Integrative taxonomy reveals two species and intraspecific differentiation in the Vipera latastei–monticola complex
Contributing authors: Inês Freitas ([email protected]), Guillermo Velo-Antón ([email protected]), Nahla Lucchini ([email protected]), Soumia Fahd ([email protected]), Said Larbes ([email protected]), Juan M. Pleguezuelos ([email protected]), Xavier Santos ([email protected]), José C. Brito ([email protected])
Zoobank links: LSID: http://zoobank.org/NomenclaturalActs/cbda977e-f8b9-45d7-85c0-96a0ef505976
Online ISSN: 1439-0469
Abstract
enModern taxonomy relies on the unified species concept and integrative approaches to delimit evolutionary coherent taxa. The western Mediterranean vipers within the Vipera latastei–monticola complex (Vipera latastei and Vipera monticola) have a rather old taxonomic history marked by the prevalence of morphological criteria in the recognition of taxonomic units. Recent phylogenetic inferences, however, contradict this taxonomic scenario, highlighting the need of integrative studies to properly evaluate the taxonomy of this complex. Here, we apply an integrative taxonomic approach, combining phylogeographic, morphological, and ecological analyses, to identify and describe evolutionary coherent taxonomic units within the Vipera latastei–monticola complex. Using dated mtDNA phylogenetic relationships, we spatially delimit two levels of evolutionary units, which are the subject of morphological and ecological comparisons. The first level corresponds to two Miocene vicariant clades, with considerable morphological distinctiveness that we identified as different species: V. latastei in the Iberian Peninsula, and V. monticola in North Africa. The second level corresponds to three Pliocene parapatric lineages within each of these species, which we recognized with subspecific status due to non-apparent geographic isolation and variable levels of phenotypic distinctiveness. Consequently, we propose distributional rearrangements in the previously recognized taxa, as well as define three new subspecies: Vipera latastei arundana ssp. nov. in southern Iberia, Vipera monticola atlantica ssp. nov. in the western High Atlas Mountains and Vipera monticola saintgironsi ssp. nov. in the eastern High Atlas, Middle Atlas, Rif, Tellian Atlas and Aurès Mountains. Our proposed taxonomic scenario anticipates important outcomes for the conservation status of these Mediterranean taxa.
Resumen
esLa taxonomía moderna se basa en el concepto de especie unificada y en enfoques integrativos para delimitar taxones evolutivos coherentes. Las víboras del Mediterráneo occidental incluidas en el complejo Vipera latastei-monticola (Vipera latastei y Vipera monticola) tienen una historia taxonómica bastante antigua, marcada por la prevalencia de criterios morfológicos en el reconocimiento de unidades taxonómicas. Sin embargo, recientes inferencias filogenéticas contradicen este escenario taxonómico, destacando la necesidad de estudios integrativos para evaluar adecuadamente la taxonomía de este complejo. Aquí aplicamos un enfoque taxonómico integrativo, combinando análisis filogeográficos, morfológicos y ecológicos, para identificar y describir unidades taxonómicas evolutivas coherentes dentro del complejo Vipera latastei-monticola. Utilizando relaciones filogenéticas datadas basadas en ADN mitocondrial, delimitamos espacialmente dos niveles de unidades evolutivas, que son objeto de comparaciones morfológicas y ecológicas. El primer nivel corresponde a dos clados vicariantes del Mioceno, con una marcada distinción morfológica y que identificamos como especies diferentes: V. latastei en la Península Ibérica y V. monticola en el norte de África. El segundo nivel corresponde a tres linajes parapátricos del Plioceno dentro de cada una de estas especies, que identificamos con nivel subespecífico debido a una aparente falta de aislamiento geográfico y niveles variables de diferenciación fenotípica. Consecuentemente, proponemos cambios en los rangos de distribución de los taxones previamente reconocidos, y definimos tres nuevas subespecies: Vipera latastei arundana ssp. nov. en el sur de Iberia, Vipera monticola atlantica ssp. nov. en las montañas del Alto Atlas occidental y Vipera monticola saintgironsi ssp. nov. en el este del Alto Atlas, Medio Atlas, Rif, Atlas Teliano y montañas de Aurès. Nuestro escenario taxonómico propuesto anticipa resultados importantes para el estado de conservación de estos taxones mediterráneos.
1 INTRODUCTION
Species are fundamental units in biology, basal to many disciplines, particularly to taxonomy and conservation. Modern taxonomy relies on the unified species concept (de Queiroz, 2007), which defines species as separately evolving lineages and their biological properties (e.g., morphological distinctiveness, differentiated ecological niches) as “operational criteria” that ultimately provide evidence for their separation. When evolutionary units show incomplete separation (e.g., lack of reproductive isolation), the subspecies rank can be proposed, particularly to recognize phenotypically distinct groups of populations distributed across geographic areas (de Queiroz, 2020; Mayr, 1969). Integrative taxonomic approaches are thus designed to combine different lines of evidence (e.g., genetic, morphological, ecological) and methodologies (e.g., phylogenetic inference, ordination methods, ecological modeling) to objectively identify taxa (Dayrat, 2005).
European vipers (fam. Viperidae, genus Vipera) are a monophyletic group of venomous snakes, distributed across Eurasia and North Africa (Sindaco et al., 2013). Taxonomic inferences have long been based on morphological traits, and consequently, European vipers have suffered from species inflation (see Freitas et al., 2020; Sindaco et al., 2013). A recent taxonomic evaluation of the group (also including other genera of vipers) analyzed phylogenetic and morphological variability across taxa and recognized the doubtful validity of some previously described species, urging for the use of integrative taxonomic approaches to re-evaluate the taxonomy of particular groups such as the Vipera latastei–monticola complex (Freitas et al., 2020).
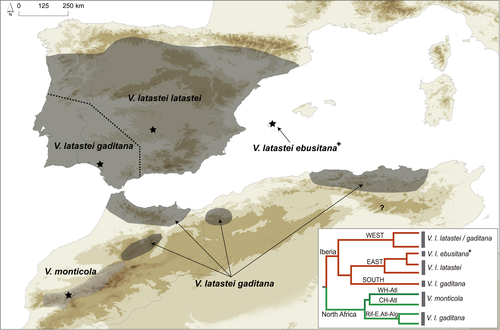
The V. latastei–monticola complex is a good example of a rather old taxonomic history marked by the prevalence of morphological criteria in the recognition of taxonomic units. The species was first described in the Mediterranean region of the Iberian Peninsula as Vipera latastei Boscá, 1878, by Eduardo Boscá and later expanded into the Mediterranean region of North Africa through the findings of the species in Morocco (Boettger, 1883), Algeria (Tourneville, 1881) and Tunisia (Chpakowsky & Chnéour, 1953). During the second half of the 20th century, Hubert Saint Girons described two subspecies: (a) V. latastei monticola Saint Girons, 1953, restricted to the high elevation ranges of the High Atlas Mountains of Morocco and characterized by its small size (i.e., dwarf), specific scalation and coloration traits; and (b) Vipera latastei gaditana Saint Girons, 1977, which differed by a number of scalation traits (e.g., ventral scales) from the nominal subspecies (Vipera latastei latastei) and occurring in the southwestern Iberian Peninsula and North Africa (Rif and Middle Atlas in Morocco, and Tellian Atlas in Algeria). Later on, and based on its morphological distinctiveness, Beerli et al. (1986) elevated V. l. monticola to full species rank, V. monticola. Using large datasets of specimens and traits, contemporary assessments of morphological variability recognized the occurrence of two and three morphologically differentiated groups within Iberia and North Africa, respectively; one of them corresponding to V. monticola (Brito et al., 2006, 2008; Santos et al., 2014). Nevertheless, no taxonomic arrangements were proposed in these studies.
Recent molecular analyses based on mtDNA have unveiled the evolutionary history of V. latastei–monticola (Freitas et al., 2018; Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012). Vipera latastei–monticola is recognized as sister group to the Para-Mediterranean Vipera aspis (Linnaeus, 1758), from which it likely diverged during the late Miocene (~9 Mya) in the Iberian Peninsula, with a subsequent expansion to North Africa during the Messinian Salinity Crisis (~6 Mya, Martínez-Freiría, Freitas, et al., 2020). At the end of this period (~5.33 Mya), the reopening of the Strait of Gibraltar led to the formation of two vicariant groups: the Iberian and the North African clades. Subsequent Plio-Pleistocene climatic oscillations produced further allopatric isolation within the Iberian and North African clades, inducing high levels of genetic structuring in both groups (Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012). As a consequence, three major Pliocene lineages with parapatric distributions are currently recognized within each of the two main clades, and most of these lineages present further levels of Pleistocene diversification (Freitas et al., 2018; Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012). Vipers from two of the three North African lineages are characterized by dwarf phenotypes and one of these two lineages corresponds to the populations of the central High Atlas, which were previously recognized as V. monticola (Freitas et al., 2018; Velo-Antón et al., 2012). More recently, an extinct subspecies within this complex has been described from subfossil remains in the island of Ibiza (Balearic Islands, Spain): Vipera latastei ebusitana Torres-Roig, Alcover & Bailon, 2021 (Torres-Roig et al., 2021). This subspecies, also characterized by a small size, is phylogenetically nested within the eastern Iberian lineage, which likely colonized Ibiza by rafting events from the Iberian coast about 1 Mya, and went extinct with the human colonization of Ibiza about 4–2 kya BP (Torres-Roig et al., 2021).
