Locomotor habits and phenotypic evolution of the appendicular skeleton in the oryzomyalian radiation in the Neotropics (Sigmodontinae, Cricetidae, Rodentia)
Contributing authors: Ludmilla Carvalho Coutinho ([email protected]), João Alves de Oliveira ([email protected])
Abstract
enSigmodontine rodents colonized South America in Late Miocene or earlier, leading to the clade Oryzomyalia, which rapidly radiated in distinct lineages and occupied almost all continental habitats, in a pattern classically interpreted as an adaptive radiation. Nevertheless, no evidence of strong influence of niche diversification on the evolution of cranial and mandibular morphology, or of deceleration in rates of phenotypic evolution in these structures over time following niche saturation, as expected according to the Ecological Opportunity model, has been detected. Here, we investigated morphometric variation among 59 oryzomyalian species using phylogenetically informed comparative analyses for testing (1) if the diversification of locomotor habits played an important role in shaping the morphology of the appendicular skeleton, and (2) if the disparification of appendicular skeleton showed high rates at the early diversification of Oryzomyalia and then has deaccelerated. Results showed that the different locomotor habits are associated with different shapes in both the forelimb and hindlimb, and selection of evolutionary models suggested that each locomotor habit was associated with their own adaptive optima. Moreover, the most extreme and specialized phenotypes, found in the semifossorial species Geoxus valdivianus, Blarinomys breviceps, and Paynomys macronyx, seem to have appeared after events of acceleration in the rates of morphological evolution. On the other hand, no evidence of a reduction in the rate of evolution over time was detected. The results suggest that the acquisition of different locomotor habits in oryzomyalians was associated with morphological specializations in the appendicular skeleton guided by natural selection and that, especially in the case of the evolution of fossoriality, there was a marked change in evolutionary regimes, generating highly modified phenotypes after acceleration of the pace of morphological changes. Despite the strong association between diversification of locomotor niches and evolution of the appendicular skeleton, the diversification of oryzomyalians does not seem to have experienced niche saturation, as noted in some other adaptive radiation events on Neotropics.
Resumo
ptRoedores sigmodontíneos colonizaram a América do Sul no Mioceno Superior ou antes, dando origem ao clado Oryzomyalia, que rapidamente se irradiou em linhagens distintas e ocupou quase todos os habitats continentais, em um padrão classicamente interpretado como uma radiação adaptativa. Entretanto, até o presente não foram detectadas evidências de forte influência da diversificação de nichos ecológicos sobre a evolução da morfologia craniana e mandibular, ou de desaceleração nas taxas de evolução fenotípica nessas estruturas ao longo do tempo após a saturação de nichos, conforme esperado pelo modelo de Oportunidade Ecológica. No presente estudo, nós investigamos a variação morfométrica entre 59 espécies de Oryzomyalia usando análises comparativas filogeneticamente informadas para testar (1) se a diversificação dos hábitos locomotores desempenhou um papel importante moldando a morfologia do esqueleto apendicular, e (2) se a disparificação do esqueleto apendicular mostrou altas taxas no início da diversificação de Oryzomyalia e, em seguida, desacelerou. Os resultados mostraram que os diferentes hábitos locomotores estão associados a diferentes formas dos membros anteriores e posteriores, e a seleção de modelos evolutivos sugeriu que cada hábito locomotor está associado a seus próprios ótimos adaptativos. Além disso, os fenótipos mais extremos e especializados, encontrados nas espécies semifossoriais Geoxus valdivianus, Blarinomys breviceps e Paynomys macronyx, parecem ter surgido após eventos de aceleração nas taxas de evolução morfológica. Por outro lado, não foram detectadas evidências de redução da taxa de evolução ao longo do tempo. Os resultados sugerem que a aquisição de diferentes hábitos locomotores em Oryzomyalia esteve associada a especializações morfológicas no esqueleto apendicular guiadas pela seleção natural e que, principalmente no caso da evolução da fossorialidade, houve mudanças marcantes nos regimes evolutivos, com surgimento de fenótipos muito especializados após acelerações pontuais no ritmo evolutivo. Apesar da forte associação entre diversificação de nichos locomotores e evolução do esqueleto apendicular, a diversificação de Oryzomyalia não parece ter experimentado saturação de nichos, como observado em alguns outros eventos de radiação adaptativa na região Neotropical.
1 INTRODUCTION
Muroid rodents, comprising rats, mice, hamsters, and allies, encompass approximately 26% of all mammalian species and compose a large fraction of mammalian diversity of all continents, except for Antarctica and New Zealand (Burgin et al., 2018, 2020; Musser & Carleton, 2005). It is estimated that they first appeared in the Eocene in Eurasia (Fabre et al., 2012; Jansa et al., 2009; Musser & Carleton, 2005; Steppan & Schenk, 2017) and, since then, dispersed and colonized the main landmasses on the planet (Alhajeri et al., 2016; McKenna & Bell, 1997; Musser & Carleton, 2005). In South America, muroid rodents are represented primarily by the cricetid subfamily Sigmodontinae, composed mainly by the Oryzomyalia, a subclade encompassing about 93% of the sigmodontine species, excluding only those of the tribes Sigmodontini and Ichthiomyini (Pardiñas et al., 2017; Patton et al., 2015). Fossil records and most recent fossil-calibrated phylogenies estimate that sigmodontines likely originated in North or Central America during the Late Miocene or even earlier (Gonçalves et al., 2020; Leite et al., 2014; Maestri et al., 2017; Martin et al., 2020; Parada et al., 2015; Prevosti et al., 2021; Schenk & Steppan, 2018; Steppan & Schenk, 2017), although the possibility of a South American origin cannot be disregarded (Martin et al., 2020). Shortly after the sigmodontine origin, still in Late Miocene (Prevosti et al., 2021), an ancestral lineage of the Oryzomyalia experienced a noteworthy diversification in South America, giving rise to the currently 439 recognized living species, distributed across 81 genera and 10 and tribes (Pardiñas et al., 2021; Ronez et al., 2021; Salazar-Bravo et al., 2016). Although it also occurs in North and Central America, the oryzomyalian current diversity is concentrated in South America, where it occurs through all biomes and explores a wide range of alimentary and locomotory ecological niches (Patton et al., 2015).
Given their extraordinary diversity, the Sigmodontinae, and, in a more restricted sense, the Oryzomyalia, have been classically understood as examples of adaptive radiation (Engel et al., 1998; Hershkovitz, 1966a; Parada et al., 2013; Reig, 1981; Steppan et al., 2004). Recently, the phenomenon of adaptive radiation has been primarily explained by the Ecological Opportunity (EO) Model (Pincheira-Donoso et al., 2015; Stroud & Losos, 2016; Yoder et al., 2010). This model postulates that explosive speciation follows the access by an ancestral lineage to diverse and abundant resources, prompting the rapid emergence of morphological, physiological, or behavioral specializations, and thus allowing wide exploitation of available resources while reducing competition (Pincheira-Donoso et al., 2015; Stroud & Losos, 2016; Yoder et al., 2010). In addition, EO model predicts that, over time, niches become saturated, resulting in deceleration of rates of speciation and phenotypic evolution (Alhajeri et al., 2016). A recent phylogenetic analysis has found an explosive acceleration in diversification after the Oryzomyalia origin, endorsing expectation of EO (Parada et al., 2015). Nevertheless, an equivalent explosive acceleration is not evident from morphological structures presumably associated with the exploration of ecological niches, such as the cranium and mandible (Maestri et al., 2017). In view of this, it has been pointed out that the diversification of alimentary habits, which most directly affects cranio-mandibular morphology, may have not been a key factor for the explosive diversification of sigmodontines in South America (Missagia & Perini, 2018; Pardiñas et al., 2021), and the diversification of locomotor niches, may have played a more relevant role (Maestri et al., 2017). Even so, phenotypic characters of the external morphology, which are supposedly more related to locomotor habits, also did not show evidence of explosive diversification followed by a slowdown in the rate of evolution (Alhajeri et al., 2016).
In this context, it would be enlightening to evaluate the effects of niche diversification and the hypothesis of early-burst disparification on structures more directly associated with locomotor functions, such as the appendicular skeleton. Studies focusing on sigmodontines have found appendicular specializations for different locomotor habits (Carrizo et al., 2014, 2021; Coutinho & Oliveira, 2017; Coutinho et al., 2013; Tulli et al., 2016), although these specializations tend to be subtle as in most muroid rodents (Coutinho & Oliveira, 2017; Hedrick et al., 2020; Nations et al., 2019). If the diversification of locomotor niches constitutes a key factor for adaptive radiation of sigmodontines in South America, it is reasonable to hypothesize that the morphological disparification of those structures would have occurred explosively, shortly after the origin of Oryzomyalia, and then deaccelerated.
To evaluate this hypothesis with respect to the Oryzomyalia radiation, we analyzed patterns of morphometric variation of the appendicular skeleton in a wide taxonomic coverage in this group using phylogenetically informed comparative methods. Specifically, our aims were to investigate if (1) the diversification of locomotor habits played an important role in evolutionary shaping the morphology of the appendicular skeleton of oryzomyalians, and if (2) the disparification of the morphology of appendicular skeleton showed a high rate at the early diversification of Oryzomyalia and then deaccelerated, as expected in an adaptive radiation scenario.
2 MATERIAL AND METHODS
2.1 Examined sample and measurements
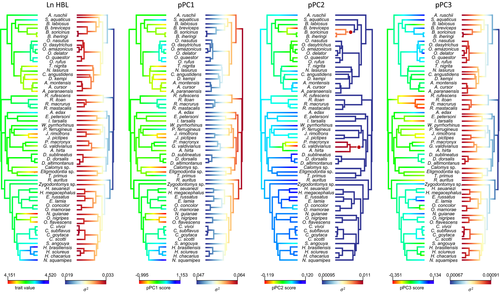
The specimens examined are the ones listed in Coutinho and Oliveira (2017), at the exclusion of those either missing any of the appendicular measurements listed below or belonging to a species lacking a previous phylogenetic assignment. These restrictions determined a total of 677 adult specimens belonging to 59 species and 37 genera, 34 of which distributed in nine oryzomyalian tribes, and three currently regarded as incertae sedis (Figure 1, Appendix). Taxonomy follows Patton et al. (2015), with updates for the genus Castoria (Pardiñas et al., 2016), for the tribe Andinomyini (Salazar-Bravo et al., 2016) and for the tribe Wiedomyini (Gonçalves et al., 2020).
