electron-dense central core surrounded by a much less electron-dense periphery (Figure 3a,b,e). Previtellogenic oocytes have a large nucleus with a distinct nucleolus and uncondensed chromatin and numerous ribosomes and mitochondria in the cytoplasm (Figure 3b). Peculiar epithelial cells have been observed around all oocytes to form a wall completely surrounding the ovary (white arrows in Figure 3c–f).
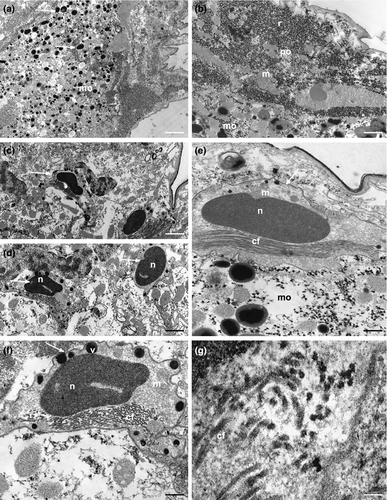
These cells forming a monolayer about 3 μm thick show an irregular shape and an ovoidal nucleus (2 × 0.8 μm) containing only heterochromatin. The cytoplasmic compartment is large and rich in mitochondria, ribosomes, strongly electron-dense vesicles, and collagen fibrils (Figure 3c–g).
3.3 Frontal organ of K. carlosrochai
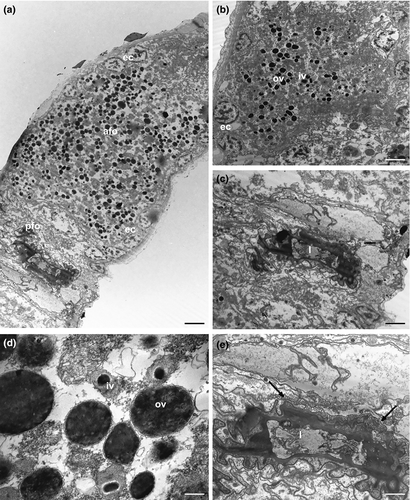
The epithelial frontal organ is roughly globose in shape (approximately 20 μm in diameter); it is located in the mid of the body (U51), anteriorly to the mature oocyte. The ovary separates the frontal organ from the caudal organ. Optical microscope observations revealed that the frontal organ is clearly formed by two parts: vacuoles fill the anterior part whereas several immotile spermatozoa have been observed in the posterior one (black arrows in Figure 1c). Electron microscope observations confirmed this internal compartmentalization (Figure 4a). The wall of the anterior part is formed by epithelial cells, with the nucleus facing outwards and abundant cytoplasm with mitochondria, Golgi apparatus, well-developed endoplasmic reticulum, and numerous vesicles (Figure 4b). The latter are of different shapes, textures, and sizes; most of them are oval and filled with strongly electron-dense, non-homogeneous material; others have a more irregular shape with a dark core surrounded by electron-transparent peripheral material. All the vesicles are lined with a curled membrane slightly detached from the internal contents (Figure 4d). The wall of the posterior part of the frontal organ is formed by vacuolated epithelial cells, surrounding a wide lumen (Figure 4c). The latter is lined by cells with small nuclei, a thick electron-dense layer of uncertain nature and long cytoplasmic processes, which form a very high number of microvilli on the side facing towards the lumen of the anterior part of the frontal organ (Figure 4e). Spermatozoa were not present in the observed sections.