Considering peer-reviewed scientific literature only (for the reasons given in Kaiser et al., 2013), the current taxonomy of the group recognizes two species (Figure 1): (a) the polytypic V. latastei (previously considered as V. latasti, but see International Commission of Zoological Nomenclature, 2017), with two extant subspecies distributed in Iberia and North Africa plus one extinct subspecies from the Balearic Islands; and (b) the monotypic V. monticola, restricted to the High Atlas. However, molecular data contradict this taxonomic classification as it nests V. monticola inside the North African clade and places V. l. gaditana in several lineages within both clades (Figure 1). A conciliation among phylogenetic, morphological, and ecological data is therefore needed to solve the current taxonomic puzzle of the V. latastei–monticola complex.
Here, we apply an integrative taxonomic approach, combining phylogeographic, morphological, and ecological analyses, to identify and describe taxonomic units within the V. latastei–monticola complex. We rely on the mtDNA phylogeography of the group to establish two cutoff points and to spatially delimit evolutionary units, which are then object of morphological and ecological comparisons. According to the level of phylogenetic divergence and geographic isolation as indicators of reproductive isolation, as well as morphological and ecological distinctiveness, we propose an updated taxonomy for the group, which is expected to enhance the delimitation of evolutionary coherent units required for conservation purposes.
2 MATERIALS AND METHODS
2.1 Data
For phylogeographic analyses, sequences for three mtDNA markers (CYTB, 517 bp; ND2, 1004 bp; and ND4, 751 bp) from 153 specimens (136 from Iberia and 17 from North Africa) covering the range of the V. latastei–monticola complex were used (Figure S1). Of them, 316 sequences (148 for CYTB, 22 for ND2, and 146 for ND4) were already published in previous works (e.g., Freitas et al., 2018, 2020; Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012) and were downloaded from the GenBank database (https://www.ncbi.nlm.nih.gov/genbank). Additionally, seven unpublished CYTB (n = 4; GenBank accession numbers: MZ712101, MZ712102, MZ712103, MZ712104) and ND4 (n = 3; GenBank accession numbers: MZ712105, MZ712106, MZ712107) sequences (obtained following protocols of previous works; see Freitas et al., 2018; Martínez-Freiría, Freitas, et al., 2020) of four vipers (one of them from a recently discovered population in Aurès Mountains, Algeria; see Bouam et al., 2019) were added to our dataset to increase geographic sampling coverage (Table S1). We rely on mtDNA only since previously used nuclear DNA genes (e.g., B-fib, Beta-fibrinogen intron 7; PRLR, Prolactin Receptor; RAG1, Recombination Activating protein 1) showed to be uninformative at the intraspecific level within V. latastei–monticola (see Freitas et al., 2018; Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012), and even at higher taxonomic levels within Eurasian vipers, likely due to incomplete lineage sorting (Freitas et al., 2020).
For morphological analyses, 26 traits from 912 specimens (832 for Iberia and 80 for North Africa), measured in 18 museum collections (n = 559; collected from 1880 to 2015) and in the field (n = 336; fieldwork campaigns developed from 1999 to 2019), or gathered from publications (n = 17), were considered (Figure S2; Table S2). Most specimens were in good conditions and when partially damaged (e.g., head smashed, tail cut), morphological measurements were not taken for the corresponding trait(s) that could be affected. Most specimens were adults (SVL >250 mm, n = 738; Pleguezuelos et al., 2007; Martínez-Freiría et al., 2010), with a similar proportion of sexes and geographically representative of both Iberia and North Africa (Table S3; Figure S2). Morphological traits included six biometric (all meristic), 12 pholidotic (nine meristic and three categorical), and eight coloration (one meristic and seven categorical) traits (Table 1). Biometric traits were mostly gathered by FM-F using a scale and digital caliper (with a precision of 0.01 mm). About 20% of specimens were measured by XS and JCB and, in this case, only body measurements (SVL and TAIL; see Table 1) were considered. Pholidotic data were gathered by FM-F, XS, and JCB, from live specimens and/or photographs taken from museum preserved specimens. Pholidotic accounts strictly followed the same protocol and, in some cases, were double-checked in order to minimize scale-count biases. Coloration data were gathered by NL from specimens' photographs taken by FM-F, XS, and JCB, according to categories previously proposed by FM-F and XS; these were later double-checked by FM-F.
| Type | Code | Meaning |
|---|---|---|
| Biometric | SVL | Snout vent length |
| TAIL | Tail length | |
| HL | Head length | |
| ML | Mouth length | |
| HH | Head height | |
| HW | Head width | |
| Pholidotic meristic | APIC | Number of apical scales |
| INTER | Number of intercanthal +intrasupraocular scales | |
| LOR | Number of loreal scales (right side) | |
| SUP | Number of supralabial scales (right side) | |
| INFR | Number of infralabial scales (right side) | |
| PERIOC | Number of periocular scales (right side) | |
| VENT | Number of ventral scales | |
| SUBC | Number of subcaudal scales | |
| DORS | Number of dorsal rows at midbody | |
| Pholidotic categorical | PARIET | Fragmentation of the parietal plates (partial / total) |
| FRONT | Fragmentation of the frontal plate (partial / total) | |
| ROWS | Scale row between periocular and supraocular scales (complete / incomplete) | |
| Coloration meristic | DMARK | Number of dorsal marks (right side) |
| Coloration categorical | DP1A | Narrow angular zigzag |
| DP1B | Wide angular zigzag | |
| DP2A | Wide rounded zigzag | |
| DP2B | Narrow rounded zigzag (balls) | |
| DP2C | Stretched rounded zigzag | |
| DP3 | Barred zigzag | |
| TAIL col | Coloration of the tip of the tail (black - yellowish) |
For ecological analyses, 12 topo-climatic variables (Table 2) were used to characterize environmental conditions for a total of 1048 occurrences of V. latastei–monticola (927 occurrences for Iberia, 121 occurrences for North Africa; Figure S3). Variables were gathered at 2.5 arcmin. of resolution (~5 × 5 km) and were downloaded from the WorldClim database (https://www.worldclim.org/) except slope, which was derived from elevation in ArcGIS v. 10.5 (ESRI, 2016). Occurrences, also gathered at 2.5 arc.min of resolution, were obtained from fieldwork carried out by the authors and collaborators (see Acknowledgements), previous publications (e.g., Freitas et al., 2018; Martínez-Freiría, Freitas, et al., 2020), and the database of the Spanish herpetological society (AHE). With the exception of some records that were gathered before 1950 (particularly for North Africa, see Freitas et al., 2018), most occurrence data span from 1950 to 2019. ArcGIS v. 10.5 was used to extract information from the 12 topo-climatic variables to each occurrence.
| Type | CODE | Meaning | Units |
|---|---|---|---|
| Topographic | ALT | Altitude | m |
| Topographic | SLOPE | Slope | % rise |
| Climatic | BIO1 | Annual mean temperature | °C |
| climatic | BIO4 | Temperature seasonality | coef |
| Climatic | BIO5 | Max temperature of warmest month | °C |
| Climatic | BIO6 | Min temperature of coldest month | °C |
| Climatic | BIO12 | Annual precipitation | mm |
| Climatic | BIO13 | Precipitation of wettest month | mm |
| Climatic | BIO14 | Precipitation of driest month | mm |
| Climatic | BIO15 | Precipitation seasonality | coef |
| Climatic | VAPOR | Average water vapor pressure | kPa |
| Climatic | SOLRAD | Total solar radiation | kJ/m2/day |
Note
- Type, code, meaning and units are depicted.