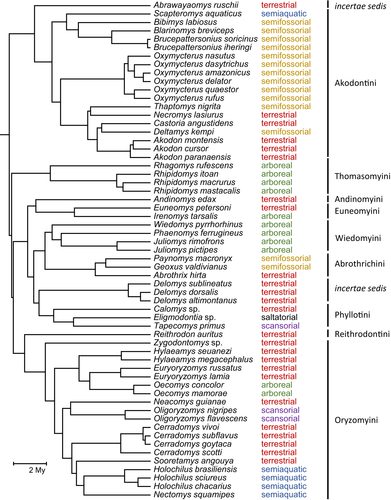
Species were assigned to six different locomotion modes (arboreal, scansorial, saltatorial, semifossorial, semiaquatic, and terrestrial; Figure 1) based on previous studies which have determined habitat preferences from frequencies of captures in different vegetation strata, as well as from the analysis of external morphological characters (references listed in Table 1 from Coutinho & Oliveira, 2017). It is worth considering that the a priori locomotor habits assignment for some taxa is conflicted and subject to revision depending on future studies on ecology and natural history. For example, Bibimys, Brucepattersonius and Oxymycterus species have external and postcranial traits typically associated with semifossorial rodents, but field observations confirming these behaviors are lacking or scarce (Carrizo, Tulli, & Abdala, 2014; Coutinho et al., 2013; Coutinho & Oliveira, 2017; Oliveira & Gonçalves, 2015; Tulli et al., 2016; Vilela et al., 2015). Deltamys kempi, despite being classified here as semifossorial and having its digging behavior already registered (Massoia, 1964), preferentially inhabits swamp areas (González & Pardiñas, 2002) and probably exhibits some natatorial behavior. Reithrodon, despite being often treated as terrestrial, also show the habit of digging tunnels (Pardiñas & Galliari, 2001). Sooretamys angouya, despite being often treated as terrestrial, are also good climbers and can produce “extensive tunnels, even in hard, rocky ground” (Chiquito et al., 2014; Olmos, 1991). Although it is more likely that locomotor habits in the Oryzomyalia vary along a continuum rather than as discrete categories, the categorization of habits is a necessity for some methods employed in this study.
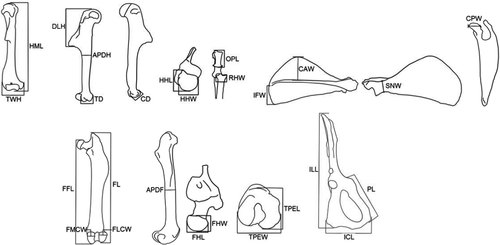
Twenty-six linear measurements were taken following Coutinho et al. (2013) with a digital caliper to the nearest 0.01 mm: HML: humeral maximum length; DLH: deltopectoral crest length of the humerus; APDH: anteroposterior diameter of the humerus; TWH: transverse width of the humerus; HHL: humeral head length; HHW: humeral head width; TD: trochlea depth; CD: capitulum depth; OPL: olecranon process length; RHW: radial head width; IFW: infraspinatus fossa width; SNW: scapula notch width; CAW: cranial angle width; CPW: coracoid process width; FFL: femoral functional length; FL: femur length; APDF: anteroposterior diameter of the femur; FHL: femoral head length; FHW: femoral head width; FMCW: femoral medial condyle width; FLCW: femoral lateral condyle width; TPEW: tibial proximal end width; TPEL: tibial proximal end length; ILL: ilium length; ICL: ischium length; PL: pubis length (Figure 2). Morphometric characters were selected from previous studies that evaluated the association between morphologic variation in the appendicular skeleton and locomotion mode in rodents and other small mammals (Candela & Picasso, 2008; Coutinho et al., 2013; Sargis, 2002a, 2002b). Distal elements of the appendicular skeleton could not be analyzed due to their unavailability, since they are usually preserved inside serial skins in scientific collections. In addition, the average head-body lengths (HBL) of each species were calculated based on data collected from specimen tags or information available in the literature and used as estimates of body size. Despite the use of head-body length data from the literature potentially inserts error in the analyses, for some species it was not possible to get such information from the analyzed specimen tags. The arithmetic means for each species were log-transformed and used in the phylogenetic comparative analyses.
2.2 Phylogenetic framework
The phylogenetic framework used in comparative analyses follows the maximum clade credibility tree recovered by Schenk and Steppan (2018), based on the genes CYTB, BRCA1, GHR, IRBP, and RAG1, by Bayesian inference. Subsequently, this tree was manually edited to insert species not included in the phylogenetic analysis. Hylaeamys laticeps was replaced by H. seuanezi, since the name laticeps was recently treated as a junior synonym for megacephalus and the sequences used as laticeps by Schenk and Steppan (2018) originate from the area of occurrence H. seuanezi (Brennand et al., 2013). Euneomys mordax was replaced by E. petersoni, the “Abrothrix clade” was replaced by Abrothrix hirta, the “Eligmodontia clade” was replaced by Eligmodontia sp., and the “Calomys clade” was replaced by Calomys sp. assuming the monophyly of these genera. Delomys altimontanus was included as sister species to D. dorsalis based on Gonçalves and Oliveira (2014), and Abrawayaomys ruschi as sister to the Akodontini based on Gonçalves et al. (2020) and Ventura et al. (2013). Phaenomys ferrugineus was included as sister to Juliomys based on Gonçalves et al. (2020); Cerradomys goytaca was inserted as sister species of Cerradomys subflavus, and Cerradomys vivoi as sister to this clade based on Tavares et al. (2016). The lengths of the grafted branches were determined from the date estimated phylogenies recovered in the above mentioned studies (Gonçalves et al., 2020; Gonçalves & Oliveira, 2014; Tavares et al., 2016; Ventura et al., 2013). The final phylogenetic framework used in this study is shown in Figure 1. All analyses were also performed using the phylogenetic framework recovered by Parada et al. (2015) and Maestri et al. (2017) based on the CYTB and IRBP genes. The results of these analysis were completely congruent with those found using the tree of Schenk and Steppan (2018), and only these will be reported here.
2.3 Multivariate ordination of data and testing phylogenetic signal
To search for main trends of variation in multivariate data taking account the phylogenetic dependence among species means (Polly et al., 2013; Revell, 2009), phylogenetic principal component analyses (pPCA) were run for the full dataset of appendicular variables, using the R package phytools (Revell, 2012).
Scores of species along phylomorphospaces were used to verify the presence of phylogenetic signal in the three major axes of morphometric variation (pPC1, pPC2, and pPC3). The presence of phylogenetic signal was tested with the Pagel's λ test (Pagel, 1999). The strength of phylogenetic signal was estimated by Blomberg's K statistic, K < 1 indicating closely related OTUs with less similar metric values than expected under a Brownian Motion model of evolution, and K > 1, resulting from more similar values than expected under a Brownian Motion model, indicating a strong phylogenetic signal (Blomberg et al., 2003).
2.4 Body size effect in appendicular morphometrics
Phylogenetic multiple correlations were conducted with Phylogenetic Generalized Least Squares (PGLS) for testing and quantifying association between body size (ln HBL) and the full set of appendicular variables. Sizes of appendicular skeleton were estimated as the first phylogenetic principal components (pPC1) obtained from pPCA with full set of variables, and with two separate sets of variables: forelimbs (pPC1forelimbs, 14 variables) and hindlimb (pPC1hindlimb, 12 variables). PGLS were conducted to test and quantify association between ln HBL, pPC1, pPC1forelimb, and pPC1hindlimb, and residuals were extracted from these correlations for estimating the deviation of each taxon from the average expectations for Oryzomyalia. PGLS were conducted in R using the caper package (Orme et al., 2018) and residuals were extracted in R using the phytools package (Revell, 2012).
Additionally, the deviations from isometry in the size variation of limb traits respective to the body size (HBL) were tested using a bivariate allometric approach. The allometric coefficient (b) of each limb variable was estimated with PGLS using the linear equation ln Y = b. ln HBL + ln a, where Y is the limb variable and a is the Y-intercept (Warton et al., 2006). Since only linear measurements were used in this study, isometry was assumed when b = 1.0, indicating that the limb trait covaries proportionally with the head-body length. Conversely, when b ≠ 1.0, the examined morphometric trait varies disproportionately to HBL and might show positive allometry (b > 1.0) or negative allometry (b < 1.0). The tests for isometry were carried out by testing for correlation between residual and fitted values (Warton et al., 2006). The eigenvector of pPC1 was also used to investigate the variation in multivariate allometric coefficients. When PC1 obtained from a Principal Component Analysis explains size, it summarizes both size isometric variation and shape variation due to allometry (Jolicoeur, 1963). In this case, the elements of the PC1 eigenvector can be interpreted as multivariate allometric coefficients, with the value for isometry = 1/n1/2, where n is the number of variables (Jolicoeur, 1963; Weston, 2003). Since in this study 26 limb variables were used, the isometric coefficient was 0.196. Unlike a bivariate allometric approach, multivariate allometry is independent of an external size variable such as HBL, and although the allometric coefficients obtained by the two methodological approaches tend to be correlated (Weston, 2003), they cannot be taken as equivalent.
2.5 Testing morphometric differences between locomotion modes
To test the morphometric difference in locomotor habits considering the phylogenetic non-independence among species means, a phylogenetic Multivariate Analysis of Variance (phyMANOVA; Garland et al., 1993) was performed with the package phytools in software R. To test whether the distribution of species in phylomorphospaces is structured by the diversity of locomotion modes (Garland et al., 1993), the species scores along pPC1, pPC2, and pPC3 were submitted to a phylogenetic Analysis of Variance (phyANOVA) using R package geiger (Harmon et al., 2008). Additionally, traditional Univariate and Multivariate Analysis of Variance (ANOVA, MANOVA), without phylogenetic information, were performed to test for morphometric differences in locomotor habits. Residuals of the correlations between ln HBL, pPC1, pPC1forelimb, and pPC1hindlimb were submitted to phyANOVA to test whether the deviation of taxa from the average expectations for Oryzomyalia is influenced by locomotor habits. Saltatorial and scansorial species were removed from phyANOVA, ANOVA, and MANOVA due to their very low sample sizes.