3.4 Caudal organ of K. carlosrochai
Optical microscopy observations show a voluminous, bullet shaped, and glandulo-muscular caudal organ extending from U73 to U82 (Figure 1a,b). Longitudinal sections observed under TEM confirm this general aspect and highlight two parts clearly recognizable: an anterior one, glandulo-muscular (54 μm long, U73–U79) corresponding to the bullet tip, and a posterior one, muscular (15 μm long, U79–U82) corresponding to the bullet base (Figure 5a,c). The anterior region has two muscular layers: the outer one thin, with circular fibers, and the inner one thick, with oblique fibers. The wall of this region is made up of three different cell types that define three distinct zones. The anterior zone is formed by cells arranged in two or three layers, which have a large central nucleus and a cytoplasm full of ribosomes and granular material; secretory granules are rare. The posterior wall cells are of two different types in the ventral and the dorsal zone, respectively. The cells of the ventral zone are full of secretory small granules (0.45 μm long), oval or piriform in shape; their nuclei are close to the cell side facing the musculature whereas the cytoplasm faces the lumen of the organ. The cells of the dorsal zone are large, with ovoid nuclei. Their cytoplasm is rich of cisternae of rough endoplasmic reticulum and round secretory granules (0.2–1 μm in diameter) with a less electron-dense central core surrounded by a strongly electron-dense sphere. The wall of the glandulo-muscular region surrounds a wide lumen full of granular material but without spermatozoa, at least in the observed sections (Figure 5a–d). The lumen extends into a long canal directed outside, which draws out from the posterior-dorsal part of the caudal organ, runs posteriorly for a long tract (40 μm), and finally bends ventrally opening anteriorly to the anus at U82 (Figure 5a). This canal is lined with vacuolated cells containing muscle fibers oriented parallel to the major axis of the organ (Figure 5c,d). The posterior muscular region of the organ is formed by tall and thin cylindrical cells with a very elongated nucleus and cytoplasm completely filled with muscle fibers arranged parallel to the main cell axis. These cells are interspersed with glandular cells in which small, electron-dense, secretory granules were visible (Figure 5a).
3.5 Spermatogenesis and spermiogenesis in K. carlosrochai
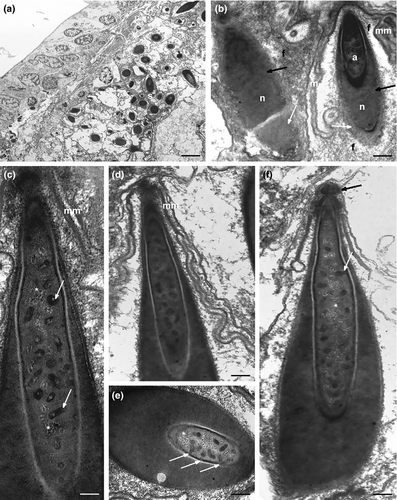
The spermatogenesis occurs in a caudo-cephalic direction so that primary germ cells are located in the posterior region of each testis while spermatids and mature spermatozoa are in the mid and the anterior parts, respectively (Figures 1a,d and 6a). The spermatocytes are large, round cells characterized by an irregular nucleus with chromatin condensed in numerous, scattered clumps of heterochromatin. In the cytoplasm of primary spermatocytes many free ribosomes, several polyribosomes and a high number of mitochondria are grouped near a cell pole. Endoplasmic reticulum and Golgi apparatus are evident in the cytoplasm of secondary spermatocytes. Moreover, a large vesicle, probably the pro-acrosome, with a highly electron-dense center is visible near the cis face of Golgi cisternae (Figure 6b).
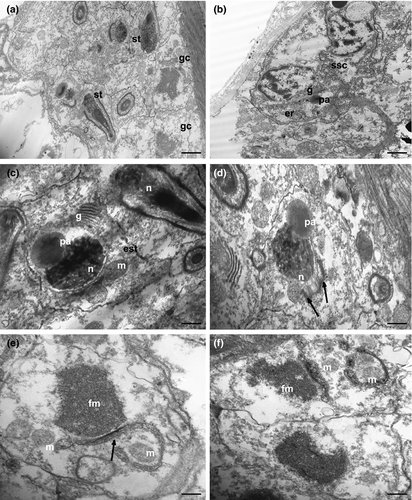
As spermiogenesis begins, spermatids start to elongate. The early spermatids are irregularly shaped, with an elongating nucleus, ovoid in cross-section (about 1 μm in diameter), and characterized by a condensed chromatin network (Figure 6c,d). Cytoplasmic organelles are numerous: mitochondria, ribosomes, Golgi complexes, and large clusters of filamentous material (Figure 6c–f). The pro-acrosomal vesicle makes contact with the nucleus and becomes wider and longer (Figures 6c,d and 7a). Mitochondria are numerous, some of which with a conventional appearance, while others appear to merge with each other (black arrows in Figure 6d,e): this process is gradual and leads to the formation of the mantle-shaped mitochondrion (Figure 7a). Intercellular bridges connect the cytoplasm of adjacent early spermatids (Figure 7a). When the mitochondrion is fully formed, several microtubules with oblique arrangement appear below it and form a bottleneck structure (Figure 7b), always close to the pro-acrosomal vesicle, which still maintains the shape of a vesicle with an electron-dense center (Figure 7b). The pro-acrosomal vesicle then moves into the bottleneck structure under the microtubules, and through numerous invagination and projections progressively forms the definitive acrosome (Figure 7d). The late spermatids are relatively elongated cells with a not fully condensed nuclear chromatin and the acrosome already located in the deep nuclear pouch. All the cytoplasmic space is occupied by the mantle-shaped mitochondrion and filamentous material, and no other cytoplasmic organelles are present (Figure 7c,d).