2.2 Phylogenetic inference and delimitation of genetic units
Sequences were manually aligned and edited using Geneious v. 4.8.5 (alignment available on Alignment S2). Phylogenetic relationships and time of divergence within the V. latastei–monticola complex were inferred using a Bayesian Inference (BI) method implemented in BEAST v. 1.7.5 (Drummond et al., 2012). The best-fitting model of molecular evolution and partition scheme was identified under the Bayesian Information Criterion using PartitionFinder (Lanfear et al., 2012). The HKY + G + I and TrN + G models applied to the CYTB and ND4 + ND2 fragments, respectively, were determined as the best-fit models and partitioning schemes. A lognormal relaxed clock and a coalescent constant size model were used as tree priors. Time for the most recent common ancestor (TMRCA) was based on the split of V. latastei–monticola and V. aspis estimated at 9.2 Mya in previous works (Freitas et al., 2020; Martínez-Freiría, Freitas, et al., 2020). A lognormal distribution with a mean of 9.2 and 0.1 of standard deviation was implemented as prior to account for uncertainty on node age estimates (Freitas et al., 2020; Martínez-Freiría, Freitas, et al., 2020). Three Vipera species (Vipera ammodytes, V. aspis, and Vipera seoanei) were included in the analyses as outgroups. Three independent runs of 100 million generations were performed, and sampling trees and parameter estimates every 10,000 generations with 10% of the trees discarded as burn-in. The quality of the runs (i.e., parameter convergence) was verified by examining the effective sample sizes (ESS >300 for all parameters) using Tracer v. 1.7 (Rambaut et al., 2018). Trees obtained from multiple runs were then combined using LogCombiner v. 1.7.5 (Drummond et al., 2012) and a summary tree was generated with TreeAnnotator v. 1.7.1 (Drummond et al., 2012). CYTB uncorrected p-distances between mtDNA clades and lineages were estimated using MEGA v. 5 (Tamura et al., 2011).
Based on major paleo-tectonic and paleo-climatic events occurred in the Western Mediterranean Basin, we established two cutoff points across the V. latastei–monticola mtDNA phylogenetic tree to delimit genetic units that were object of morphological and ecological comparisons. The first cutoff was set in the transition Miocene-Pliocene (ca. 5.33 Mya), coincident with the end of the Salinity Crisis of the Mediterranean, which produced the connection/disconnection between Europe and Africa (Duggen et al., 2003) and leads to the formation of vicariant species at each side of the Mediterranean (Hewitt, 2012). The second cutoff was established in the Pliocene—Pleistocene transition (ca. 2.6 Mya), periods characterized by strong climatic oscillations, which are known to have produced a deep impact at the intraspecific level of many western Palearctic taxa (Hewitt, 2004).
Morphological and environmental data were assigned to the distinct units delimited by these two cutoff points through spatial interpolations of the genetic distances obtained from the mtDNA tree and using the R package Phylin (Tarroso et al., 2019). In brief, genetic distances were interpolated from each genetic unit to the remaining samples, using the kriging interpolator with a spherical model. The background area for interpolations consisted of the area including all occurrence data (at 2.5 arc min of resolution). Each interpolation resulted in a raster depicting the probability of membership to one of the genetic units. A threshold of 0.75 of probability was used in GIS to delimit the areas of occurrence for each genetic unit and thus, to assign corresponding georeferenced morphological and environmental data to each genetic unit. This threshold, although arbitrarily selected, guarantees that all samples assigned to a specific genetic unit were included in its range and also that pixels with ambiguous membership, mostly falling in contact zones, were excluded.
2.3 Univariate morphological and environmental analyses
Univariate statistical analyses were performed on four datasets: three morphological, including (a) biometric meristic, (b) pholidotic and coloration meristic, and (c) pholidotic and coloration categorical traits of specimens; and one environmental, encompassing (d) topo-climatic variables characterizing evolutionary units' ranges.
In the morphological datasets, specimens with more than 25% of traits with missing values were not considered in the analyses (see Data S1–S3). Biometric, pholidotic, and coloration meristic traits were log transformed to meet assumptions of most statistical tests. All morphological traits were first tested for sexual dimorphism, using ANOVA or Kruskal–Wallis tests for quantitative data, and Fisher tests for categorical data. Traits that were significantly dimorphic were analyzed separately for females and males. Analyses on single biometric traits consisted of ANCOVA tests, using snout vent length (SVL) as a covariate for the analyses of the remaining biometric traits and restricting data to adult vipers (specimens with SVL >250 mm, n = 499). Additionally, differences in SVL among evolutionary units were addressed by restricting analyses to the longest specimens, that is, specimens included within the 0.25 higher quantile of SVL (see Brito et al., 2006 for a similar approach). Welch two-sample t-test was used for such purpose. Depending on the sample size, ANOVA or Kruskal–Wallis tests were used to address differences among evolutionary units in pholidotic and coloration meristic traits, while Fisher tests were used for such purpose in categorical data.
Univariate comparisons in topo-climatic characteristics for evolutionary units' ranges were addressed using ANOVA tests prior log-transformation of variables.
2.4 Multivariate morphological and environmental analyses
Multivariate statistical analyses were conducted on morphological traits and environmental variables that showed significant differences among evolutionary units and if a minimum of three traits/variables within each of the four datasets showed significant results. Specimens with missing data in morphological traits were not considered (see Data S1–S3).
For quantitative data (i.e., biometric, pholidotic and coloration meristic traits, and topo-climatic variables), we first ran a principal component analysis (PCA) to understand which traits/variables best explain morphological (i.e., biometric, and pholidotic and coloration meristic traits) and ecological (i.e., topo-climatic variables) variability of evolutionary units. Then, the three first PCA axes were used to: (a) represent morphological and ecological variability within each evolutionary unit, using the R package pca3d; and (b) calculate the size of the morphological and environmental variability for each evolutionary unit and the overlap between pairs of evolutionary units, using the R package hypervolume (Blonder et al., 2014). Hypervolumes were built using a Silverman bandwidth estimator, a quantile threshold of 95% that includes almost the total probability density while maximizing the overlap between units, and a set of 1000 random points to sample the kernel density. We derived the Sørensen Index (K) as a measure of overlap among variabilities of pairs of evolutionary units, as well as the overlap index (OI), as the ratio of the intersection of the hypervolumes to the size of the smallest hypervolume. Both indexes range between zero (no overlap) and one (maximum overlap). The OI is related to the Sørensen index and is particularly informative when niches have very different sizes (see Simó-Riudalbas et al., 2018). To test the significance of the overlap, a randomization procedure similar to Warren et al. (2008) was followed. The hypothesis tested was if the observed overlap is more different than the overlap obtained from the random combination of data from both units. Datasets have the same number of observations as the originals, and in each comparison, Sørensen index and OI were measured. This procedure was performed 999 times to generate the null distribution and calculate a p-value of the observed overlap. Finally, we ran a Linear Discriminant Analysis (LDA) to measure the discriminant power of using these quantitative traits/variables to characterize each unit. A confusion matrix was generated to measure the number of individuals assigned to each group and thus, obtaining a percentage of correct classification rate (CCR) for the employed discriminant function. The R package MASS was used for this purpose.
For morphological pholidotic and coloration categorical traits, correspondence analyses (CA) were run to find if the combination of studied traits support the recognition of evolutionary units. The R package candisc was used for this purpose.
3 RESULTS
3.1 Mitochondrial phylogenetic inference and delimitation of genetic units
We obtained a well-supported mtDNA phylogenetic tree (i.e., most of nodes with pp >0.95), which is in agreement with mtDNA trees published in previous works (e.g., Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012) and summarizes distinct levels of diversification since the late Miocene period (Figure 2). MtDNA sequences from four additional samples fell inside the expected lineages, with the sample collected in the Aurès Mountains of Algeria (MNCN50497; Table S1) constituting a new sublineage within the Algerian populations (Figure S4).
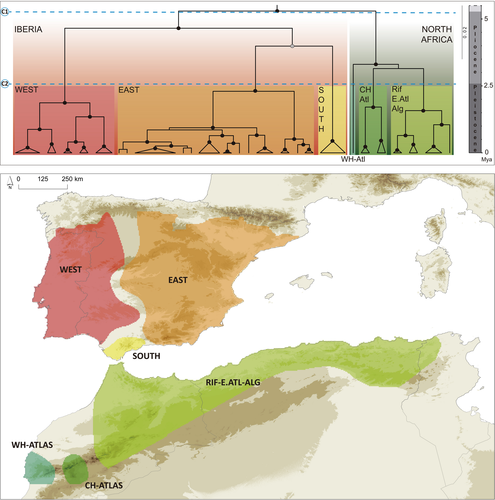
The two cutoff points established along the phylogenetic tree delimited genetic groups that arose from distinct evolutionary transitions (Figure 2): (a) the first cutoff (Miocene–Pliocene transition) delimited two main clades, one in the Iberian Peninsula and another one in North Africa; (b) the second cutoff (Pliocene—Pleistocene transition) delimited three lineages within both the Iberian (West, East, and South) and the North African clades (WH-Atlas, CH-Atlas, and Rif-E.Atl-Alg). Both clades show an allopatric distribution, while lineages are parapatrically distributed (Figure 2).
CYTB uncorrected p-distances among clades and lineages are available in Table S4.