2.6 Testing deacceleration in disparification rates
The node-height test (NHT; Freckleton & Harvey, 2006) was used for testing whether the evolution of oryzomyalian appendicular traits had slowed through time (Slater et al., 2010). We computed the absolute value of standardized independent contrasts (Felsenstein, 1985) of ln HBL and pPCA scores on our tree and correlated them with the height of the node at which they were generated. Because independent contrasts are Brownian rate parameters for the branches over which they are calculated (McPeek, 1995), a significant positive relationship between node age and absolute contrast value would indicate that rates of trait evolution slowed through time, consistently with niche-filling (Freckleton & Harvey, 2006). We also calculated mean subclade disparity through time (DTT) for body size and appendicular morphology (Harmon et al., 2003) and compared disparity of ln HBL and pPCA scores across our tree with that expected under a pure Brownian process by simulating body size evolution 10,000 times across our tree. The mean subclade disparity values for the observed and simulated data were plotted against node age and the morphological disparity index (MDI) calculated. MDI quantifies the overall difference of relative disparity of a clade compared with expectations under the null Brownian Motion model (Harmon et al., 2003). Negative MDI values indicate lower subclade disparity than expected under Brownian motion and are a common property of adaptively radiating clades. NHT and DTT analyses were conducted in R using the geiger package (Harmon, Weir, et al., 2008).
2.7 General trends of oryzomyalian appendicular trait evolution
Several regimes of phenotypic disparification have been formally described by a series of models with different parameters, including rate of evolution, variation of this rate over time, presence or absence of either a single or multiple adaptive optima, and variation in stabilizing selection force around optimal adaptations (Butler & King, 2004; Cadotte & Davies, 2016; Schweizer et al., 2014). These parameters reflect evolutionary forces and their effects on morphological disparification in a given clade. In order to test whether locomotor habits impose particular evolutionary regimes on size and shape of the appendicular skeleton of oryzomyalians, we compared the suitability of different evolutionary models to the patterns of morphological disparity currently found in the subfamily.
We estimated likelihood of 13 evolutionary models (Table 1) of continuous traits by fitting them to the recovered pPCA scores and comparing their Akaike information criterion values corrected for small sample sizes (AICc), using the packages geiger (Harmon, Weir, et al., 2008) and OUwie 2 (Beaulieu et al., 2012) in R. Three models assumed a single evolutionary regime in the entire oryzomyalian tree: (1) Brownian Motion (BM1), (2) the Ornstein–Uhlenbeck (OU1) and a time-dependent Early-Burst (EB) (Beaulieu et al., 2012; Butler & King, 2004; Cadotte & Davies, 2016; Harmon et al., 2010). In Brownian Motion models (BM), traits diverge independently over time without constraints, in a manner analogous to a random walk, and the evolutionary rates (σ2) are the only parameters to be estimated; in the BM1 model, a single σ2 is assumed to be shared by all oryzomyalian branches. The Ornstein–Uhlenbeck models (OU) are more complex than BM, by incorporating evolutionary constraints assuming that traits are pulled back toward some optimal trait value; the attraction force toward the optimum (α), often understood as a result of stabilizing selection, and the optimum itself (θ) are additional estimated parameters, assumed to be single, constant and shared by all oryzomyalian branches in OU1. The time-dependent early-burst model (EB) proposes that the evolutionary rate σ2 changes exponentially by a factor b through time (Harmon et al., 2010); so, if the estimated parameter b is negative, the rate of evolution slows through time.
In addition, variations of the BM and OU models were also compared, assuming the possibility of varying the parameters between branches with different locomotor habits. BMM models are BM models in which the σ2 evolution rates might vary between locomotor habits and OUM models are OU models incorporating multiple optima (α) (Beaulieu et al., 2012; Cadotte & Davies, 2016). Saltatorial and scansorial species were removed from model selection due to their very low sample sizes. The list of tested models can be found in Table 1.
| Model | Parameters variable between groups | Number of estimated parameters | Differences between locomotor groups |
|---|---|---|---|
| BM1 | — | 2 | All habits share parameters |
| OU1 | — | 3 | All habits share parameters |
| EB | — | 3 | All habits share parameters |
| BMM.all | σ 2 | 5 | All habits differ each other |
| OUM.all | α | 6 | All habits differ each other |
| BMM.arb | σ 2 | 3 | Arboreal lineages differ from the others ones |
| OUM.arb | α | 4 | Arboreal lineages differ from the other ones |
| BMM.fos | σ 2 | 3 | Semifossorial lineages differ from the other ones |
| OUM.fos | α | 4 | Semifossorial lineages differ from the other ones |
| BMM.aqu | σ 2 | 3 | Semiaquatic lineages differ from the other ones |
| OUM.aqu | α | 4 | Semiaquatic lineages differ from the other ones |
| BMM.ter | σ 2 | 3 | Terrestrial lineages differ from the other ones |
| OUM.ter | α | 4 | Terrestrial lineages differ from the other ones |
- Abbreviations: BM1, Brownian motion model with a single evolutionary rate; BMM, Brownian motion model with multiple evolutionary rates; EB, Early-Burst Model; OU1, Ornstein–Uhlenbeck process model with a single optimum; OUM, Ornstein–Uhlenbeck process model with multiple optima; σ2, evolutionary rates; α, evolutionary optima.
The calculation of likelihood of models with multiple parameters for different locomotor habits (BMM and OUM) depends on the mapping of habits along the phylogenetic framework. The stochastic mapping of locomotor habit states, including estimates of the ancestral states of each branch, was carried out via likelihood using the make.simmap function in the R package phytools (Revell, 2012). As this method can be a source of significant amount of random variation affecting the result of the likelihood calculation of the models, the character mapping was performed 1000 times independently and the likelihood and AICc values of each model were calculated for all 1000 mappings. Then, the modes of likelihood and AICc values for each model were calculated. Increase in model fit was significant when reduction of the AICc score of the most adequate model (i.e., the one with the lowest mode AICc score) equaled ≥3.5 (Burnham & Andersen, 2002).
2.8 Testing shifts in rates of phenotypic evolution
To investigate whether the emergence of one or more evolutionary branches of the oryzomyalian tree was associated with changes in the rates of evolution in the appendicular skeleton, the macroevolutionary dynamics of size and shape of these structures (pPCA scores) was investigated following Bayesian analysis of macroevolutionary mixtures (BAMM) (Rabosky, 2014; Rabosky et al., 2014; Rabosky, Grundler, et al., 2014). Quantification of the tempo and mode of phenotypic evolution, using reversible-jump Markov chain Monte Carlo (rjMCMC), was implemented in the program BAMM with relevant priors chosen with BAMMtools (Rabosky, 2014; Rabosky, Grundler, et al., 2014). We conducted 120 x 106 generations of rjMCMC, sampling every 24 × 103 generations, using four Markov chains and a “minimum clade size for shift” of one. Convergence of BAMM runs was assessed by computing effective sample size for the likelihood of the data and for the number of distinct regimes, obtaining more than 2900 independent samples after a “burn-in” of 10%. For each trait, configurations of rate shifts with highest posterior probabilities were retrieved with the getBestShiftConfiguration function of BAMMtools; the frequency of these configurations in rjMCMC generations was treated as F1. The “core shifts” were determined based on marginal odds ratios above 20.
All analyses were also performed separately for two sets of morphometric variables, the forelimb and hindlimb datasets, in order to avoid problems related to singular matrices in multivariate analyses due to the number of variables exceeding the number of examined taxa (Hair, Black, Babin, & Anderson, 2014; Mundry, 2014). Since these analyses presented results completely congruent to those obtained with pooled forelimb and hindlimb variables, we only report results from the analyzes with the full set of variables.
3 RESULTS
3.1 Correlation of appendicular morphology with body size and evolution of appendicular size
A PGLS showed that 87.3% of the variance of appendicular skeleton was explained by body size (ln HBL; p < 0.001). A principal component analysis found that 91.0% of all variation in limbs was summarized by the first axis of variation (pPC1), which can be interpreted as size variation added to shape variation as a function of size (= shape change due to allometry), since all characters were positively correlated with it (Table 2). A PGLS showed that roughly 90% of the variation in limb size (pPC1) was explained by the variation in body size (p < 0.001; Figure 3a). The correlations between body size and the subsequent pPCs axes were not significant (p > 0.05).
