Integrative systematics of Neotropical porcupines of Coendou prehensilis complex (Rodentia: Erethizontidae)
Contributing authors: Anderson Feijó ([email protected]), Hugo Fernandes-Ferreira ([email protected]), Itayguara Ribeiro da Costa ([email protected])
Zoobank link: LSID: http://zoobank.org/References/59456ada-15ec-4f4e-a185-ad9cee33cc6e
Online ISSN: 1439-0469
Abstract
enCoendou comprises the most speciose genus in Erethizontidae, with 15 currently recognized species. Although several taxonomic studies in the last two decades have unveiled part of its diversity, the most widespread Neotropical taxon Coendou prehensilis has received limited attention. Here, we combined morphological and molecular datasets to infer the phylogenetic relationships of the species in the genus and revise the taxonomy of the C. prehensilis complex. We found four morphotypes and three well-supported monophyletic clades within C. prehensilis. These three clades represent valid species: C. prehensilis (restricted to the north of the Atlantic Forest), C. baturitensis (occurring in the eastern Amazonian and montane forests enclaves in the Caatinga), and C. longicaudatus (two subspecies, C. l. longicaudatus from the Amazon and C. l. boliviensis from Cerrado and Chaco). Furthermore, we recovered three morphologically diagnosable clades within Coendou, for which we assigned subgeneric names. Coendou (Coendou) comprises six species (C. baturitensis, C. longicaudatus, C. mexicanus, C. prehensilis, C. quichua, and C. rufescens), Coendou (Sphiggurus) includes five taxa (C. bicolor, C. insidiosus, C. nycthemera, C. speratus, and C. spinosus), and the third subgenus we named Coendou (Caaporamys) subgen. nov, comprising C. melanurus (type species), C. vestitus, C. pruinosus, C. ichillus, and C. roosmalenorum.
Resumo
ptCoendou é o gênero de Erethizontidae mais especioso, com 15 espécies reconhecidas. Nas últimas duas décadas, diversos estudos taxonômicos revelaram parte da sua diversidade. Todavia, a espécie neotropical com maior distribuição, Coendou prehensilis, recebeu pouca atenção. Aqui, nós combinamos bancos de dados morfológicos e moleculares para inferir relações filogenéticas das espécies dentro do gênero para avaliar o estado taxonômico do complexo C. prehensilis. Nós encontramos quatro morfótipos e três clados monofiléticos bem-suportados dentro de C. prehensilis. Estes três clados representam espécies válidas: C. prehensilis (restrito ao norte da Mata Atlântica), C. baturitensis (ocorrendo no leste Amazônico até áreas florestadas de altitude da Caatinga) e C. longicaudatus (com duas subespécies, C. l. longicaudatus da Amazônia e C. l. boliviensis do Cerrado e Chaco). Além disso, nós reconhecemos três clados morfologicamente diagnosticáveis dentro de Coendou, os quais nós atribuímos nomes subgenéricos. Coendou (Coendou) com seis espécies (C. baturitensis, C. longicaudatus, C. mexicanus. C. prehensilis, C. quichua e C. rufescens), Coendou (Sphiggurus) com cinco táxons (C. bicolor, C. insidiosus, C. nycthemera, C. speratus e C. spinosus) e o terceiro subgênero nós nomeamos Coendou (Caaporamys) subgen. nov, composto por C. melanurus (espécie-tipo), C. vestitus, C. pruinosus, C. ichillus e C. roosmalenorum.
1 INTRODUCTION
The New World porcupines, family Erethizontidae, are a group of at least 17 species of large herbivorous arboreal rodents, ranging from 1 to 10 kg, with their fur modified into quills, lacking thumbs, presenting a bulbous snout and a dorsally coiling prehensile tail (Eisenberg & Redford, 1999; Ellerman, 1940; Emmons, 1997; Feijó & Langguth, 2013; Voss, 2015). Erethizontids are distributed from northern Argentina to northern Canada (Emmons, 1997; Voss, 2015) and are classified into three genera: Chaetomys Gray, 1850, Erethizon F. Cuvier, 1823, and Coendou Lacépède, 1799.