3.2 Morphological variability
3.2.1 Biometric traits
Tail length (TAIL) was found sexually dimorphic across the two evolutionary levels; that is, males' tail was significantly longer than females' tail across clades and lineages (Table 3; Data S1).
| Dataset | Cutoff | Comparison | Sex dim | Single traits | PCA-K | % CCR |
|---|---|---|---|---|---|---|
| Biometry | c1 | Iberia – North Africa | TAIL | TAIL, HL, ML, HH, HW | 0.55 (f)–0.39 (m) | 94.03 (f)–96.51 (m) |
| c2 | Iberian lineages, WEST – EAST – SOUTH | TAIL, HW | ML | /// | /// | |
| c2 | North African lineages, WH-Atl – CH-Atl – Rif-E.Atl-Alg | TAIL | HH | /// | /// | |
| Pholidotic/coloration (meristic) | c1 | Iberia – North Africa | APIC, INTER, SUP, INFR, SUBC | APIC(f), INFR, VENT, SUBC, DORS, DMARKS | 0.3 (m)–0.5 (f) | 95.49 (m)–96.53 (f) |
| c2 | Iberian lineages, WEST – EAST – SOUTH | APIC, INTER, SUP, INFR, VENT, SUBC | APIC(m), INFR(m), PERIOC, SUBC(m), DMARKS | 0.3–0.6 (m)–/// (f) | 76.89 (m)–/// (f) | |
| c2 | North African lineages, WH-Atl – CH-Atl – Rif-E.Atl-Alg | APIC, SUBC | INTER, LOR, PERIOC, VENT, DORS, DMARKS | 0.34–0.56 | 96.15 |
Note
- Dataset, cutoff, evolutionary units compared (comparison), traits showing sexual dimorphism (sex dim), traits showing significant differences among units (single traits), Sørensen (K) index derived from a Principal Component Analyses of traits showing significant differences among units (PCA—K) and % of correct classified occurrences according to a linear discriminant function (% CCR) are depicted. Significant tests (p < 0.05) for K are marked in bold. “///” means that analyses were no conducted due to not enough traits with significant differences in the previous analysis. “f” and “m” means for females and males, respectively. See Data S1 and S2 for further details on these analyses and Table 1 for trait codes.
Considering SVL as a covariate, all biometric traits showed significant differences between Iberian and North African clades. However, only one trait showed significant differences among lineages within both the Iberian and North African clades (Table 3). Accordingly, PCA and LDA were performed at clade level only, considering females and males separately (because of sexual dimorphism in TAIL). The three first components of the PCA explained more than 92% of the total variance for both sexes, with the first axis related to all measured traits. Nevertheless, in both females and males a moderate overlap among the biometric variability of both clades was obtained (Table 3). LDA reported a high percent of CCR for both females and males (Table 3).
Considering individuals within the 0.25 higher quantile, significant differences were found across the two levels. Iberian vipers are significantly larger than North African vipers (t = 8.005, df = 16.621, p-value <0.001; Table S5). Within Iberia, vipers from the EAST lineage are also significantly larger than vipers from the WEST lineage (t = −2.552, df = 169.950, p-value = 0.012; Table S6), while within North Africa, vipers from Rif-E.Atl-Alg are significantly larger than vipers from WH-Atl (t = −3.390, df = 7.991, p-value = 0.009; Table S7) and CH-Atl lineages (t = −2.443, df = 9.301, p-value = 0.036; Table S7).
3.2.2 Pholidotic and coloration meristic traits
Across the two evolutionary levels, two to six traits were found significantly sexually dimorphic, and five to six traits showed significant differences between clades and among lineages (Table 3; Data S2).
Principal component analysis analyses, with an explained total variance ranging from 64.6% to 81.5%, showed a general pattern of moderate overlap among the morphological variability of the distinct units, with no marked differences between the two cutoff levels (Table 3). In general, body traits (e.g., SUBCAU, DORS, DMARK) are related to the morphological variability of clades, while head traits (e.g., INFRA, PERI, LOR) seem to explain the morphological variability of lineages within each clade (Data S2). LDA revealed higher CCR between clades and among North African lineages than among Iberian lineages (Table 3).
3.2.3 Pholidotic and coloration qualitative traits
The degree of fragmentation of parietal and frontal plates of the head, and the dorsal coloration of the body were significantly sexually dimorphic in both clades, and in the three Iberian lineages (Table 4; Data S3).
| Cutoff | Comparison | Sex dim | Single traits | CA—head | CA—col |
|---|---|---|---|---|---|
| c1 | Iberia — North Africa | PARIET, FRONT, DP2C | PARIET(m), FRONT, ROWS, DP1B, DP2B, DP3, TAIL col | p < 0.001 | p < 0.001 |
| c2 | Iberian lineages, WEST — EAST — SOUTH | PARIET, FRONT, DP1B, DP2C | FRONT(m), ROWS, DP1A, DP1B, DP2A, DP2B, TAIL col | p = 0.08 | p < 0.001 |
| c2 | North African lineages, WH-Atl - CH-Atl - Rif-E.Atl-Alg | // | DP1B, DP2B, DP3 | /// | p < 0.001 |
Note
- Cutoff, evolutionary units compared (comparison), traits showing sexual dimorphism (sex dim), traits showing significant differences among units (single traits), and probability for correspondence analyses performed for the head (CA—head) and coloration (CA—col) are depicted. “//” means no traits with significant results. “///” means no conducted due to not enough traits with significant differences in the previous analysis. “f” and “m” means for females and males, respectively. See Data S3 for further details on these analyses and Table 1 for trait codes.
Pholidotic and coloration traits were significantly different between Iberian and North African clades, as well as among Iberian lineages. However, only coloration traits were significantly different among North African lineages (Table 4). Canonical discriminant analyses reported significant differences when considering the head pholidosis of Iberian and North African clades, and the dorsal coloration of clades and lineages (Table 4).
3.3 Ecological niches
Nine to eleven topo-climatic variables showed significant differences across the two evolutionary levels (Table 5; Data S4). PCAs developed with these variables explained more than 85% of the topo-climatic variance in all comparisons. BIO 6, BIO 13, and BIO 14 were the variables with higher importance across the topo-climatic niches of evolutionary units.
| Cutoff | x–y | Variables | Vol x | Vol y | Intersection | K | OI | % CCR |
|---|---|---|---|---|---|---|---|---|
| c1 | Iberia — North Africa | ALT, SLOPE, BIO4, BIO6, BIO13, BIO14, BIO15, VAPOR, SOLRAD | 175.892 | 244.505 | 67.894 | 0.338 | 0.406 | 94.847 |
| c2 | Iberia, WEST – EAST | 189.774 | 105.119 | 22.204 | 0.147 | 0.214 | ||
| c2 | Iberia, WEST – SOUTH | ALT, BIO1, BIO4, BIO5, BIO6, BIO12, BIO13, BIO14, BIO15, VAPOR, SOLRAD | 190.199 | 46.457 | 22.716 | 0.201 | 0.518 | 94.002 |
| c2 | Iberia, EAST – SOUTH | 105.207 | 46.022 | 1.681 | 0.022 | 0.037 | ||
| c2 | North Africa, CH-Atl - Rif-E.Atl-Alg | ALT, SLOPE, BIO1, BIO4, BIO5, BIO6, BIO13, BIO14, BIO15, VAPOR, SOLRAD | 21.187 | 172.511 | 13.901 | 0.152 | 0.704 | 96.639 |
Note
- Cut-off, evolutionary units compared (x–y), traits showing significant differences and considered in niche overlap test (variables), volume of the first (x) and second (y) evolutionary units in comparison, intersection of both x and y volumes, and Sørensen (K) and overlap (OI) indexes, and % of correct classified occurrences according to a linear discriminant function (% CCR) are depicted. Significant tests (p < 0.05) for K and OI are marked in bold. See Data S4 for further details on these analyses and Table 2 for variable codes meaning.
The degree of volume intersection ranged from low to moderate between most pairwise comparisons. All permutation tests were significant (p < 0.05). Niche overlap as measured by the Sørensen Index (K) ranged from low to moderate, with the lowest values occurring for the comparisons East and South Iberian lineages. The OI was moderate in most cases, except between East and South Iberian lineages, where it was low, indicating very different sizes in niche volumes (Table 5). LDA revealed high CCR for most of the comparisons (>94% in all cases; Table 5).
3.4 Taxonomic implications
Considering the old and the relatively high mtDNA divergence between the Iberian and the North African clades (ca. 5.5 Mya, Figure 2; 7% of CYTB uncorrected distance, Table S4), as well as their allopatric distribution and impermeable barrier to dispersal between the two continents (Figure 2), we recognize both clades as evolutionary independent units and propose them to be recognized as different species. Although we found a moderate overlap for some topo-climatic and morphological variables between Iberian and North African species (Tables 3 and 5), vipers from these clades present marked differences in biometric, pholidotic and coloration traits (Table S5), as well as in the topo-climatic variables that characterize their ranges (Table S8). Indeed, LDA analyses for both morphologic and topo-climatic traits allow a correct classification of ca. 95% of measured animals and occurrences.