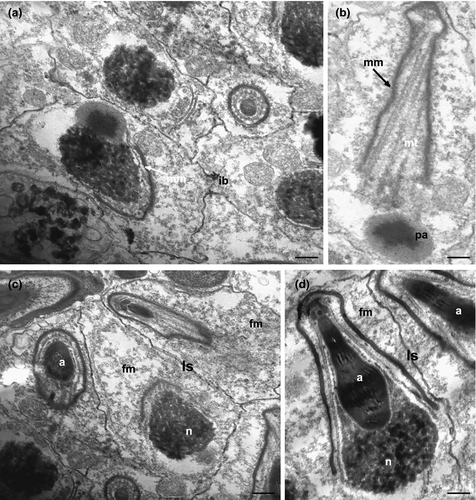
3.6 Mature spermatozoon of K. carlosrochai
Under optical microscopy, mature spermatozoa of K. carlosrochai have the shape of pumpkin seeds with a well-evident pointed anterior extremity (4 μm long and 1.5 μm wide) but no discernible flagellum (Figure 1c,d). The observations under the electron microscope confirm this general shape and the absence of a flagellum. Longitudinal sections of the sperm cell showed an electron-dense central structure core (2 μm long and 0.5 μm wide) composed of two well-distinct parts: the acrosome, corresponding to the pointed anterior region, and the nucleus, respectively (Figures 8a,b and 9). The acrosome (0.6 μm long ×0.3 μm wide) shows a highly convoluted club-shaped acrosomal vesicle connected through a narrow connection to an apical opercular structure similar to a bottle cap (white arrow in Figure 8b). The acrosomal vesicle appears electron dense in longitudinal sections, with rod-shaped and tubular structures, the latter regularly arranged in parallel rows (black and white arrows in Figure 8e,f). The nucleus (1 μm long and 0.5 μm in diameter) is rod shaped with a deep anterior pouch hosting the acrosome (Figures 8a–d and 9). The nuclear chromatin is organized in two concentric regions: the external one (black arrows in Figure 8c,d) surrounds the other, more electron dense (white arrows in Figure 8c,d), in the form of thin line for the whole length of the nucleus, and extends posteriorly for about 0.5 μm creating a well-developed nuclear base (Figures 8a,c,d and 9). A peculiar, single, mitochondrion covers the two anterior thirds of the nucleus like a mantle. The real nature of this organelle can only be interpreted in the light of the spermatogenesis (Figures 8b–d and 9). Numerous filaments adherent to the nuclear membrane protrude into the cytoplasm reaching the sperm plasma membrane (Figures 8b and 9). In addition, the space between the internal side of plasma membrane and the external membrane of the underlying mitochondrion is full of filaments (Figure 8b); the plasma membrane has a corrugated appearance.