| pPC1 | pPC2 | pPC3 | ||||
|---|---|---|---|---|---|---|
| Eigenvector | Loading | Eigenvector | Loading | Eigenvector | Loading | |
| HML | 0.184 | 0.964 | 0.196 | 0.193 | 0.090 | 0.055 |
| DLH | 0.183 | 0.978 | 0.015 | 0.015 | 0.173 | 0.107 |
| APDH | 0.206 | 0.933 | −0.235 | −0.201 | −0.138 | −0.072 |
| TWH | 0.185 | 0.908 | −0.426 | −0.395 | −0.027 | −0.015 |
| HHL | 0.183 | 0.961 | −0.090 | −0.089 | −0.085 | −0.052 |
| HHW | 0.191 | 0.977 | 0.092 | 0.089 | −0.153 | −0.091 |
| TD | 0.180 | 0.956 | −0.181 | −0.182 | −0.052 | −0.032 |
| CD | 0.184 | 0.969 | −0.170 | −0.168 | 0.002 | 0.001 |
| OPL | 0.198 | 0.879 | −0.471 | −0.394 | 0.420 | 0.216 |
| RHW | 0.182 | 0.952 | −0.211 | −0.207 | 0.067 | 0.041 |
| IFW | 0.206 | 0.907 | 0.266 | 0.221 | −0.399 | −0.204 |
| SNW | 0.205 | 0.962 | −0.175 | −0.155 | −0.157 | −0.086 |
| CAW | 0.216 | 0.949 | 0.009 | 0.007 | 0.202 | 0.103 |
| CPW | 0.149 | 0.859 | −0.150 | −0.163 | −0.524 | −0.349 |
| FFL | 0.197 | 0.953 | 0.288 | 0.262 | 0.179 | 0.100 |
| FL | 0.200 | 0.952 | 0.295 | 0.265 | 0.186 | 0.103 |
| APDF | 0.206 | 0.965 | 0.066 | 0.058 | 0.049 | 0.027 |
| FHL | 0.202 | 0.985 | 0.041 | 0.037 | −0.161 | −0.091 |
| FHW | 0.187 | 0.955 | 0.058 | 0.055 | −0.183 | −0.108 |
| FMCW | 0.210 | 0.983 | 0.052 | 0.046 | −0.012 | −0.007 |
| FLCW | 0.195 | 0.971 | 0.085 | 0.080 | −0.098 | −0.057 |
| TPEW | 0.200 | 0.989 | 0.047 | 0.044 | −0.028 | −0.016 |
| TPEL | 0.206 | 0.980 | 0.104 | 0.093 | −0.021 | −0.012 |
| ILL | 0.212 | 0.979 | 0.156 | 0.136 | 0.194 | 0.104 |
| ICL | 0.206 | 0.970 | 0.045 | 0.040 | 0.166 | 0.091 |
| PL | 0.211 | 0.960 | 0.166 | 0.143 | 0.155 | 0.082 |
| Eigenvalue | 0.0283 | 0.0010 | 0.0004 | |||
| % of variation | 91.012 | 3.237 | 1.225 | |||
- Abbreviations: APDF, anteroposterior diameter of the femur; APDH, anteroposterior diameter of the humerus; CAW, cranial angle width; CD, capitulum depth; CPW, coracoid process width; DLH, deltopectoral crest length of the humerus; FFL, femoral functional length; FHL, femoral head length; FHW, femoral head width; FL, femur length; FLCW, femoral lateral condyle width; FMCW, femoral medial condyle width; HHL, humeral head length; HHW, humeral head width; HML, humeral maximum length; ICL, ischium length; IFW, infraspinatus fossa width; ILL, ilium length; OPL, olecranon process length; PL, pubis length; RHW, radial head width; SNW, scapula notch width; TD, trochlea depth; TPEL, tibial proximal end length; TPEW, tibial proximal end width; TWH, transverse width of the humerus.
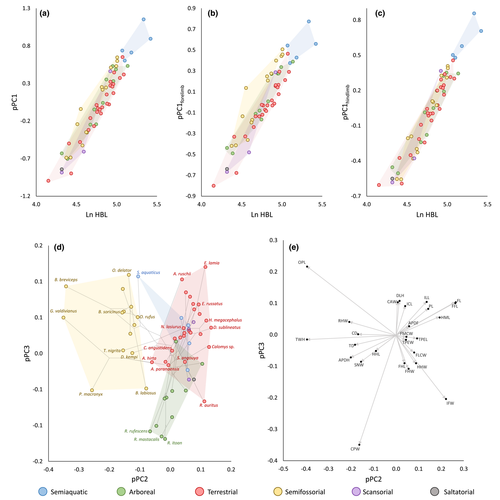
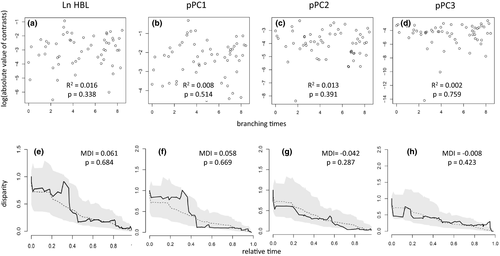
Ln HBL and pPC1 showed significant phylogenetic signals (Table 3). Semiaquatic taxa presented higher ln HBL values and pPC1 scores (Figure 3a), reflecting their absolute larger body size and limb size; however, phyANOVA did not detect significant variation among scores of species showing distinct locomotor habits (Table 3). The NHT found no correlation between divergence time and standardized phylogenetic contrasts of size (ln HBL and pPC1; Figure 4a,b), and DTT tests found no deviation from null BM expectations (Figure 4e,f). The evolutionary model that best fitted the variation of both body size (ln HBL) and limb size (pPC1) was OUM.aqu, showing that semiaquatic lineages have their own adaptive optimum, with a higher value (i.e., larger size) than other oryzomyalian lineages (Table 4). BAMM found no evidence of changes in disparification rate of size, indicating that the most likely evolutionary scenarios are those with only one evolutionary regime throughout the phylogeny (Table 5, Figure 5).
| Phylogenetic signal | phyANOVA | ||||||
|---|---|---|---|---|---|---|---|
| Blomberg's K | Pagel's λ | F | p (ANOVA) | p (phyANOVA) | |||
| K | p | λ | p | ||||
| ln HBL | 0.808 | <0.001 | 0.989 | <0.001 | 5.651 | 0.001 | 0.148 |
| pPC1 | 0.871 | <0.001 | 1.000 | <0.001 | 5.165 | 0.002 | 0.164 |
| pPC2 | 1.203 | <0.001 | 1.000 | <0.001 | 43.154 | <0.001 | 0.001 |
| pPC3 | 0.783 | <0.001 | 0.888 | <0.001 | 16.727 | <0.001 | 0.003 |
Note
- K: Blomberg's K estimate. λ: Pagel's λ estimate. p = p-value. Bold: p-values < 0.05.
| Model | ln HBL | pPC1 | ||||
|---|---|---|---|---|---|---|
| loglik | AICc | ΔAICc | loglik | AICc | ΔAICc | |
| BM1 | 0.069 | 4.089 | 26.835 | −33.075 | 70.377 | 27.191 |
| OU1 | 11.365 | −16.268 | 6.477 | −21.816 | 50.093 | 6.907 |
| EB | 11.329 | −16.195 | 6.550 | −21.795 | 50.052 | 6.866 |
| BMS_all | 12.540 | −13.880 | 8.866 | −20.874 | 52.948 | 9.762 |
| OUM_all | 15.688 | −17.662 | 5.084 | −17.347 | 48.409 | 5.223 |
| BMS_arb | 11.372 | −16.282 | 6.463 | −21.803 | 50.068 | 6.882 |
| OUM_arb | 11.385 | −13.987 | 8.759 | −21.769 | 52.323 | 9.137 |
| BMS_ter | 11.529 | −16.596 | 6.149 | −21.537 | 49.536 | 6.350 |
| OUM_ter | 11.639 | −14.494 | 8.252 | −21.267 | 51.318 | 8.132 |
| BMS_fos | 11.397 | −16.332 | 6.413 | −21.729 | 49.919 | 6.733 |
| OUM_fos | 12.050 | −15.316 | 7.429 | −21.456 | 51.696 | 8.510 |
| BMS_aqu | 12.341 | −18.220 | 4.526 | −21.148 | 48.757 | 5.571 |
| OUM_aqu | 15.765 | −22.746 | 0.000 | −17.201 | 43.186 | 0.000 |
| Model | pPC2 | pPC3 | ||||
|---|---|---|---|---|---|---|
| loglik | AICc | ΔAICc | loglik | AICc | ΔAICc | |
| BM1 | 55.251 | −106.276 | 33.957 | 77.754 | −151.282 | 36.532 |
| OU1 | 66.511 | −126.561 | 13.672 | 89.886 | −173.311 | 14.503 |
| EB | 66.618 | −126.775 | 13.458 | 89.014 | −171.566 | 16.248 |
| BMS_all | 73.599 | −135.998 | 4.235 | 90.896 | −170.591 | 17.223 |
| OUM_all | 76.653 | −139.591 | 0.642 | 99.373 | −183.032 | 4.782 |
| BMS_arb | 69.562 | −132.663 | 7.570 | 89.052 | −171.642 | 16.172 |
| OUM_arb | 66.558 | −124.332 | 15.901 | 98.299 | −187.814 | 0.000 |
| BMS_ter | 67.764 | −129.067 | 11.166 | 90.234 | −174.007 | 13.807 |
| OUM_ter | 70.776 | −132.767 | 7.466 | 91.771 | −174.757 | 13.057 |
| BMS_fos | 73.203 | −139.945 | 0.288 | 90.467 | −174.472 | 13.342 |
| OUM_fos | 75.309 | −140.233 | 0.000 | 90.075 | −171.366 | 16.448 |
| BMS_aqu | 71.374 | −136.287 | 3.947 | 89.030 | −171.599 | 16.215 |
| OUM_aqu | 66.520 | −124.256 | 15.977 | 90.158 | −171.531 | 16.283 |
Note
- Bold: best fitted models (AAIc ≤3.5). Loglik: log-likelihood. AICc: Akaike information criterion corrected for small sample sizes.
| ENshifts | ENLLik | Shifts | Iterations with best shift configuration | Clade/lineage | F 1 | |
|---|---|---|---|---|---|---|
| ln HBL | 10,700 | 8856 | 0 | 0.998 | — | 0.989 |
| pPC1 | 10,801 | 8875 | 0 | 0.988 | — | 0.999 |
| pPC2 | 10,487 | 7966 | 2 | 0.190 | Rate increasing in origin of Abrotrichini and in the lineage leading to Blarinomys and Brucepattersonius | 0.111 |
| 2 | 0.190 | Rate increasing in the origin of Abrotrichini and in lineage leading to the origin of Blarinomys | 0.076 | |||
| pPC3 | 10,801 | 10,292 | 0 | 0.450 | — | 0.795 |
Note
- Effective size of shift number (ENshifts) and log-likelihood (ENLLik) following Markov chain Monte Carlo runs are listed. Shifts: estimated number of shifts in the macroevolutionary dynamics of the evolutionary configuration with highest posterior probability. Clade/lineage: clades or lineages showing macroevolutionary dynamic shifts in the evolutionary configuration with highest posterior probability. F1: frequency of the best estimated macroevolutionary configuration.