The main lineages that compose the Iberian (i.e., West, East, and South) and North African (i.e., WH-Atl, CH-Atl, and Rif-E.Atl-Alg) clades also present a rather old divergence (ca. 4.5–3 Mya; Figure 2) and a considerable genetic distance (4.7%–6.4% of CYTB uncorrected distance within Iberian and North African clades, respectively; Table S4). These lineages show differences in body size, and some pholidotic and coloration traits (Tables S6 and S7), as well as in topo-climatic variables that characterize their ranges (Tables S9 and S10). Pholidotic and coloration traits highly overlap among Iberian lineages. In contrast, these traits plus body size allow the recognition of two major morphotypes in North African lineages (Tables 3 and 4): a “dwarf” morphotype (i.e., small body size, 19 dorsal rows, and barred dorsal pattern) characteristic of WH-Atl and CH-Atl lineages; and another “regular-sized” morphotype (i.e., larger body size, 21 or 23 dorsal rows, and non-barred dorsal pattern), which is the most frequent in the Rif-E.Atl-Alg lineage (91.4% of measured animals). Furthermore, topo-climatic niches marginally overlap and so there is a high level of correct classification for both Iberian and North African lineages (94% and 96%, respectively; Table 5). Geographic isolation among lineages is probably incomplete across some mountain chains for both Iberian and North African taxa (Figure 2, Figure S5). Within Iberia, West and East lineages present continuous populations across the Iberian Central System and Sierra Morena, while the South lineage is apparently isolated from the West lineage by the Guadalquivir river basin but not from the East lineage since adjacent populations of both lineages occur across the central Penibaetic System, with no apparent topographic barriers (Figure 2, Figure S5). Despite an apparent geographic isolation of the three North African lineages, no evident topographic barriers exist among them (Figure 2, Figure S5). Based on these evidences, we propose each of these lineages as different subspecies within corresponding species.
3.5 Taxonomic update
Following the law of priority of the International Commission on Zoological Nomenclature, V. latastei–monticola populations within the Iberian Peninsula should be considered as V. latastei Boscá, 1878, whereas populations in North Africa must be now recognized as V. monticola Saint Girons, 1953 since the epithet “monticola” prevails as the oldest given name to North African populations (Figure 3). Detailed information on the variability and differences for morphological traits and ecological characteristics of each species occurrence are available in Tables S5 and S8. Below, we detail a taxonomic update based on subspecies, given that previous taxonomy contemplated the recognition of several subspecies within this species complex.
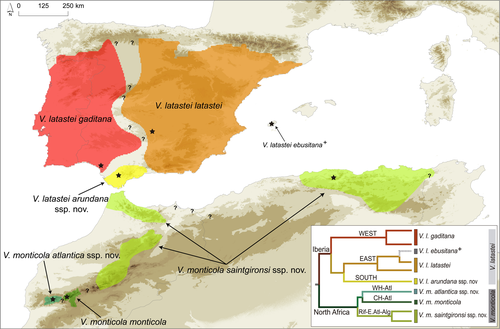
Vipera latastei Boscá, 1878—species restricted to the Iberian Peninsula and composed of the following three extant subspecies (Figure 3):
(1) Vipera latastei latastei Boscá, 1878
Types
The species (and therefore the nominal subspecies) was originally described by E. Boscá using specimens from the surroundings of Burgos, Valencia del Cid (= Valencia), and Ciudad Real (Spain). Due to lack of a holotype designated by Boscá (1878), Saint Girons (1977) designated as lectotype the specimen BMNH 1946.1.21.5 (formerly BMNH 1920.1.20.252), deposited in the Natural History Museum of London (UK), which was sent by Boscá to Lataste in 1878 and constitutes the only known specimen from the series used to describe the species (Saint Girons, 1977). However, previously to Saint Girons (1977), Grün in 1931 and 1932 collected in Mosqueruela (Teruel, Spain) the specimens ZSM 1809/1–2 (subadult male and female), ZSM 3201/1–2 (two adult males), and ZSM 4722 (adult female), deposited in the Bavarian State Collection of Zoology (Germany), which were designated as syntypes at the time. After Saint Girons designation of the lectotype in 1977, these specimens collected by Grün should be therefore considered as paralectotypes.
Terra typica
The specimen BMNH 1946.1.21.5 was collected in Ciudad Real (Spain) by E. Boscá.
Diagnosis
In relation to the other Iberian Lataste's viper subspecies, specimens of V. l. latastei reach in average larger sizes (SVL), present lower number of periocular scales and higher number of subcaudal scales (both in females and males), and are characterized by the predominance of angular zigzag dorsal patterns with higher number of dorsal marks (see Figure 4a–e; Table S6).

Etymology
E. Boscá named V. latastei after the French naturalist M. Fernand Lataste; H. Saint Girons (1977) applied this epithet to the subsequent subspecies.
Distribution
According to our results, the nominal subspecies V. l. latastei is now allocated to the populations of the East lineage, which is restricted to the eastern half of Spain, and therefore, northwestern Iberian populations are excluded from this subspecies (Figure 3).
Topo-climatic niche
This subspecies occurs from sea level to 2800 m of elevation (in Sierra Nevada, Granada), although most of the occurrences are between 500 and 1500 m of elevation (Table S9). This region is characterized by a marked temperature seasonality, with very warm summers and cold winters, and low levels of annual precipitation (95% of occurrences in areas of <790 mm).
Habitat description
Habitat varies from high elevation rocky areas (e.g., in Sierra Nevada, Granada), agriculture cereal crops (e.g., in La Rioja Alta and Burgos), Mediterranean scrublands, pastures, and cleared oak (e.g., in southern Iberian System, Soria; in Sierra Morena, Jaén) or pine forest (e.g., in Serranía de Cuenca, Cuenca; in Garraf Mountains, Barcelona), and littoral and arid scrublands (e.g., in Cabo de Gata, Almería).
(2) Vipera latastei gaditana Saint Girons, 1977
Types
The specimen MHNG 1352.99 (male), collected by H. Saint Girons and deposited in the Natural History Museum of Geneva (Switzerland) was designated as holotype. No paratypes were designated.
Terra typica
The specimen MHNG 1352.99 was collected in Coto de Doñana (Huelva, Spain) and the subspecies was described by Saint Girons (1977) for the populations of the south-western quarter of Iberia, as well as Rif and Middle Atlas in Morocco and Tellian Atlas in Algeria (Figure 1).
Diagnosis
In relation to the other Iberian subspecies, individuals of V. l. gaditana reach in average smaller sizes (SVL), present intermediate number of periocular scales and number of subcaudal scales (both in females and males), and are characterized by the predominance of rounded zigzag dorsal patterns with low number of dorsal marks (see Figure 4f–i; Table S6).
Etymology
The adjective “gaditana” comes from the Latin name Gades (=Cadiz) making reference to the inhabitants of the southernmost region of Spain within the Iberian Peninsula, in the eastern bank of the Guadalquivir river mouth (i.e., Saint Girons confounded the toponymy). Although this region is now excluded from the range of this subspecies, where a newer subspecies is described (see below), “gaditana” has prevalence to design this subspecies since the holotype was assigned to the specimen MHNG 1352.99 collected in Coto de Doñana (Huelva, Spain).
Distribution
According to our results, V. l. gaditana must be restricted to the populations of the West lineage (Figure 2), and therefore, the range of this subspecies includes the whole Portugal and western Spain, while excludes populations of the South lineage in Iberia (here described as another subspecies) and those from North Africa (see Figure 3).
Topo-climatic niche
This subspecies occurs from sea level to 2100 m of elevation (in the Central System, Spain), although most of the occurrences are between 500 and 1100 m of elevation (Table S9). This region is characterized by low temperature seasonality, since summers and winters are not very drastic, and high levels of annual precipitation (up to 2200 in P.N. Peneda-Gerês, northern Portugal) and moderate precipitation seasonality.
Habitat description
Habitat includes open forest of Quercus sp. with Mediterranean scrubs and rocks (e.g., in Peneda-Gerês N. P., northern Portugal; in the Western Central System, Spain), scrublands and grasslands with abundant rocks (e.g., in Ourense and Zamora, Spain), particularly in hilly and mountain depopulated areas, and coastal sandy areas (e.g., Doñana National Park, Huelva, Spain).
(3) Vipera latastei arundana subsp. nov. Martínez-Freiría, Velo-Antón, Santos & Pleguezuelos.
Zoobank registration
LSID: http://zoobank.org/NomenclaturalActs/cbda977e-f8b9-45d7-85c0-96a0ef505976
Holotype
14VL018 (adult, male), collected by F. Jiménez-Cazalla in 2013, deposited in the Estación Biológica Doñana—CSIC (Sevilla, Spain) with code EBD 38308H. For specimen description, and sequence and tissue availability, see Appendix 1.