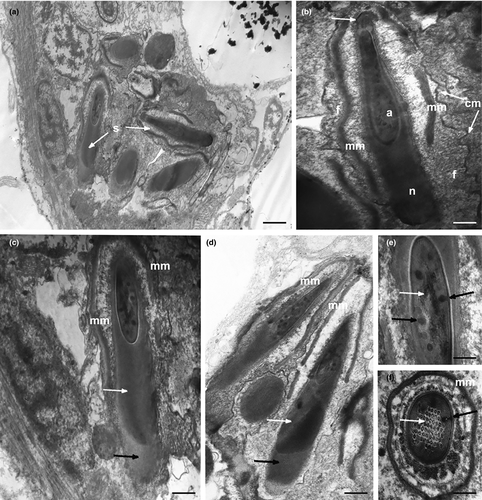
3.7 Mature spermatozoon of Kryptodasys sp.
The mature spermatozoa of Kryptodasys sp. have the shape of a pumpkin seed with the pointed anterior end not very prominent (4.5 μm long and 3.5 μm wide). A long electron-dense structure (4 μm long and 2 μm wide) composed of the acrosome and the nucleus (Figure 10a–f) lies in the cell center. The acrosome (2 μm long ×400 nm wide) is a long, almost cylindrical club with a short grip, which is topped by an opercular structure (black arrow in Figure 10f). The acrosomal vesicle rises in many points, forming rod-shaped and tubular structures (white arrows and asterisks in Figure 10c,e,f). The nucleus (3 μm long and 1.5 μm in diameter) surrounds the acrosome and in longitudinal section is U-shaped with the two U wings very close at the apex (Figure 10c,d,f). The posterior part of the nucleus is made up of chromatin arranged into two regions clearly distinguishable for a different electron density: the external region (white arrows in Figure 10b) forms a thin hood surrounding the more electron-dense inner one. A peculiar, single mantle-shaped mitochondrion covers the nucleus for almost its entire length (Figure 10b–d,f). The sperm cell, wrapped by a significantly folded cytoplasmic membrane, has a large amount of cytoplasm full of numerous filaments (Figure 10b–d). No flagellum is visible.
4 DISCUSSION
4.1 Reproductive apparatus: general arrangement
The ultrastructural observations carried out in this study confirm the general arrangement of the reproductive system of Kryptodasys described by Todaro, Dal Zotto, Kånneby, et al. (2019), thus providing support to the establishment of this genus based on morphological characteristics. It is worth mentioning that Kryptodasys has been erected by Todaro, Dal Zotto, Kånneby, et al. (2019) primarily on the basis of the reproductive system and spermatozoa peculiarities, which clearly distinguish the members of this genus from those of Macrodasys. The present study confirms the frontal and the caudal organ of Kryptodasys species being separated by the female gonad, whereas in Macrodasys the two organs are positioned next to each other (e.g., Evans, 1994; Todaro & Leasi, 2013). Moreover, our study clearly shows that the male gametes of Kryptodasys are clearly aflagellate in contrast with those of the species of Macrodasys, which possess a regular flagellum (Marotta et al., 2005). A phylogenetic analysis based on molecular data placed Kryptodasys within the family Macrodasyidae but on a clade clearly separated from Macrodasys, despite the overall general morphological similarity exhibited by the two genera (Todaro, Dal Zotto, Kånneby, et al., 2019).
4.2 Ovary and oogenesis of K. carlosrochai
As in most gastrotrichs, in K. carlosrochai, the oocytes mature in a caudo-cephalic direction (Balsamo et al., 2002; Guidi et al., 2011; Kieneke & Schmidt-Rhaesa, 2015; Ruppert, 1978a; Todaro et al., 2012). The presence of an epithelial wall lining the entire female gonad (early oocytes plus mature egg/s) is a feature that Kryptodasys shares with other macrodasyidan species, for example, Macrodasys sp. 1 and 2 (Ruppert, 1978a), Oregodasys (= Platydasys) cf. ocellatus, Thaumastoderma heideri, Acanthodasys sp., and Diplodasys ankeli (Ruppert, 1978b). The collagen fibrils, observed within the cells of the epithelial wall, will most likely form the thick layer of protein material covering the eggshell, already observed in some Macrodasyida (Teuchert, 1968).
The lack of the epithelial wall covering the early oocytes has been reported in Tetranchyroderma bunti (Ruppert, 1978b), Dactylopodola typhle (Kieneke et al., 2008), Neodasys chaetonotoideus (Kieneke et al., 2009), Paraturbanella teissieri (Balsamo et al., 2002), Crasiella diplura (Guidi et al., 2011), and Dinodasys mirabilis (Todaro et al., 2012).