Body size explained a higher percentage of the total hindlimb variation (91.3%) than of forelimb variation (82.6%). For both the anterior and posterior appendicular skeletons, when analyzed separately, pPC1s (pPC1forelimb and pPC1hindlimb) could be characterized as size variation axes. Appendicular size explained a higher percentage of total morphometric variation in hindlimb (pPC1forelimb: 95.8%) than in forelimb (pPC1hindlimb: 89.2%). Congruently, body size (ln HBL) explained a higher percentage of the hindlimb size variation (92.4%; Figure 3c) than of forelimb size variation (84.9%; Figure 3b).
The residuals of the linear regression between pPC1 and ln HBL (F = 3.821; p = 0.321; Figure 3a), and between pPC1hindlimb and ln HBL (F = 0.099; p = 0.995; Figure 3c) were not significantly different among locomotor habits according to the phyANOVA. Conversely, the residuals of the linear regression between pPC1forelimb and ln HBL (F = 10.564; p = 0.031; Figure 3b) were significantly different among locomotor habits. Pairwise post hoc comparisons showed that terrestrial, semiaquatic, and arboreal species shared similar residual values, significantly lower than semifossorial forms (Figure 3b). This finding reveals that semifossorial species have more developed hindlimbs than those with other habits respective to their body size. The taxon that had the highest residuals for the regression of pPC1forelimb on ln HBL was Geoxus valdivianus, followed by Blarinomys breviceps, Paynomys macronyx, and Thaptomys nigrita.
All univariate traits showed significant correlation with body size (ln HBL), the highest for DLH (R2 = 0.920; p < 0.001) and the lowest for OPL (R2 = 0.509; p < 0.001). In the bivariate allometric analysis, 14 traits did not deviate significantly from isometry, 12 traits showed negative allometry and no trait showed positive allometry (Table 6). Negative allometry was found in a greater proportion in forelimb (8/14 = 57.1%) than in hindlimb variables (4/12 = 33.3%). The length of the humerus (HML) varied with negative allometry, while the length of the femur (FFL and FL) varied isometrically. All traits of the scapular and pelvic girdles varied isometrically, with the only exception for CPW. The multivariate allometric approach was congruent with bivariate allometric analysis in showing that low allometric coefficients (pPC1 eigenvector <0.196) were more recurrently found in forelimb (9 of 14 measurements) than in hindlimb variables (two of 12 measurements; Table 2).
| Measurement | Bivariate allometric coefficient | Test for isometry (b = 1.0) | |||
|---|---|---|---|---|---|
| b (95% CI) | R 2 | R | p | Result | |
| HML | 0.851 (0.783–0.925) | 0.901 | −0.457 | <0.001 | Negative allometry |
| DLH | 0.828 (0.768–0.892) | 0.920 | −0.557 | <0.001 | Negative allometry |
| APDH | 0.938 (0.824–1.069) | 0.757 | −0.128 | 0.334 | Isometry |
| TWH | 0.906 (0.769–1.068) | 0.614 | −0.157 | 0.237 | Isometry |
| HHL | 0.824 (0.740–0.918) | 0.835 | −0.432 | 0.001 | Negative allometry |
| HHW | 0.876 (0.804–0.954) | 0.896 | −0.381 | 0.003 | Negative allometry |
| TD | 0.818 (0.709–0.944) | 0.705 | −0.350 | 0.007 | Negative allometry |
| CD | 0.840 (0.739–0.955) | 0.765 | −0.339 | 0.009 | Negative allometry |
| OPL | 1.045 (0.869–1.257) | 0.509 | 0.063 | 0.638 | Isometry |
| RHW | 0.851 (0.748–0.967) | 0.764 | −0.318 | 0.014 | Negative allometry |
| IFW | 0.943 (0.824–1.078) | 0.742 | −0.116 | 0.383 | Isometry |
| SNW | 0.920 (0.803–1.055) | 0.734 | −0.159 | 0.228 | Isometry |
| CAW | 0.990 (0.887–1.106) | 0.825 | −0.023 | 0.862 | Isometry |
| CPW | 0.734 (0.633–0.852) | 0.684 | −0.488 | < 0.001 | Negative allometry |
| FFL | 0.937 (0.855–1.026) | 0.882 | −0.187 | 0.156 | Isometry |
| FL | 0.944 (0.862–1.034) | 0.883 | −0.166 | 0.209 | Isometry |
| APDF | 0.942 (0.860–1.031) | 0.883 | −0.173 | 0.191 | Isometry |
| FHL | 0.895 (0.821–0.976) | 0.893 | −0.322 | 0.013 | Negative allometry |
| FHW | 0.866 (0.783–0.958) | 0.855 | −0.354 | 0.006 | Negative allometry |
| FMCW | 0.945 (0.868–1.030) | 0.896 | −0.172 | 0.193 | Isometry |
| FLCW | 0.873 (0.803–0.950) | 0.900 | −0.395 | 0.002 | Negative allometry |
| TPEW | 0.901 (0.830–0.978) | 0.905 | −0.320 | 0.013 | Negative allometry |
| TPEL | 0.933 (0.852–1.022) | 0.882 | −0.198 | 0.133 | Isometry |
| ILL | 0.957 (0.883–1.038) | 0.906 | −0.141 | 0.287 | Isometry |
| ICL | 0.921 (0.847–1.002) | 0.900 | −0.251 | 0.055 | Isometry |
| PL | 0.959 (0.880–1.045) | 0.895 | −0.129 | 0.329 | Isometry |
Note
- b: estimated bivariate allometric coefficient. 95% C.I.: 95% confidence interval. R2: Determination coefficient for correlation between ln limb variables and ln HBL. R: correlation coefficient for correlation between the residual and the fitted values assuming b = 1.0. p = p-value.
- Abbreviations: APDF, anteroposterior diameter of the femur; APDH, anteroposterior diameter of the humerus; CAW, cranial angle width; CD, capitulum depth; CPW, coracoid process width; DLH, deltopectoral crest length of the humerus; FFL, femoral functional length; FHL, femoral head length; FHW, femoral head width; FL, femur length; FLCW, femoral lateral condyle width; FMCW, femoral medial condyle width; HHL, humeral head length; HHW, humeral head width; HML, humeral maximum length; ICL, ischium length; IFW, infraspinatus fossa width; ILL, ilium length; OPL, olecranon process length; PL, pubis length; RHW, radial head width; SNW, scapula notch width; TD, trochlea depth; TPEL, tibial proximal end length; TPEW, tibial proximal end width; TWH, transverse width of the humerus.
3.2 Evolution of appendicular non-allometric shape
The pPC2 and pPC3 accounted, respectively, for 3.2% and 1.2% of total limb variation (Table 2) and can be interpreted as axes of size-independent shape variation (= non-allometric shape) since univariate morphometric characters were correlated with them in different directions and, by definition, both axes are orthogonal to the size axis pPC1. Furthermore, pPC2 and pPC3 showed no significant correlation with body size (ln HBL). Distribution of species scores along them (Figure 3d) showed significant phylogenetic signals, with pPC2 showing the K estimate much greater than 1.0 (Table 3).
The characters with the highest loadings for pPC2 were the negatively correlated TWH, OPL, RHW, and APDH and the positively correlated FL, FFL, IFW, and HML (Table 2; Figure 3e). The phyANOVA detected that the scores of semifossorial species were in average significantly lower than other locomotor habits along pPC2 (Table 3). In fact, there was no overlap between the semifossorial oryzomyalians and the other locomotor habits along pPC2, except for the akodontine species Scapteromys aquaticus, here treated as semiaquatic, which shared low scores with them. Despite overlap, most terrestrial species tended to show higher pPC2 scores than the arboreal and semiaquatic ones (Figure 3d). Given the structure of the pPC2 eigenvector, this result shows that semifossorial species, with their low scores, are characterized by presenting a transversely wide humeral epicondyle, a long olecranon process, a wide radial head, an anteroposteriorly wide humeral diaphysis, short humeral and femoral diaphyses, and a narrow humeral head. Species with other locomotory habits, mainly the terrestrial ones, tend to present opposite conditions regarding these morphological characters. Geoxus valdivianus, followed by Blarinomys breviceps and Paynomys macronyx, stood out from all other species in the morphospace for presenting very low scores and, therefore, the most specialized limb morphology among semifossorials (Figures 3d and 5). Notably, among the species treated here as terrestrial, most abrothrichines and akodontines are those showing the lowest pPC2 scores. The species with the highest pPC2 scores were Delomys sublineatus, Calomys sp., Hylaeamys megacephalus, and Euryoryzomys lamia (Figures 3d and 5).
The NHT found no correlation between divergence time and standardized phylogenetic contrasts along pPC2 (Figure 4c), as well as along pPC3 (Figure 4d). Congruently, DTT tests did not deviate from null BM expectations for both pPC2 and pPC3 scores (Figure 4g,h).
The evolutionary model that best fitted the species distribution along pPC2 was OUM.fos, followed by BMM.fos (with ΔAICc = 0.288) and OUM.all (with ΔAICc = 0.642; Table 4). These models showed that the fossorial lineages differ from all the other lineages in having their own adaptive optimum and their own rate of morphological evolution. These three models fitted to the species distribution along pPC2 better than all other models, which presented ΔAIC ≥3.9 (Table 4). BAMM found that the most likely evolutionary scenarios had two shifts in evolutionary rates throughout the phylogenetic history of Oryzomyalia (Table 5; Figure 5), with an acceleration event occurring in the lineage that led to the Abrothrichini and another in the origin of the clade that includes Blarinomys and Brucepattersonius or in the lineage that gave rise to Blarinomys.