Paratypes
MNCN 15921 (adult, male), collected by an unknown collector in 1980, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain), MNCN 38917 (adult, female) from Yunquera, Málaga (Spain) collected by F. Palacios in 1981, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Spain), and EBD 23705H (adult, male) from Cortes de la Frontera—El Colmenar, Málaga (Spain) collected by J. Braza and I. Varela in 1987, deposited in Estación Biológica de Doñana—CSIC (Spain). For specimen descriptions, see Appendix 1.
Terra typica
The specimen 14VL018 was collected in La Jarda, Montes Propios de Jerez de la Frontera, Cádiz (Spain).
Diagnosis
In relation to the other Iberian subspecies, vipers of V. l. arundana reach in average intermediate size (SVL), present higher number of periocular scales and lower number of subcaudal scales (both in females and males), and present both wide angular and rounded zigzag dorsal patterns with intermediate number of dorsal marks (see Figure 4j,k; Table S6).
Etymology
The adjective “arundana” is the demonym of the inhabitants of Arunda (=Ronda, Málaga province; designation used by the Romans in the antiquity), locality in the central range of this subspecies.
Distribution
This new subspecies corresponds to populations of the South lineage in Iberia, which is restricted to the southernmost region of Spain, including Cádiz and Málaga provinces. It occupies Serranía de Ronda and surrounding areas, being limited to the west and north by the Guadalquivir river basin and reaching through Torcal Mountain in the central part of the Málaga province (Western Penibaetic System) (Figure 3). This subspecies is therefore endemic to Spain.
Topo-climatic niche
This subspecies occurs from sea level to 1800 m of elevation (Table S9). Subspecies distributional range is characterized by low temperature seasonality, with both warm summers and winters, moderate to high levels of annual precipitation (from 600 mm in Málaga to 1800 mm in Grazalema Mountains) and high precipitation seasonality.
Habitat description
Habitat varies from open Quercus forests with sandy and rocky substrate (e.g., in Alcornocales Natural Park, Cádiz) to scrublands and grasslands of mountain areas (in the Serranía de Ronda and surrounding small mountain ranges, Cádiz and Málaga provinces).
Vipera monticola Saint Girons, 1953, restricted to North Africa and composed of the following three subspecies (Figure 3):
(1) Vipera monticola monticola Saint Girons, 1953
Types
Seven specimens from the Toubkal Massif in the Central High Atlas (Morocco) were collected by H. Saint Girons and designated as syntypes: MNHN 8959 (adult, male), MNHN 2012.0433 (formerly MNHN-RA 8959A; adult, male), MNHN 2012.0434 (formerly MNHN-RA 8959B; adult, female), MNHN 8410 (juvenile, female), MNHN 2012.0412 (formerly MNHN-RA 8410A; adult, male), MNHN 1973.0079-0080 (two adult males) [deposited in Muséum national d'Histoire naturelle—Paris (France)] and ZMA.RENA 11154 [deposited in the Naturalis Biodiversity Centre—Leiden (The Netherlands)].
Terra typica
Toubkal Massif in the Central High Atlas, region of Marrakesh-Safi, Morocco. The nominal subspecies was originally described as a subspecies of V. latastei by Saint Girons (1953) and posteriorly elevated to species range by Beerli et al. (1986).
Diagnosis
In relation to the other North African Mountain viper subspecies, V. m. monticola presents small size (but larger than the specimens from Western High Atlas), low number of intercanthal + intrasupraocular, loreal and periocular scales, moderate number of ventral scales, 19 dorsal rows (a characteristic also shared with vipers from other lineages), high number of dorsal marks and frequently, a barred dorsal pattern (a characteristic also shared with specimens from the Western High Atlas). Only dwarf specimens are known (see Table S7). See Figure 5a–c.

Etymology
The species and subspecies name, “monticola,” is Latin for “inhabitant of the mountains” (Latin “colere” =inhabiting).
Distribution
According to our results, V. m. monticola corresponds to the populations of the CH-Atl lineage, and thus, it is restricted to the central High Atlas Mountains, including Toubkal, Oukaimeden, Tircht, and Sirwa Mountains (Marrakesh-Safi, Souss-Massa and Drâa-Tafilalet regions of Morocco); this subspecies is therefore endemic to Morocco (Figure 3).
Topo-climatic niche
This subspecies occurs in moderate-steep slope areas, from 1500 to 3500 m of elevation (Table S10), although most of the observations gathered by the authors during the last 15 years are located above 2600 m. Its distributional range is characterized by low mean annual temperatures and a marked temperature seasonality, with low precipitation in both dry and wet seasons and high levels of incident solar radiation.
Habitat description
Habitat is composed of high elevation xeric thorny scrubs and grasslands in rocky areas.
(2) Vipera monticola atlantica subsp. nov. Martínez-Freiría, Lucchini & Freitas
Zoobank registration
LSID: http://zoobank.org/NomenclaturalActs/11a2c689-6612-4108-8d56-d9d88f946709
Holotype
Although no specimens were sacrificed and deposited in a collection, we designate as holotype the specimen 15VM073 (male, adult), sampled in the Nfis meadows of the Tichka Plateau (Morocco) by F. Martínez-Freiría, A. Pimenta, and I. Freitas in 2015. For specimen description, and sequences and tissue availability, see Appendix 2.
Paratypes
We designate the following three paratypes, all of them sampled in the Nfis meadows of the Tichka Plateau in Morocco: 15VM074 (adult, female) sampled by F. Martínez-Freiría, A. Pinto, and I. Freitas in 2015; and 19VM053 (adult, female) and 19VM059 (adult, male) sampled by F. Martínez-Freiría, I. Avella, F. Corga, U. Enríquez-Urzelai, and N. Lucchini in 2019. For specimens description, and sequences and tissue availability, see Appendix 2.
Terra typica
The holotype and paratypes were sampled in the Nfis meadows of the Tichka Plateau (region of Marrakesh-Safi, Morocco).
Diagnosis
In relation to the other North African Mountain viper subspecies, Vipera m. atlantica presents the smallest size, the highest number of intercanthal + intrasupraocular and ventral scales, moderate number of loreal and periocular scales, 19 dorsal rows (a characteristic shared with V. m. monticola and few specimens from the Rif-E.Atl-Alg lineage), moderate number of dorsal marks and frequently, a barred dorsal pattern (a characteristic also shared with V. m. monticola). Only dwarf specimens are known, resembling V. m. monticola but with previous mentioned differences (see Figure 5d,e; Table S7).
Etymology
The adjective “atlantica” refers to the location of this subspecies range, in the westernmost distribution range of the species, close to the Atlantic Ocean (at about 120 km in a straight line).
Distribution
This new subspecies corresponds to the single known population of the WH-Atl lineage, which is located in the Nfis meadows of the Tichka Plateau (Marrakesh-Safi region), in the Western High Atlas Mountains; it is therefore endemic to Morocco (Figure 3).
Topo-climatic niche
Only one population is known, occurring at 2750 m a.s.l. in climatic conditions, which are perfectly included within the range of V. m. monticola (Table S10). Therefore, the known distributional range (limited to 3 km2) is characterized by low mean annual temperature (8ºC) and moderate-high temperature seasonality, with low precipitation in dry (7 mm) and wet seasons (70 mm), and high levels of incident solar radiation.
Habitat description
Habitat is composed of high elevation xeric thorny scrubs in rocky areas, nearby meadows (see Appendix 2).
(3) Vipera monticola saintgironsi subsp. nov. Martínez-Freiría, Fahd, Larbes & Brito
Zoobank registration
LSID: http://zoobank.org/NomenclaturalActs/cfdd9eb2-157b-44e2-b397-e1c20660c967
Holotype
We designate as holotype the specimen MNCN 50498 (adult, female) collected in Djurjurá Mts (Algeria) by S. Larbes in 2010, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain). For specimen description, and sequences and tissue availability, see Appendix 3.
Paratypes
We designate the following three paratypes: BEV.11978 (juvenile) collected in Tislit lake, Imilchil (region Draa-Tafilalet, Morocco) by P. Geniez and A. Miralles in 2012, deposited in the Centre d'Ecologie Fonctionnelle & Evolutive (Montpellier, France); BMNH 1894.3.22.5 (adult, male) collected near Tangier (Morocco) by an unknown collector in 1894, deposited in the Natural History Museum (London, UK); and MNCN 50497 (adult, male), collected in Aurès Mts, Batna (Algeria) by S. Larbes in 2018, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain). For specimens description, and sequences and tissue availability, see Appendix 3.
Terra typica
The holotype MNCN 50498 was collected Djurjurá Mts (Algeria).
Diagnosis
In relation to the other North African Mountain viper subspecies, V. m. saintgironsi presents larger size, moderate number of intercanthal + intrasupraocular scales, high number of loreal and periocular scales, low number of ventral scales, 19 to 23, frequently 21 dorsal rows at midbody, low number of dorsal marks, and variable dorsal pattern (including the three general types; see Figure 5f,j; Table S7). Specimens with dwarf characteristics (i.e., small size and 19 dorsal rows) are known for the Eastern High Atlas Mountains in Morocco (Figure 5H) and the Aurès Mountains in Algeria (i.e., paratype MNCN 50498, Appendix 3).