The presence of the epithelial wall around the mature oocyte, forming the uterus section of the female reproductive system, is believed to be the plesiomorphic condition in Gastrotricha, whereas its presence around the entire female gonad (early oocytes + mature oocyte) is considered a derived condition given that basal taxa such as Neodasys and Dactylopodola lack it (Kieneke et al., 2009).
4.3 Frontal organ of K. carlosrochai
Electron microscopy observations confirm that the frontal organ of K. carlosrochai is compartmentalized in two well-distinct parts as observed by Todaro, Dal Zotto, Kånneby, et al. (2019). Within Macrodasyidae, Kryptodasys shares a similar compartmentalization of the frontal organ with the members of Macrodasys, where the two parts are functionally described as a seminal receptacle and a spermatheca (Evans, 1994; Ruppert, 1978a). Among Macrodasyida, two morpho-functional compartments are reported also for the frontal organ (bursa) of Xenodasys riedli and Chordodasiopsis antennatus (Xenodasyidae; Rieger et al., 1974; Schoepfer-Sterrer, 1969). However, the real function of this putative frontal organ in these species is poorly understood (see note by Rieger et al., 1974). A simple rounded epithelial sac-like organ, without a clear morpho-functional partitioning, is reported for many other taxa, for example, Dolichodasys carolinensis (Fam. Cephalodasyidae), Thaumastodermatinae, Turbanellidae, and Planodasyidae species (Guidi et al., 2014; Ruppert, 1978b; Ruppert & Shaw, 1977; Todaro et al., 2012).
The different structures and contents of the two compartments of the frontal organ of Kryptodasys leave little doubt that these two portions perform different functions, which presumably can be assimilated to those described for Macrodasys, that is, anterior spermathecal and posterior seminal receptacle. However, the apparent absence of external and internal pores of the frontal organ in Kryptodasys may raise doubts on the homologous nature of the two compartments in the frontal organ of Macrodasys and Kryptodasys. The different anatomical positions of the frontal organ in the two taxa must be highlighted further on: it is anterior to the largest egg in Kryptodasys and posterior to the largest egg in Macrodasys, which would lead to hypothesize an independent evolutionary origin of the frontal organ in the two genera. If the latter hypothesis is correct, then caution should also be taken while scoring the frontal organ trait in a more comprehensive phylogenetic analysis of Gastrotricha based on morphological characteristics.
The independent evolutionary origin of the frontal organ of most taxa and the “frontal sac” of some taxa of the Macrodasyida (e.g., Acanthodasys and Diplodasys) was already discussed by Kieneke et al. (2009). The “frontal organ” of Kryptodasys could either be homologous to either to the frontal organ or to the “frontal sac” of other gastrotrichs or it may represent another (third) solution of a seminal receptacle in Gastrotricha.
4.4 Caudal organ of K. carlosrochai
The glando-muscular nature of the caudal organ of Kryptodasys is in line with that of the other Macrodasyida and equates it to a copulatory organ as in the other Macrodasysida. The structure of caudal organ, however, may vary considerably even among genera/taxa of the same family (e.g., Kieneke & Schmidt-Rhaesa, 2015; Todaro et al., 2014, 2015). This is especially the case in Macrodasyidae, which show a wide array of structurally different caudal organs. In Thaidasys, for example, the caudal organ is a bulky cylindrical structure that opens at both ends and contains an internal canal surrounded by slightly spirally arranged musculature. Inside the canal, there is a hook-shaped sclerotic process that protrudes externally from the ventral side (Todaro et al., 2015). In Urodasys species, the caudal organ is V-shaped and formed by two distinct muscular regions parallel to each other, one glandular in nature and the other with a funnel-like, copulatory stylet (Todaro, Dal Zotto, & Cesaretti, 2019). The two regions are connected by a thin anterior duct opening to the outside into a common pore (Fregni et al., 1998, 1999; Schoepfer-Sterrer, 1974). The structural complexity of the caudal organ of Kryptodasys approaches most closely that of the caudal organ of Macrodasys. In fact, in species of Macrodasys, similarly to those of Kryptodasys, the caudal organ is divided into a voluminous anterior glandular region and a small posterior muscular region (antrum femininum, Ruppert, 1978a) in which an internal non-branched canal is present, opening outside with a single pore.