The characters that most contributed to pPC3 were the negatively correlated CPW, IFW, FHW, and FHL, and the positively correlated OPL, DLH, ILL, CAW, FL, FFL, ICL, and PL (Figure 3e). According to the phyANOVA, arboreal species show significantly lower pPC3 scores than species of all other locomotor habits, which largely overlapped each other with high scores (Table 3, Figure 3d). The pPC3 loadings reveal that most arboreal oryzomyalians, in comparison with species of other locomotor habits, present a wide coracoid process, scapula with a wide infraspinatus fossa and a narrow cranial angle, humerus with both the shaft and the deltoid tuberosity short, ulna with short olecranon process, relatively small pelvis with short ilium, ischium and pubis, large femoral head, and short femoral diaphysis, including a short functional length of femur reflecting a reduced major trochanter. Opposite conditions regarding these characters characterize non-arboreal species. Arboreal species with the lowest pPC3 scores, and therefore possibly the most specialized limb morphology, were Rhipidomys itoan, Rhipidomys mastacalis, Rhagomys rufescens, Irenomys tarsalis, and Rhipidomys macrurus. On the other extreme, species with the higher scores were Euryoryzomys lamia, Oxymycterus delator, Scapteromys aquaticus, and Abrawayaomys ruschi (Figures 3d and 5).
The evolutionary model that best fitted the species distribution along pPC3 was OUM.arb (Table 4), a model that shows that the arboreal lineages have their own adaptive optimum. This model fits pPC3 scores better than all other compared models (ΔAICc >4.8; Table 4). BAMM found that the most likely evolutionary scenarios have a single evolutionary regime across the entire oryzomyalian phylogeny, without rate shifts along pPC3 scores (Figure 5; Table 5).
4 DISCUSSION
4.1 Locomotor specializations in oryzomyalian appendicular morphology
The phylogenetic signal recovered in all multivariate axes indicates that morphometric variation of oryzomyalian appendicular skeleton is partially structured by shared evolutionary history. This finding highlights the importance of considering the phylogenetic structure of comparative, morphometric data (Felsenstein, 1985; Harvey & Pagel, 1991). Consistently for all multivariate axes analyzed, the evolutionary models that best explained the variation in size and shape of the appendicular skeleton were the Ornstein–Uhlenbeck models with particular adaptive optima for one or more locomotor habits (OUM). These results suggest that natural selection is a relevant evolutionary force shaping the morphological variation of the appendicular skeleton of oryzomyalian (Butler & King, 2004; Cadotte & Davies, 2016).
A previous study based on a larger taxonomic sample (176 species) showed that the cranio-mandibular morphology of sigmodontines evolved under regimes more similar to the Brownian Motion than to the Ornstein–Uhlenbeck model (Maestri et al., 2017), even with notable cases of evolutionary convergence related to feeding habits (Missagia et al., 2021; Pardiñas et al., 2021) and with some overlap among distinct habits along the phylomorphospaces (Maestri et al., 2017). The difference between selection models for appendicular and cranio-mandibular morphologies suggests that ecological and functional pressures might be more influential selective factors in the evolution of the appendicular skeleton than in the evolution of the skull and mandible. Alternatively, our smaller sample size (59 species) may have facilitated the computational bias already reported that artificially favors highly parametric models, such as OUM, over simpler models, such as BM and EB (Cooper et al., 2016; Uyeda et al., 2015). Future studies with broader and equivalent sampling for cranial and appendicular morphology may test this possibility. Given this possibility, it is important to consider that the results of the selection of evolutionary models must be observed carefully. However, the consistency of our findings in view of the results of phyANOVA and phyMANOVA, showing that the diversity of locomotor habits is a relevant factor structuring the examined variation both in forelimb and hindlimb, suggests that throughout the oryzomyalian diversification in fact each locomotor habit has its own adaptive peaks, as predicted in the OUM models.
Body size is an intrinsic factor with great ecological implications at the organismal level and therefore is potentially subject to several selective pressures (e.g. Monteiro et al., 2003; Patton & Brylski, 1987), and due to allometric correlation between body parts, size of appendicular skeleton responds indirectly to selection on body size (Christiansen, 2002). However, the association between locomotor habits and the size of body or of limbs was not found to be strong in oryzomyalians, and only significant in the absence of a phylogenetic framework. Our results suggest that semiaquatic oryzomyalians tend to have larger body size and appendicular skeletons than other species. The size increase occurred in the two oryzomyalian lineages that independently evolved the semiaquatic habit: the oryzomyine clade here represented by the genera Nectomys and Holochilus (Clade D sensu Weksler, 2006) and the akodontine lineage represented by the genus Scapteromys. Considering that adaptations for semiaquatic lifestyle in several mammals often result in increase of body size, thus saving energy by facilitating thermoregulation and avoiding predation, it is reasonable to propose that their large size is partially adaptive (Gearty et al., 2018; Harrington et al., 2012; Osburn, 1903; Wolff & Guthrie, 1985). Despite the pattern suggested here, increase in body size in semiaquatic lineages cannot be taken as an absolute rule for the Sigmodontinae, as there are some non-semiaquatic taxa not sampled here with large body size (e.g., Kunsia) as well as specialized semiaquatic forms with small- to medium-sized body (e.g., Ichthyomys).
In Oryzomyalia, the size of the hindlimbs was more tightly associated with body size than the size of the forelimbs, a finding consistent with a general pattern already reported for several eutherian clades (Schmidt & Fischer, 2009). This stronger association might be due to genetic and developmental constraints, and selectively maintained by the more intense functional commitment of the hindlimbs to support and propel body mass, while the forelimbs are associated with additional functions, like handling and grasping objects (Schmidt & Fischer, 2009). Our results also showed that most variables of the hindlimb, such as femur length, covariate with body size isometrically, while those of the forelimb, such as humeral length, mostly covariate with negative allometry. Thus, larger oryzomyalian species will tend to have disproportionately larger hindlimbs than forelimbs due to an allometric rule. Many semiaquatic mammals have well-developed hindfeet that favor powerful propulsion during swimming (Fish, 1992; Osburn, 1903; Torres et al., 2020), and it could be argued that the large hindlimbs of semiaquatic oryzomyalians are a result of selection associated with this locomotor function. However, due to the strong association between body and hindlimb sizes and due to the allometric patterns reported here, it is not possible to distinguish whether the large hindlimb of semiaquatic oryzomyalians is (1) a direct result of selection on limb size, (2) a correlated outcome of selection on body size, or (3) the result of simultaneous selection on both characters. In contrast to hindlimbs, the relationship between forelimbs and body size was shown to vary depending on locomotor habits. In species of semifossorial oryzomyalians, mainly Geoxus valdivianus, Paynomys macronyx, Blarinomys breviceps, and Thaptomys nigrita, the forelimbs were larger than in non-semifossorial species with similar body size. These species use their large forelimbs to dig tunnels (Diório et al., 2011; Matson & Abravaya, 1977; Pearson, 1984; Teta et al., 2015), a condition convergent to several lineages of scratch-digging rodents (Elissamburu & Vizcaíno, 2004; Tavares et al., 2020).
The variation in non-allometric shape was shown to be more strongly structured by locomotor habits than the variation in size and in allometric shape, since both axes of non-allometric shape (pPC2 and pPC3) were significantly affected by the habits according to phyANOVA. Semifossorial species presented the most differentiated appendicular shape. This result is in agreement with previous studies showing that among muroids, digging species are those presenting the most remarkable locomotor specializations in appendicular bones (Hedrick et al., 2020; Samuels & Van Valkenburgh, 2008). This is probably due to the markedly distinct biomechanical demands for digging, which imposes severe selective pressures on phenotype toward very restricted adaptive peaks (Hildebrand, 1988; Nevo, 1979). Forelimb attributes associated with semifossoriality in oryzomyalians, such as well-developed humeral epicondyles (TWH) and olecranon process (OPL), increase the efficiency of forelimbs for excavation, either by increasing the attachment area of forearm flexor muscles or by increasing the mechanical advantage of the triceps brachii complex (Candela & Picasso, 2008; Hildebrand, 1988). In addition, the reduction in length of humerus increases the mechanical advantage for muscles primarily involved in excavation (Morgan & Álvarez, 2013; Morgan & Verzi, 2006; Steiner-Souza et al., 2010). In several mammalian families, these characteristics appeared independently in semifossorial, scratch-digging species characterized by flexing and extending alternately the forelimbs to break and loosen the soil to open underground tunnels (Candela & Picasso, 2008; Hildebrand, 1988; Nevo, 1979; Samuels & Van Valkenburgh, 2008; Sargis, 2002a, 2002b). Their independent occurrence in semifossorial akodontines and abrothrichines illustrates the presence of locomotor specializations in oryzomyalians.
The abrothrichines Geoxus valdivianus and Paynomys macronyx and, independently, the akodontine Blarinomys breviceps showed the most extreme appendicular morphologies. In line with previous analyzes of cranial, postcranial, and claw morphology, this result reveals these species as some of the most specialized among sigmodontines (Carrizo et al., 2014; Coutinho et al., 2013; Maestri et al., 2017; Missagia & Perini, 2018; Tulli et al., 2016). The remarkable morphological specializations of Geoxus, Paynomys, and Blarinomys seem to have arisen as a result of sudden changes in the evolutionary regimes of the lineages that gave rise to these taxa. The results of BAMM showed that the only acceleration events of phenotypic evolution among Oryzomyalia occurred precisely in lineages giving rise to these three taxa. The increase in evolutionary rates suggests that the selective pressures associated with fossoriality became more intense in these evolutionary branches.
The akodontine Scapteromys aquaticus stands out as the only non-semifossorial taxon disposed within the semifossorial morphospace range, relatively close to other akodontines. Although here classified as semiaquatic, some authors have proposed that Scapteromys are semiaquatic rodents with considerable fossorial ability (Barlow, 1969; Hershkovitz, 1966b; Massoia & Fornes, 1964). Thus, the Scapteromys position within the semifossorial akodontine morphospace reflects both their multipurpose locomotor abilities, as well as their phylogenetic history. The akodontines Thaptomys, Oxymycterus, Brucepattersonius and Bibimys showed intermediate morphology between semifossorial taxa with extreme specializations and species classified as terrestrial. Although the burrowing habit of Thaptomys is well documented (Teta et al., 2015), the same is not true for Oxymycterus, Brucepattersonius and Bibimys. Considering their appendicular morphology, as well as other external and postcranial traits (Carrizo, Tulli, & Abdala, 2014; Coutinho & Oliveira, 2017; Coutinho et al., 2013; Tulli et al., 2016), it is likely that species of these genera have tunnel-digging ability, although not as developed as in Blarinomys, Geoxus and Paynomys. Among the taxa classified as terrestrial, Abrothrix, Akodon, Castoria, and Necromys are the ones that are closest to the morphospace occupied by the semifossorial species (low pPC2 scores). This result suggests that all abrothrichines and akodontines, even those often treated as terrestrial, may have some morphological predisposition to fossoriality. Likely for this reason, these two tribes experienced the evolution of highly specialized burrowing lineages. In contrast, the reithrodontine Reithrodon auritus, that has been reported living in tunnel systems dug by itself (Pardiñas & Galliari, 2001; Pearson, 1988), has one of the most different appendicular morphologies from highly specialized fossorial species, with a very high pPC2 score. This result suggests that burrowing behavior of Reithrodon is not associated with the acquisition of fossorial morphofunctional specialization in the appendicular skeleton.