Etymology
The adjective “saintgironsi” refers to Hubert Saint Girons, French herpetologist who extensively worked in biogeography, ecology, and taxonomy of European vipers, also describing V. monticola.
Distribution
This new subspecies is designated for the populations of the Rif-E.Atl-Alg lineage, and thus, its distribution includes the Eastern High Atlas, Middle Atlas, and the Rif Mountains in Morocco, and the Tellian Atlas and Aurès Mountains in Algeria. Populations in between Rif and Tellian Atlas (e.g., Saidia, Morocco; Tlemcen, Algeria), as well as from Tunisia are believed to pertain to this subspecies, although no information about genetic variability is available (Figure 3).
Topo-climatic niche
This subspecies presents the widest distribution in North Africa, occurring across Morocco, Algeria, and probably Tunisia, and consequently a wide ecological niche. Altitudinal range goes from sea level to 3400 (in the Eastern High Atlas, Morocco; Table S10), although coastal populations are believed to be currently extinct (Brito et al., 2011). Annual mean temperature varies from 3 to 19°C, precipitation occurs in both winter (profusely) and summer, and solar radiation ranges from moderate to high.
Habitat description
There are populations in areas dominated by evergreen (Cedrus atlantica, Abies maroccana) and deciduous (Quercus sp.) forest (e.g., in the Rif and Middle Atlas Mountains, Morocco; in Jebel Chélia, Algeria), as well as scrubs and herbaceous vegetation (e.g., in the Middle and Eastern High Atlas, Morocco), usually with abundant rocky substrate. Costal populations inhabiting the surroundings of sand dunes (e.g., Saidia, Morocco) are likely extinct.
4 DISCUSSION
In this work, we use an integrative taxonomic approach, bringing together information derived from mtDNA, morphological and ecological inferences, to update the taxonomy of the V. latastei–monticola complex. Following the unified species concept (de Queiroz, 2007) and an updated subspecies concept (de Queiroz, 2020), we propose the recognition of two species, represented by the vicariant Iberian and North African clades that diverged in the late Miocene, each one composed of three subspecies, corresponding to lineages that diverged during the Pliocene. Our proposal is largely supported by the mtDNA phylogenetic relationships and the distribution patterns of evolutionary units (Martínez-Freiría, Freitas, et al., 2020; Velo-Antón et al., 2012) and, to a smaller extent, by our morphological and ecological inferences, which must be considered as “operational criteria” to support our taxonomic decisions (de Queiroz, 2007, 2020). The newly proposed taxonomy will have important outcomes for the conservation status of these taxa, since it completely changes the current taxonomic scenario resulting from past morphological assessments (e.g., Saint Girons, 1977).
4.1 An evolutionary-based taxonomic update
Paleo-tectonic events, such as the Messinian Salinity Crisis of the Miocene (6–5.33 Mya; Duggen et al., 2003), have led to the formation of vicariant taxa in low dispersal vertebrates at both sides of the Mediterranean (e.g., salamanders, Rodríguez et al., 2017; frogs, Dufresnes et al., 2020; lizards, Paulo et al., 2008). Yet, Pleistocene oversea dispersal events across the Strait of Gibraltar likely occurred for many vertebrates, including some reptiles (e.g., freshwater turtles, Velo-Antón et al., 2015; Veríssimo et al., 2016; lizards, Mendes et al., 2017; snakes, Carranza et al., 2006). Our results point to an effective barrier of the Mediterranean Sea separating Iberian and North African viper populations since the separation of European and African continents. This resulted in a level of mtDNA divergence high enough to consider them as distinct species (see Freitas et al., 2020 and references therein), and in line with similar mtDNA divergence estimates found between vicariant species occurring at both sides of Strait of Gibraltar. Indeed, European vipers are sedentary, low dispersal animals (Brito, 2003; Martínez-Freiría et al., 2010), which hardly would be able to cross the Mediterranean Sea by rafting (but see Torres-Roig et al., 2021). Following the unified species concept (de Queiroz, 2007), viper populations belonging to Iberian and North African clades represent separately evolving units and consequently, must be recognized as different species. Morphological differentiation between these species also occurs in the three types of traits here studied (i.e., biometric, pholidotic and coloration), thus providing another line of evidence to support our proposed taxonomic update.
Genetic divergence among lineages is also high within each taxa. These lineages diverged during the Pliocene, likely due to geographic restriction mediated by the response of viper populations to climatic oscillations and topographic barriers (Martínez-Freiría, Freitas, et al., 2020). These lineages were previously suggested as candidate species in a recent taxonomic revision (Freitas et al., 2020), and fall within the Plio-Pleistocene diversification that resulted in the description of incipient species within both the Iberian Peninsula (e.g., Pelodytes frogs, Dufresnes et al., 2020; Lissotriton newts, Sequeira et al., 2020; Psammodromus lizards, Fitze et al., 2012) and North Africa (e.g., Hyla frogs, Dufresnes et al., 2019). We also described variable levels of phenotypic distinctiveness among lineages, with Iberian lineages showing moderate morphological differentiation and low-moderate overlap in topo-climatic niches, while North African lineages display high morphological differentiation and high overlap in topo-climatic niches. However, some of these lineages (i.e., subspecies) likely meet in secondary contact zones across Iberia (e.g., V. l. gaditana– V. l.latastei) and North Africa (across central-western Atlas Mountains; Figure 3). Consequently, these phenotypically different lineages (either in morphological traits or in topo-climatic niches) might not have achieved complete reproductive isolation and thus, fit in the subspecies concept as proposed by Mayr (1969) and later updated by de Queiroz (2020).
4.2 Sources of phenotypic variation
Phenotypic differentiation can result from selective forces such as natural selection, which can run in parallel to allopatric speciation, and favor local adaptation (Zamudio et al., 2016). At the same time, micro-evolutionary processes such as gene flow can act in an opposite way, leading to a homogenization of phenotypic differences among taxa when populations meet in secondary contact (e.g., Watson et al., 2019). Considering these factors, it should be expected a higher phenotypic differentiation at the species level than at the subspecies level. This expectation is fulfilled for morphological variability, but not when topo-climatic variability characterizing taxonomic units' ranges is considered. Our results on topo-climatic variability of vipers largely agree with Martínez-Freiría, Freitas, et al. (2020), while extending the multivariate niche analyses to other variables and additional occurrences; diversification of V. latastei and V. monticola was largely absent from climatic adaptation, as topo-climatic niches were mostly conserved across the two evolutionary levels addressed in our study.
Regarding morphological variability, marked differences occurred in biometric, scalation and dorsal coloration traits, particularly between species and among V. monticola subspecies. Several studies addressing morphological variability in Vipera species highlighted the adaptive and plastic character of several morphological traits, as they were found varying in accordance to environmental gradients (e.g., Martínez-Freiría & Brito, 2013; Martínez-Freiría et al., 2009; Martínez-Freiría, Toyama, et al., 2020; Santos et al., 2014). As in previous studies using ecological modeling tools (Brito et al., 2008; Freitas et al., 2018; Santos et al., 2006), we found that V. monticola occurs across a narrower topo-climatic niche than V. latastei (Data S4), which makes the former more sensitive to extreme and stochastic changes than the later. Topo-climatic restrictions experienced by V. monticola in North Africa, therefore, emerge as potential selective forces responsible for morphological differentiation at both specific and subspecific levels.
Prey availability and consumption could also play a role in the patterns of morphological distinctiveness between species and within V. monticola. Studies on diet conducted on the Iberian V. latastei (Santos et al., 2008) and other Vipera species (e.g., in Vipera berus, Forsman, 1991) have reported that vipers size is correlated with prey size and that prey availability plays a role in the biometric differentiation of populations. Some animal communities, such as small mammals, drastically differ between the Iberian Peninsula and North Africa, as well as across each region (Wilson & Reeder, 2005). Microtus and Apodemus are two of the most common genera of small mammals in Iberia, and also the most consumed, largest prey in V. latastei (Santos et al., 2008). However, the first genus does not exist across the distributional range of V. monticola and only the species Apodemus sylvaticus occurs in North Africa, approximately limited to the V. m. saintgironsi ssp. nov. range (Wilson & Reeder, 2005). It is therefore plausible that morphological differences in biometric traits across these taxa could be related to the consumption of distinct species, acting as a main ecological driver. Although the diet of V. monticola is known from anecdotic records only (mostly limited to High Atlas populations), prey preference for lizards together with isolation in montane environments were suggested as main factors driving dwarfism in High Atlas populations (Freitas et al., 2018).