A possible difference between the caudal organs of the two taxa is that Macrodasys species are reported to develop a thin copulatory tube inside the canal in which sperm are packed just prior copulation. This “copulatory” tube is then everted into the frontal organ of the partner during mating (Evans, 1994; Ruppert, 1978a). The presence of such a tube has not been ascertained during our investigation of Kryptodasys.
4.5 Spermatogenesis in K. carlosrochai
Within the Macrodasyida, few species present aflagellate spermatozoa (see the following section 4). Among these, a TEM study has been conducted only on Dolichodasys sp., which, in addition to the description of spermatozoa, also reports some information on the spermatogenesis (Guidi et al., 2017). Therefore, the spermatogenesis of K. carlosrochai can sensibly be compared only with that of Dolichodasys sp. In both species, the definitive mitochondrion arises from the coalescence of small conventional mitochondria, but it wraps around the nucleus and then penetrates inside it in Dolichodays sp., whereas in K. carlosrochai it remains in the cytoplasm and covers the anterior part of the nucleus like a mantle.
The cytoplasm of Dolichodasys spermatids is occupied by large numbers of microtubules, whereas in K. carlosrochai by filaments that are here interpreted as F-actin (see the following section 4).
The acrosome of Dolichodasys sp. is formed by numerous ampulla-shaped vesicles that are arranged in a layer covering the microtubules of the anterior half of the mature sperm. In K. carlosrochai, the formation of the acrosome is completely different and is preceded by the appearance of a bottle-shaped microtubular structure into which the proacrosomal vesicle enters. The proacrosomal vesicle is arranged under the microtubules, and then its internal membrane is introflected to form the rod-shaped and tubular structures, typical of the mature spermatozoon.
Neither basal body nor developing flagella are observed during spermatogenesis in both K. carlosrochai and Dolichodasys sp. (Guidi et al., 2017).
4.6 Mature spermatozoa of Kryptodasys
The spermatozoa of the two Kryptodasys species have the same general shape similar to pumpkin seeds, but under optical microscope, they are well distinguishable from each other based on the width and length of the pointed apical part that is much larger and shorter in Kryptodasys sp. than in K. carlosrochai, respectively. When observed under TEM, they show the same general architecture: a club-shaped acrosome surrounded by the nucleus showing chromatin with two different electron densities, a unique mantle-shaped mitochondrion, cytoplasm full of filaments, and no flagellum. Therefore, the spermatozoa of the two Kryptodasys species differ deeply from the general Macrodasyida sperm model, which is represented by a filiform cell with a corkscrew-shaped acrosome, a spring-shaped nucleus surrounding a mitochondrial axis, and an ordinary flagellum (Balsamo et al., 2002; Ferraguti & Balsamo, 1995; Fischer, 1994; Guidi et al., 2004, 2011, 2014, 2017; Teuchert, 1976; Todaro et al., 2012). The ultrastructural observations of K. carlosrochai and Kryptodasys sp. confirm the absence of a flagellum in the spermatozoa of these two species, thus providing support to the observations of Todaro, Dal Zotto, Kånneby, et al. (2019) on the existence of further species of Kryptodasys on the basis of optical microscopy. It can therefore be said that a key characteristic (autapomorphy) of the genus Kryptodasys is the absence of the flagellum in the spermatozoa. Phylogenetic analysis based on molecular data found Kryptodasys allied with the members of the family Macrodasyidae (Todaro, Dal Zotto, Kånneby, et al., 2019), within which only for Macrodasys hexadactylis aflagellate spermatozoa have been reported, too (Figure 5; Rao, 1970). However, Todaro, Dal Zotto, Kånneby, et al. (2019) showed that M. hexadactylis was misidentified in the first place and relocated it to the genus Kryptodasys (i.e., M. hexadactylis = K. hexadactylis); thus, this latter taxon is the only one in the family Macrodasyidae characterized by aflagellate sperm.