Studies on Coendou in the last decades resulted in many taxonomic changes and new species descriptions. Leite et al. (2011) designated the neotype of Coendou prehensilis (Linnaeus, 1758). Three monographs were published about the family's taxonomy (Voss, 2011, 2015; Voss & Angermann, 1997; Voss & da Silva, 2001). Geographic variation was examined and detailed for hairy dwarf porcupines of eastern Brazil, Coendou spinosus (F. Cuvier, 1823) and Coendou insidiosus (Olfers, 1818) (Caldara Júnior & Leite, 2012). Four species were described as follows: Coendou ichillus Voss & da Silva, 2001, Coendou roosmalenorum Voss & da Silva, 2001, Coendou speratus Mendes Pontes et al., 2013, and Coendou baturitensis Feijó & Langguth, 2013. Additionally, phylogenetic relationships among some erethizontid species have been inferred by molecular data (Bonvicino et al., 2002; Leite et al., 2011; Mendes Pontes et al., 2013). However, limited taxonomic and geographic sampling hampered a comprehensive phylogenetic hypothesis for the family. Only one work included 13 of the 17 known erethizontid species (Voss et al., 2013), but it lacked sequences of C. baturitensis, C. speratus, C. insidiosus, and C. roosmalenorum.
Coendou prehensilis is the recognized species with the broadest distribution, ranging from Northern Argentina to the Guianas and reported in all tropical South American biomes (Ramírez-Chaves et al., 2020; Torres-Martínez et al., 2019; Voss, 2015). It exhibits remarkable morphological variation (Leite et al., 2011; Voss, 2011) especially in the color pattern of the quills (Feijó & Langguth, 2013). Individuals from the Atlantic Forest biome are medium to small size, while animals from other regions are medium to large size (Feijó & Langguth, 2013). Such variation raised a suspicion that the name C. prehensilis might have been applied to a complex of closely related species (Cabrera, 1961; Husson, 1978; Leite et al., 2011; Voss, 2011). For example, Cabrera (1961) recognized morphological variation within C. prehensilis, which led him to recognize three subspecies, followed by Husson (1978). Feijó and Langguth (2013), based on external and cranial traits, described C. baturitensis, as part of the C. prehensilis complex. Later, Voss (2015) treated it as a junior synonym of C. prehensilis.
Previous phylogenetic studies on the C. prehensilis complex have consistently recovered two monophyletic lineages: one with central and northwestern South American specimens and another with a single sequence of the neotype of the C. prehensilis from northeastern Brazil (Leite et al., 2011; Voss et al., 2013). Accordingly, unique morphological traits were reported for each lineage (Feijó & Langguth, 2013; Leite et al., 2011). Nevertheless, comprehensive analyses of the geographic variation of the whole C. prehensilis complex are lacking. Here, we combined morphological and molecular datasets to infer the phylogenetic relationships of the genus Coendou and revise the taxonomy of the C. prehensilis complex through an integrative approach.
2 MATERIALS AND METHODS
2.1 Examined specimens
We examined morphologically 280 specimens of erethizontids, of which 140 are diagnosable specimens with complete skins and/or skulls of the C. prehensilis complex (see Appendix A1 for a list of all examined specimens). The specimens are housed in the collection of Mammals of the Universidade Federal da Paraíba, João Pessoa, Brazil (UFPB), collection of Mammals of the Universidade Federal de Pernambuco, Recife, Brazil (UFPE), collection of Mammals of the Universidade de Brasília, Brasília, Brazil (UnB), Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil (MZUSP), Museu Nacional do Rio de Janeiro, UFRJ, Rio de Janeiro, Brazil (MNRJ), Museu Paraense Emílio Goeldi, Belém, Brazil (MPEG), American Museum of Natural History, New York, USA (AMNH), and Field Museum of Natural History, Chicago, USA (FMNH).
Furthermore, living specimens from the Baturité’s Range (the type locality of C. baturitensis), Mulungu, Ceará, Brazil, were examined and had blood and quills samples collected under Brazilian license SISBIO no. 44678-3.
2.2 Morphological description
We scored morphological character states of the skin and the skull. A new nomenclature for the porcupine body regions, band colors, and mechanical regions of the quills is proposed to score the external morphological character states. Specimens of the C. prehensilis complex that share most of the character states were grouped in morphotypes and their localities plotted on a map to assess their distribution. The morphotypes are interpreted as species, subspecies, or intraspecific variation. Heuristic age determination follows Voss and da Silva (2001).