Morphological evolution in appendicular skeleton of the terrestrial, arboreal, semiaquatic, and saltatorial oryzomyalians seems to have occurred more slowly than in the case of semifossorial species, without significant changes in their rates, as suggested by the results of BAMM. Among the appendicular structures examined here, the differentiation between arboreal and terrestrial oryzomyalians were concentrated in the scapular shape. In arboreal species, the wider coracoid process provides greater stability for shoulder movements, as well as wider areas for insertion of the muscles coracobrachialis and biceps brachii; the wider infraspinal fossa increases the area of origin of the infraspinatus muscle, which is one of the main responsible for the abduction and external rotation of the humerus, in addition to stabilizing the shoulder join (Coutinho et al., 2013; Woods, 1972). These modifications are reported in several groups of arboreal small mammals and are assumed to be important for moving the arms in three-dimensional spaces, and eventually for supporting and pulling body weight by the hindlimbs while climbing (Coutinho et al., 2013; Flores & Díaz, 2009; Woods, 1972).
Regarding hindlimbs, in arboreal species the larger femoral head suggests greater amplitude and stability of hip movements, while an anteroposteriorly wide femoral diaphysis derives from a more expanded lesser trochanter, associated with a more developed iliopsoas muscle (Candela & Picasso, 2008; García-Esponda & Candela, 2010; Tavares & Pessôa, 2020; Wilson & Geiger, 2015). This muscle is associated with hip rotation and abduction capacity (Candela & Picasso, 2008; Soames & Palastanga, 2018). These changes, also reported from other arboreal mammals, are associated with increased maneuverability and the ability to explore three-dimensional space (Candela & Picasso, 2008; Sargis, 2002b; Tavares & Pessôa, 2020; Wilson & Geiger, 2015). On the other hand, in terrestrial species, a well-developed greater trochanter is related to an increased mechanical advantage of the hip extension muscles (Coutinho et al., 2013; Tavares & Pessôa, 2020). Congruently, the increase of the pubis, ischium, and ilium favors a greater area of origin of the muscles associated with the extension and flexion of the hip (Coutinho et al., 2013; Tavares & Pessôa, 2020). In this way, morphological specializations associated with the terrestrial habit increase the amplitude and speed of the gaits.
4.2 Lacking evidence for early-burst phenotypic disparification
One of the expected conditions for adaptive radiation is the divergence of characters with ecological relevance, allowing a specialized use of the multiple resources available and thus avoiding niche overlap (Gavrilets & Losos, 2009; Schluter, 1996). According to the EO model, ecological specializations, by reducing competition, favor accelerated speciation until niches became saturated. The accumulation of disparity in characters with ecological relevance is expected to be early concentrated in phylogenies, and more rarefied toward terminal taxa, as a result of disparity deceleration after niches saturation (Liedtke et al., 2016; Schweizer et al., 2014; Yoder et al., 2010). As already pointed out, our results support ecological specializations in the morphology of appendicular skeleton, allowing the occupation of several locomotor niches among oryzomyalians. Nevertheless, we found no support for an early accumulation of locomotor specializations in appendicular morphology followed by deceleration in evolutionary rates, the EB model was not an adequate model to explain the morphological variation among species, and NHT and DTT did not indicate that the disparity has slowed down over time. These results suggest that rates of disparity are independent of the time since South American colonization by oryzomyalians.
Despite evidence that speciation rate has accelerated at the origin of Oryzomyalia (Parada et al., 2015), our study found no evidence that morphological disparity rates of appendicular skeleton exhibited a similar behavior, in congruence with previous results for cranio-mandibular and external morphology (Alhajeri et al., 2016; Maestri et al., 2017). While the EO model postulates a positive correlation between speciation and phenotypic evolution rates (Harmon et al., 2003; Mahler et al., 2010; Rabosky et al., 2013; Weir & Mursleen, 2013; Yoder et al., 2010), our data suggest that these rates were decoupled along the diversification of the oryzomyalian appendicular skeleton, similarly to their external and cranio-mandibular morphologies (Alhajeri et al., 2016; Maestri et al., 2017). Similar results in Neotropical lizards and cichlid fish showed constant rates of morphological divergence despite explosions of diversification in select strains (López-Fernández et al., 2013; Pincheira-Donoso et al., 2015). On the other hand, in highly diversified groups such as furnariid birds, parrots, and primates, phenotypic evolution rates corroborated the EO model, while the speciation rates did not (Aristide et al., 2015; Derryberry et al., 2011; Schweizer et al., 2014). Outside the Neotropics, the dissociation between rates of phylogenetic and phenotypic diversification has been documented for plants, salamanders, murine rodents, squirrels, cetaceans, snakes, and lizards (Alhajeri et al., 2016; Burbrink et al., 2012; Kozak et al., 2006; Slater et al., 2010; Zelditch et al., 2015). These studies point out the difficulties in proposing general and predictive models of adaptive radiation (Burbrink et al., 2012).
At least two factors can be hypothesized to explain the lack of association between speciation and morphological disparity rates in South American sigmodontines: the continental dimension of Oryzomyalia radiation and the lack of association between speciation and locomotion. The particularities of Oryzomyalia diversification can be better understood considering the size, environmental complexity, and habitat heterogeneity of South America (Harmon et al., 2008; Liedtke et al., 2016). Biogeographic complexity and partitioning of South America seem to have played an important role in the adaptive radiation of oryzomyalians, with subclades having experienced regional bursts of speciation correlated with expansion in geographic distribution (Maestri et al., 2018; Schenk & Steppan, 2018). Considering that subclade diversification remained mostly regionally limited, saturation of resources determining the decline of diversification is unlikely to have been achieved with the high ecological complexity and wide continental area (Liedtke et al., 2016). In this regard, oryzomyalians may still be diversifying and may not have reached the deceleration phase (Alhajeri et al., 2016). Continuous and gradual phenotypic and phylogenetic diversification can be favored by complex geographic histories of large continental areas that prevent the stabilization of environmental conditions. From the Late Miocene to Late Pliocene, South America has repeatedly experienced profound large-scale environmental reconfigurations (Hartley & Chong, 2002; Hoorn et al., 2010; Kleinert & Strecker, 2001; Mora et al., 2011; Ortiz-Jaureguizar & Cladera, 2006). Additionally, during the Pleistocene, cyclical climatic oscillations drastically changed the ranges of main phytogeographic formations, affecting rodent distributions (Carnaval & Moritz, 2008; Costa & Leite, 2012; Gonçalves et al., 2007; Lessa et al., 2010; Oliveira et al., 1999; Werneck, 2011; Werneck et al., 2010, 2012). This dynamic scenario corroborates the proposition of the continuous appearance of new ecological opportunities, with the gradual occupation of niches throughout oryzomyalian diversification, (Liedtke et al., 2016; Schweizer et al., 2014). In addition, it must be considered that small terrestrial mammals, presenting a high predisposition for allopatric speciation by isolation when colonizing a new continent, can experience an accelerated rate of speciation without the appearance of ecological specializations, characterizing a non-adaptive radiation (Czekanski-Moir & Rundell, 2019; Rundell & Price, 2009). Even if Oryzomyalia diversification could be characterized as an adaptive radiation, it is also possible that the diversification of locomotor niches, as investigated here, was not a key factor in the exploitation of resources. In this case, other ecological factors would have a preponderant importance and might better explain its explosive diversification.
With this study, we conclude that different locomotor habits compose relevant ecological factors shaping morphological evolution of South American sigmodontines. However, despite this ecological relevance, we found no evidence that the disparification of appendicular skeletal in this group of rodents took place with a clear slowdown over time, as would be expected in a classical pattern of adaptive radiation.
It is important to consider that all conclusions raised here are restricted to the proximal portion of the appendicular skeleton. Autopodial structures showed prominent morphofunctional specializations in several groups of rodents already studied (Boivin et al., 2018; Candela et al., 2017; Ginot et al., 2016; Morgan & Verzi, 2011; Nations et al., 2019). Therefore, it is likely that investigations including information from limb distal elements might detect a different influence of locomotor habits on the morphological evolution of the oryzomyalians appendicular skeleton. We hope that further studies with a denser taxonomic sampling, also including non-oryzomyalian cricetid lineages, and with detailed information on entire appendicular skeleton will allow us to reconstruct a more detailed scenario of the morphological disparification of sigmodontines after their colonization in South America.
ACKNOWLEDGMENTS
We are very thankful to Andrés Parada, Renan Maestri, and John Schenk for promptly making available the newick files with phylogenetic reconstructions recovered in their studies. We thank Gisele Lessa (Museu de Zoologia João Moojen de Oliveira, Universidade Federal de Vicosa, Minas Gerais, Brazil), Maria Rita Silvério Pires (Laboratório de Zoologia de Vertebrados, Universidade Federal de Ouro Preto, Minas Gerais, Brazil), and Ulyses Pardiñas (Colección de Mamíferos—Colección de material de egagrópilas y afines “Elio Massoia,” Centro Nacional Patagónico, Puerto Madryn, Argentina), who generously allowed access, approved loans and helped us to analyze specimens under their care. We are also thankful to Gustavo S. Libardi, Julio Torres, and Carola Cañón Valenzuela for the amazing hospitality and all their help during the LCC stay in Puerto Madryn, Argentina. We thank the editor and reviewers whose comments helped to improve this work. A preliminary version of this study was developed as part of the PhD Dissertation presented by LCC to the Graduate Program in Biodiversity and Evolutionary Biology (PPGBBE) at Universidade Federal do Rio de Janeiro. It was partially supported by a graduate fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) to LCC and by a research fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) to JAO.