The aflagellate spermatozoa are unusual within the order Macrodasyida; in fact, they have been reported only in selected members of the family Cephalodasyidae in addition to the family Macrodasyidae (Kieneke & Schmidt-Rhaesa, 2015).
In detail, Ruppert and Shaw (1977), on the basis of light microscopy data, suggested that the semicolon-shaped sperm of Do. carolinensis and D. delicatus very likely lack a flagellum. Guidi et al. (2017) analyzed the ultrastructure of the pod-like spermatozoa of Dolychodasys sp. and showed that they are in fact aflagellate, as they firstly appeared under optical microscopy.
Sperm without flagellum have been reported also for Dactylopodola baltica (Fischer, 1996). Unfortunately, in this case, only penetrated sperm (i.e., allospermatozoa of presumably foreign origin) were investigated, leaving doubt that loss of the flagellum could occur during penetration. However, an aberrant sperm morphology may also correlate with the formation and transfer of spermatophores as is the case of D. typhle (Kieneke et al., 2008). As Dactylopodola has been reported to occupy a basal position within the Macrodasyida evolutionary branch (e.g., Hochberg & Litvaitis, 2001), additional study on spermatozoa of this taxon is advisable to shed light on the ground pattern of the Gastrotricha sperm cell.
Aflagellate spermatozoa have also been reported in species of the order Chaetonotida, within which the presence of flagellate spermatozoa is the rule in clades considered to be basal, that is, Neodasyidae (suborder Multitubulatina), Muselliferidae, and Xenotrichulidae (suborder Paucitubulatina) (Guidi et al., 2003). Neodasyidae have rather small, bizarre spermatozoa termed commaform, because tail and head appear orthogonally oriented with respect to each other. Muselliferidae and Xenotrichulidae possess rather large, filiform spermatozoa with acrosome, nucleus, and a rather long flagellum in a linear sequence (e.g., Balsamo et al., 2010; Ferraguti et al., 1995; Guidi et al., 2003). By contrast, aflagellate spermatozoa are found in Chaetonotida Paucitubulatina taxa bearing derived characteristics, currently classified within the families Chaetonotidae and Dasydytidae (e.g., Kieneke & Schmidt-Rhaesa, 2015; Weiss, 2001). Peculiarly, these aflagellate spermatozoa are reported to appear together with growing oocytes in specimens that previously reproduced by parthenogenesis, making the reproductive biology of these gastrotrichs an unicum within Metazoa (e.g., Balsamo et al., 2020). Usually, sperm are condensed in single or bilateral bundles, and under light microscopy, their shape varies from rod-like to spindle-like, round, oval, or filiform (Balsamo & Todaro, 1988; Kieneke & Schmidt-Rhaesa, 2015; Weiss, 2001). Ultrastructural data are scanty but concordant in describing these spermatozoa as cells containing a nucleus but no acrosome, no mitochondria, and, obviously, no flagellum (Balsamo, 1992). Whether or not these sperms are functional (i.e., capable of fertilizing) remains an open question: certainly, they are ultrastructurally different from the aflagellate spermatozoa found in Macrodasyida.
4.7 Spermatozoa motility
The complex ultrastructure of the sperm cell of the two species of Kryptodasys parallel that of the spermatozoa of Dolichodasys, and in both cases, suggests that they are functional (i.e., capable to fertilize the egg). However, the lack of the flagellum raises the question whether and how these aflagellate sperm can move in order to reach the mature oocyte and fertilize it. A plausible explanation of the capacity of movement of the aflagellate spermatozoa of Dolichodasys sp. was proposed by Guidi et al. (2017) on the basis of previous observations carried out on aflagellate spermatozoa of platyhelminthes and coccid insects (Baccetti et al., 1982; Ehlers, 1981; Justine et al., 1985; Newton, 1980). These authors showed that sperm motility is due to the presence of a system of microtubule singlets linked to each other by dynein and the activity level of the ATPase. Therefore, the motility would be the result of dynein–singlet interaction, as it occurs in the flagellum axoneme between the doublets.
In the two studied Kryptodasys species, microtubules are not present in the sperm cytoplasm, whereas numerous microfilaments are observed instead. We interpret these microfilaments as F-actin filaments of the cytoskeleton that could be able to confer motility to the spermatozoon.