Intraspecific gene flow could be another factor explaining the low morphological distinctiveness among subspecies, particularly within V. latastei, where morphological differentiation among subspecies is reduced and is mostly limited to V. l. gaditana and V. l. latastei (as similarly recovered in previous works, Brito et al., 2008; Santos et al., 2014). Bidirectional gene flow between lineages would favor morphological homogenization and thus obscure the patterns of morphological distinctiveness of each subspecies. Several contact zones are currently noticeable (Figure 3 and Figure S5) and could have been more pronounced or extended in the past, when viper populations were supposed to be less restricted to mountain ranges (Santos et al., 2006). Vipera latastei hybridizes with its phylogenetically related species V. aspis in northern Iberia contact zones, leading to individuals with intermediate morphological traits (Martínez-Freiría et al., 2006, 2009; Tarroso et al., 2014). Thus, gene flow across contact zones likely has a high and extensive impact on the morphological variability of Iberian and North African subspecies, reducing morphological differences among them.
4.3 Conservation implications and future research
Current global (i.e., IUCN Red List; Miras et al., 2006; Miras et al., 2009) and regional assessments (e.g., Brito, 2008; Martínez-Freiría et al., 2014; Pleguezuelos et al., 2010) list V. latastei as Vulnerable (VU) and V. monticola as Near Threatened (NT). Our proposed taxonomic scenario entails new distributional range delimitations for the species (e.g., V. latastei becomes endemic to the Iberian Peninsula, while V. monticola is now widespread but with isolated populations in North Africa). Furthermore, it proposes new intraspecific units within them (e.g., V. l. arundana ssp. nov. and V. m. atlantica ssp. nov. restricted to 54 and 1 UTM 10x10 km squares, respectively). Consequently, the global and regional conservation contexts of V. latastei and V. monticola will drastically change. Anthropogenic impacts such as habitat loss, climate change or intentional killing due to aversion are negatively affecting populations of V. latastei and V. monticola, which are mostly isolated in mountain ranges (Brito et al., 2011; Freitas et al., 2018; Martínez-Freiría et al., 2013; Santos et al., 2006). In the light of these threats, we advocate for a more detailed management unit than the subspecies here proposed that considers genetic structure and diversity, as well as geographic isolation of populations. The concept of Evolutionary Significant Units (ESU; Moritz, 1994; Ryder, 1986) has been designed for such purposes and might be relevant for these taxa.
Our taxonomic scenario reckons on an extensive sampling covering most of known populations (particularly for the Iberian Peninsula) and in the evolutionary signal recovered with mtDNA inferences. Although nuclear DNA genes, commonly used in other taxa, have been identified as uninformative at both interspecific and intraspecific level in Eurasian vipers (Freitas et al., 2020), it is important to acknowledge that further evolutionary, taxonomic and conservation inferences should be considered in the light of nuclear data. Genomic approaches, accounting for many loci, are expected to unveil further evolutionary relationships within both species (e.g., Dinis et al., 2019), while microsatellite data could address reproductive isolation among populations or lineages (e.g., Dufresnes et al., 2020), as well as recover patterns of genetic diversity required to infer ESUs (e.g., Hutama et al., 2017). These approaches will be particularly relevant for V. latastei, as sampling in Iberian Peninsula is very detailed and there is comprehensive information already gathered on distinct ecological aspects of populations (e.g., Brito, 2003; Martínez-Freiría et al., 2010; Santos et al., 2008). However, V. monticola requires additional fieldwork campaigns to increase available data from populations, and better delimit distributional ranges of evolutionary/taxonomic units and contact zones. Information on this species is quite limited, and it is essential to improve life history and ecological knowledge on populations to support further evolutionary and taxonomic inferences, as well as conservation decisions.
ACKNOWLEDGEMENTS
Authors want to acknowledge to all friends and collaborators who provided help during fieldwork and/or provided samples, road-killed animals or pictures, in particular to J. Álvarez, I. Avella, E. Benmokhtar, W. Beukema, I. Bouam, M. Caetano, F. Corga, U. Enríquez-Urzelai, M. Feriche, L. García-Cardenete, P. Geniez, G. Giner, L. Hadji, F. Jiménez-Cazalla, G. Martínez del Marmol, M. Meijide, X. Pardavila, A. Pimenta, E. Ramírez, B. Rebollo, R. Vázquez, and O. Zuazo, as well to curators and staff from the visited museum collections. Morphological measurements in live specimens were carried out in accordance with the current ethics and regulation on the use of animals for scientific research (EU Directive 2010/63/EU), with permits from the national or regional environmental authorities where the fieldwork was developed (e.g., for Portugal: 558/2016/CAPT, 858/2018/CAPT, 14150/2019/DRNCN/DGEFF; for Spain: A/2017/021, A/2018/022, A/2019/028, EP/CyL/31/2017, EP/CyL/56/2018, EP/CyL/27/2019; for Morocco: HCEFLCD/DLCDPN/DPRN/CFF N°19/2015, 35/2018). This work was supported by SYNTHESYS projects of the EU (ref. ES-TAF-5874, FR-TAF-6626, GB-TAF-5886). FM-F, IF, GV-A, NL, and JCB are supported by FCT (ref. DL57/2016/CP1440/CT0010, SFRH/BD/148514/2019, IF/01425/2014, SFRH/BD/06609/2020 and CEECINST/00014/2018/CP1512/CT0001, respectively).
DATA AVAILABILITY STATEMENT
The morphological and occurrence data that support the findings of this study are available on request from the corresponding author, while DNA sequences are available on GenBank (see Table S1.1 for sequences accession numbers).
APPENDIX 1
Holotype and paratypes of Vipera latastei arundana ssp. nov.
Description of the holotype
14VL018—from Finca La Jarda, Montes Propios de Jerez de la Frontera, Cádiz (Spain), collected by Francisco Jiménez Cazalla in 2013, deposited in the Estación Biológica Doñana—CSIC (Sevilla, Spain) with code EBD 38308H. CYTB sequence available in GenBank (accession number MT193742) and tissue sample deposited in the tissue bank from Estación Biológica Doñana—CSIC (Sevilla, Spain).
Description of the paratypes
MNCN 15921—from Sierra de Grazalema, Cádiz (Spain), deposited in the MNCN (Madrid, Spain) collected by an unknown collector in 1980.
MNCN 38917—from Junquera, Málaga (Spain) collected by Fernando Palacios in 1981.
EBD 23705H—from Cortez de la Frontera—El Colmenar, Málaga (Spain) collected by J. Braza and I. Varela in 1987.
APPENDIX 2
Holotype and paratypes of Vipera monticola atlantica ssp nov.
Description of the holotype
15VM073—sampled in the Nfis meadows of the Tichka Plateau (Morocco) by Fernando Martínez-Freiría, António Pimenta, and Inês Freitas in 2015. No sacrificed and therefore not available in collections. CYTB, ND2, and ND4 sequences available on GenBank (accession numbers MG875539, MT157192, and MG875559). DNA deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain) with code MNCN-ADN 119034.
Description of the paratypes
15VM074—sampled in the Nfis meadows of the Tichka Plateau (Morocco) by Fernando Martínez-Freiría, António Pimenta and Inês Freitas in 2015. No sacrificed and therefore not available in collections. CYTB and ND4 sequences available on GenBank (accession numbers MG875540 and MG875560). DNA deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain) with code MNCN-ADN 119035.
19VM053—sampled in the Nfis meadows of the Tichka Plateau (Morocco) by Fernando Martínez-Freiría, Ignazio Avella, Frederico Corga, Urtzi Enríquez-Urzelai and Nahla Lucchini in 2019. No sacrificed and therefore not available in collections. DNA deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain) with code MNCN-ADN 119036.
19VM059—sampled in the Nfis meadows of the Tichka Plateau (Morocco) by Fernando Martínez-Freiría, Ignazio Avella, Frederico Corga, Urtzi Enríquez-Urzelai and Nahla Lucchini in 2019. No sacrificed and therefore not available in collections. DNA deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain) with code MNCN-ADN 119037.
Pictures of the habitat
APPENDIX 3
Holotype and paratypes of Vipera monticola saintgironsi ssp. nov.
Description of the holotype
MNCN50498—collected in Djurjurá Mts (Algeria) by Said Larbes in 2010, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain). CYTB and ND4 sequences available on GenBank (accession numbers MZ712101 and MZ712105).
Description of the paratypes
BEV.11978—collected in Tislit lake, Imilchil (Midelt, Morocco) by Philippe Geniez and Aurélien Miralles in 2012, deposited in the Centre d'Ecologie Fonctionnelle & Evolutive (Montpellier, France). CYTB and ND4 sequences available on GenBank (accession numbers MG875542 and MG875556).
BMNH 1894.3.22.5—collected near Tangier (Morocco) by an unknown collector in 1894, the Natural History Museum (London, UK).
MNCN 50497—collected in Aurès Mts, Batna (Algeria) by Said Larbes in 2018, deposited in the Museo Nacional de Ciencias Naturales—CSIC (Madrid, Spain). CYTB and ND4 sequences available on GenBank (accession numbers MZ712102 and MZ712106).