APPENDIX
List of examined specimens. The specimens are deposited in the collections of mammals of the following institutions: MN: Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil. LZV-UFOP: Laboratório de Zoologia de Vertebrados, Universidade Federal do Ouro Preto, Ouro Preto, Brazil. CNP: Colección de Mamíferos, Centro Nacional Patagónico (CENPAT-CONICET), Puerto Madryn, Chubut, Argentina. CNP-E: Colección de material de egagrópilas y affines “Elio Massoia,” CENPAT-CONICET, Puerto Madryn, Chubut, Argentina.
Incertae sedis: Abrawayaomys ruschi: MN 67557, LZV-UFOP (62M, CAC89, and CAC121). Delomys altimontanus: MN 60573, 60574, 60584, 60585 and 79013. Delomys dorsalis: MN 60572, 60577, 60578, 60580, 60581, 60582, 60583, 60586, 78501, 78540, 78562, 78564, 78682, 78686, 78687, 78690, 78691,78698, 78909, 78915 and 79021. Delomys sublineatus: MN 78652, 78660, 78674, and 78676.
Tribe Abrotrichini: Abrothrix hirta: CNP-E 502, 1015, 1257, 1435, 2736, 2744, 2747, 2745, 2796, 2850, 2858, 2862, 3663, 3833, 3892, 5100, 5469, 5471, and 5468. Geoxus valdivianus: CNP-E 437. Paynomys macronyx: CNP-E 441, 445, 446, 1896, 2373, 3624, 3888, 3889, 3890, 3891, 3896, and 5365.
Tribe Akodontini: Akodon cursor: MN 78917, 78930, 78962, 78974, 78980, 78984, 78985, 78996, and 78997. Akodon montensis: MN 68211, 68327, 68243, 68244, 68287, 68290, 68301, 68317, 68318, 68319, 68348, 79022, 78419, 78420, 78428, 78431, 78634, 78636, and 78658. Akodon paranaensis MN 79011. Bibimys labiosus: MN 78988. Blarinomys breviceps: MN 70223, 70224, 70225, and 70226. Brucepattersonius iheringi: MN 78542. Brucepattersonius soricinus: MN 48017, 68344, MN 78421, 78659; Castoria angustidens: MN 78422, 78424, 78425, 78432, 78433, 78434, 78437, 78456, 78459, 78460, 78461, 78465, 78479, and 78921. Deltamys kempi: MN 78491, 78496, 78502, 78510, and 78579. Necromys lasiurus: MN 36343, 36349, 36373, 36470, 36510, 36523, 36671, 37264, 37316, 37317, 64358, 64454, 64456, 64475, 64479, 64692, 67561, 67625, 67636, 67652, 68341, 74922, 74923, 74924, 74925, 74926, 74927, 74928, 74929, 74933, 74934, 74935, 74938, 74939, 74940, 74943, 74944, 74945, 74947, and 74950. Oxymycterus amazonicus: MN 79724, 79725, and MZUSP M-968449. Oxymycterus dasytrichus: MN 46648, 46652, 46653, 47985, 62241, 62242, 62243, 62245, 62246, 62247, 62248, 62249, 62251, 62252, 62253, 62257, 62258, 62263, 62313, 72094, 72097, and 77810. Oxymycterus delator: MN 69691, 69692, 69696, 69702, 69703, 69704, 69707, 69713, 69718, 69728, 69730, 69733, 69734, 69740, 69742, and 69748. Oxymycterus nasutus: MN 46683, 46691, 46708, 46709, 46712, 48748, 48752, 48753, 48754, 48758, 48759, 48761, 78443, 78451, 78466, 78486, 78487, 78492, 78507, 78519, 78521, and 78537. Oxymycterus quaestor: MN 46646, 46647, 48082, 48083, 67490, 67491, 67495, 67496, 68339, 75361, 75362, 75363, 78454, 78470, 78471, 78473, 78477, 78523, 78556, and 79072. Oxymycterus rufus: MN 65527 and 65538. Scapteromys aquaticus: CNP-E 710, 1496, 1911, 4146, 4608, 4639, 4641, 4644, 4652, 4653, 4654, 5245, 5246, 5247, 5303, 5308, and 5310. Thaptomys nigrita: MN 68219, 68225, 68227, 68261, 68264, 68270, 68277, 68278, 68300, 68302, 68304, 68308, 68309, 68310, 69617, 69631, 69632, 69641, 69642, 69670, 78423, 78458, 78475, 78478, 78633, 78641, 78661, 78663, and 78675.
Tribe Andinomyini: Andinomys edax: CNP-E 2364 and 5491.
Tribe Euneomyini: Euneomys petersoni: CNP-E 2406, 2411, 2413, 2414, 2415, 2416, 3674, 3700, 3780, 4420, 4429, 5406, and 5467. Irenomys tarsalis: CNP-E 5295.
Tribe Oryzomyini: Cerradomys goytaca: MN 67536, 70229, 73178, 73184, 73187, and 73193. Cerradomys scotti: MN 61667, 61668, 61670, 61671, 61679, and 67089. Cerradomys subflavus: MN 35903, 42861, 44545, 63350, 63351, 63390, 63426, 63429, 78968, and 78969. Cerradomys vivoi: MN 63381, 63382, 67528, 67529, 67533, 67534, 67535, 67541, 67543, 67544, 67545, 67546, 67565, 67567, 67629, 67631, 67634, 67691, 67736, 67749, 67794, 67800, 67805, 67809, 75924, 75927, and 75928. Euryoryzomys lamia: MN 67090. Euryoryzomys russatus: MN 48050, 68213, 68214, 68217, 68233, 68234, 68238, 68239, 68241, 68266, 68281, 68284, 68285, 68291, 68292, 68293, 68299, 68306, 70114, 70117, 70118, 73765, 75185, 78632, 78640, 78643, and 78649. Holochilus brasiliensis: MN 75679, CNP-E 3964, 3965, 5269, and 5321. Holochilus chacarius: CNP-E 1890, 1894, 2391, 3946, 3947, 3949, 3951, 3952, 3954, 3957, 3958, 3959, 3961, and 4637. Holochilus sciureus: MN 70399. Hylaemys megacephalus: MN 36288, 36487, 36519, 37263, 37287, 37290, 37315, 37354, 37390, 64115, 64255, 64501, 64530, 70262, 70353, 70358, 70411, 70433, 70490, 70493, 70498, 70505, 70511, 70513, 70613, 70618, 70649, 70727, 70736, and 70930; Hylaemys seuanezi: MN 35904 70052, 70058, and 70059; Neacomys guianae: MN 70398, 70404, 70405, 70408, 70563, 70759, 70764, 70805, 70836, 70857, 70925, 70928, 70933, and 70955. Nectomys squamipes: MN 50550, 50551, 50552, 53386, 53387, 59009, 61798, 62198, 62199, 62200, 62201, 62202, 62203, 62205, 62207, 62208, 62211, 62212, 62213, 62215, 66175, 67029, 67032, 67034, 67035, 67037, 67039, 67044, 67045, 67046, 67053, 67054, and 78983. Oecomys concolor: MN 36231, 36301, 36399, and 37280. Oecomys mamorae: MN 63911. Oligoryzomys flavescens: MN 69577, 69578, 69579, 69697, 69706, 69709, 69722, 69736, 69749, 78447, 78448, and 78464. Oligoryzomys nigripes: MN 43836, 43837, 61715, 61716, 61717, 61718, 61728, 66152, 66153, 66156, 66157, 66159, 66217, 68335, 69638, 69919, 69921, 69924, 69926, 69932, 70227, 72096, 72137, 75328, 75337, 78449, 78457, 78467, 78516, 78533, 78566, 78570, 78598, 78963, 78965, 78966, 78967, 78970, 78971, 79018, and 79850. Sooretamys angouya: MN 50234, 68220, 68267, and 71849. Zygodontomys sp.: MN 69023, 69029, 69077, 69078, 70316, 70329, 70336, 70337, 70347, 70350, 70351, 70352, 70388, 70391, 70400, 70581, 70582, 70588, 70737, 70740, 70741, 70742, 70743, 70747, 70761, 70777, 70778, and 70934.
Tribe Phyllotini: Calomys sp.: MN 76135, 76139, 76141, 76156, 76158, 76180, 76781, 76783, 76787, 76789, 76793, 76795, 76796, 76797, 76798, 76799, 76800, 76801, 76802, 76803 and 76804, 76809, 76812, 76814, 76822, 76823, 76825, 76826. Eligmodontia sp.: CNP-E 939, 1067, 1142, 1164, 1244, 1636, 2250, 3197, 4398, and 5436. Tapecomys primus: CNP-E 828 and 829.
Tribe Reithrodontini: Reithrodon auritus: CNP-E 1161, 2001, 2004, 2008, 2010, 2011, 4831, 5441, and 5849.
Tribe Thomasomyini: Rhagomys rufescens: LZV-UFOP (215R) and MN 65545. Rhipidomys itoan: MN 63603, 63606, 63609, 63613, 63614, 63615, 63616, 63620, 71199, 71204, and 71205. Rhipidomys macrurus: MN 4323, 4333, 4335, 5196, 30016, 30017, 34408, 34409, 34410, 34411, 34429, 63317, 63318, 63321, and 63324. Rhipidomys mastacalis: MN 34430, 36358, 46564, 67751, 67826, 67827, 67875, 72704, 72705, 72706, and 72707.
Tribe Wiedomyini: Juliomys pictipes: MN 68336, 68347, and 69765. Juliomys rimofrons: MN 77793. Phaenomys ferrugineus: MN 53614. Wiedomys pyrrhorhinos: MN 62180, 63357, 63420, 73442, 73453, 73461, 73477, 75110, and 75126.