Ultrastructural studies suggested the presence of F-actin microfilaments in the aflagellate spermatozoa of Argas polonicus (Ixodida, Acari) and Armadillidium peraccae (Isopoda, Oniscidea), in particular in the region of the sperm head (Trovato et al., 2011; Witalinski & Dallai, 1994). The initial hypothesis was confirmed in A. peraccae by cytochemical and immunocytochemical methods, which found a massive presence of F-actin and also allowed to fix its exact localization inside the spermatozoon. In both cases, the authors argue that the presence of such a large amount of F-actin inside the spermatozoa could reasonably be linked with the need of motility acquisition by the male gamete, even if only temporary, for example, at the time of its interaction with the female gamete (Trovato et al., 2011; Witalinski & Dallai, 1994).
It seems therefore plausible that also the spermatozoa of the two studied Kryptodasys species, like those of Dolichodasys sp., would have a short-time amoeboid movement attributable to cytoskeleton components, although the cellular elements involved in the process would be different: microtubules in Dolichodasys and microfilaments in Kryptodasys. These differences would indicate that the acquisition of ameboid mobility in the two types of spermatozoa has arisen two times independently in these two genera of Gastrotricha. Such a hypothesis get support by phylogenetic analyses carried out so far, which placed the two genera in distinct, unrelated clades (e.g., Kieneke & Todaro, 2020; Todaro, Dal Zotto, Kånneby, et al., 2019; Todaro et al., 2014). Being Kryptodasys and Dolichodasys part of two clades deeply nested within the Macrodasyida phylogenetic tree, a secondary origin of this type of motility in both taxa involved appears the most plausible one.
As an alternative hypothesis, both types of sperms could be actually immobile. Aflagellated, immobile spermatozoa, yet functional (i.e., capable to fertilize the egg), have been frequently reported in the literature (Morrow, 2004). In these cases, passive movement is generally referred to as the mechanism that brings the sperm into contact with the egg allowing fertilization to occur (e.g., Berruti et al., 1978; see also Morrow, 2004).
If the aflagellate spermatozoa of Kryptodasys and Dolichodasys are actually immobile cells, then the only possible way for them to come into contact with the mature oocytes is that they may be passively conveyed to the organ in which they are then stored. The absence of muscular fibers in the wall of the frontal organ of both Kryptodasys and Dolichodasys species seems to rule out that the sperm may be squeezed out by contraction of the organ itself. Consequently, sperm may exit the frontal organ either by a yet unknown mechanism generated by the organ itself or as a consequence of the general movement/contractions of the body, which may cause a compression of the sack-like frontal organ and the extrusion of the spermatozoa, as it is for instance suggested for the caudal organ functioning of D. typhle (Kieneke et al., 2008).
In our opinion, available evidences seem to favor the movement hypothesis: future studies (e.g., live cell imaging) could support or disprove it.
4.8 Prospecting evolutionary considerations
Aflagellate spermatozoa have probably evolved several times within Gastrotricha: the challenging question is, however, why? According to Morrow (2004), one of the selective forces in determining sperm shapes is sperm competition, which occurs when the spermatozoa of different males compete to fertilize the eggs of a single female. In strictly monandrous species, sperm competition is absent; consequently, the selective pressure on individuals to produce motile spermatozoa may be less strong. Because aflagellate spermatozoa are less expensive to produce, over time selection could then result in the loss of the flagellum.
If this is true, then gastrotrich species bearing aflagellate spermatozoa should be considered strictly monandrous. This would mean that these gastrotrich species mate only once, while species possessing flagellate spermatozoa may be subject to multiple inseminations. This is an interesting scenario whose likelihood will have to be proved by future research, because currently data in this regard just do not exist.
ACKNOWLEDGEMENTS
The research benefitted from Scientific Research grants from the Italian Ministry of University (MIUR, 2020) of Loretta Guidi and Maria Balsamo. We would like to thank A. Kieneke and two anonymous reviewers whose suggestions improved significatively the readability and meaning of the manuscript. Open Access Funding provided by Universita degli Studi di Urbino Carlo Bo within the CRUI-CARE Agreement.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.