We also assessed the external and cranial morphometric variation of the morphotypes. External measurements were obtained from the specimens’ labels: weight (W), head and body length (HBL), length of tail (LT), and length of hindfoot with claws (HF). Cranial measurements were taken with digital calipers to the nearest 0.01 mm following the criteria described by Voss and da Silva (2001), and we added two new measurements: the anterior height of rostrum (AHR, anterior extreme of upper incisive foramen to nasal tip), and the posterior height of rostrum (PHR, anterior base of the zygomatic process of the jugal to the nasal above the lacrimal location; see Figure S1 for details). We assumed there is no sexual dimorphism in porcupine species following the findings of Caldara Júnior and Leite (2012) for the C. insidiosus complex. We considered only adult specimens for the description of morphotypes.
2.3 Living specimens and sampling collection
Quills, blood samples, measurements, and external morphology datasets were collected from two living individuals from Mulungu municipality in Baturité’s Range, Ceará State, Brazil, the type locality of C. baturitensis. The individuals were lifted by the tail, contained in a box and sedated via intramuscular injection in the tail with ketamine 20 mg/kg and midazolam 2 mg/kg by the responsible veterinarian. The quills are housed in the Collection of Mammals of the Universidade Federal da Paraíba under numbers UFPB 9390 (juvenile female) and UFPB 9391 (adult male).
2.4 DNA purification, amplification, and sequencing
The DNA from blood samples of UFPB 9391 was obtained through the Wizard® Genomic DNA Purification Kit. The DNA of UFPB 9412 (an adult female), UFPB 9780 (a sex-undetermined adult), and UFPB 9781 (an adult female) were extracted from fresh muscle samples preserved in PA ethanol at 4℃ through a standard CTAB extraction protocol (Doyle & Doyle, 1987). The polymerase chain reaction (PCR) was performed with the GoTaq® Green Master Mix 2X kit with primers MVZ 05 (sense) 5′ CGAAGCTTGATATGAAAAACCATCGTTG (Smith & Patton, 1993) and UMMZ 04 5′ TCTTCATTTYWGGTTTACAAGAC (antisense) (Jansa et al., 1999) to amplify the complete cytochrome b (cyt b) sequence. PCR conditions were initial denaturation at 95°C for 2 min, 39 cycles at 95°C/30 s, 44°C/45 s, and 73°C/90 s with final extension of 73°C/8 min. The expected amplicon length is about 1200 bp. The PCR product with the primers used for the amplification was sent for sequencing in the Laboratory of Histopathology at Instituto de Ciências do Mar (Labomar) of the Universidade Federal do Ceará, Ceará, Brazil. For sequencing, we used an additional primer, MZV16 (antisense) 5′ AAATAGGAARTATCAYTCTGGTTTRAT (Smith & Patton, 1993). The new sequences are deposited in GenBank with accession numbers KY784123-KY784126.
2.5 Morphometric analyses
We explored the cranial morphometric variation in C. prehensilis complex through univariate and multivariate analyses. Only adult individuals were included in the statistical analyses. In order to increase the sample size in multivariate analyses, we imputed missing data using the Amelia R package (Honaker et al., 2011) with species as a cross-factor. To increase the predictive power of imputations, we excluded specimens with numerous missing values, the final dataset included 72 specimens with only 3.8% of missing data. We evaluated the reliability of the estimation using the overimpute function which treats observed values as missing and estimates the observed values and their confidence intervals with the model used to estimate missing values. The breadth of morphospace variation within C. prehensilis was assessed via principal component analysis (PCA) using the log-transformed cranial measurements. The separation among morphotypes was quantified via discriminant function analysis (DFA) with leave-one-out cross-validation. We obtained similar results in the multivariate analyses when removing all individuals with missing data.
2.6 Phylogenetic analyses
Phylogenetic relationships were inferred based on morphological and molecular data, as separate and combined partitions.
2.6.1 Morphological data
The coding of morphological characters was made in a nexus file following Maddison et al. (1997). We performed maximum parsimony analysis (MP1) in PAUP4.0a150 (Swofford, 2002; Wilgenbusch & Swofford, 2003) with jack-knife resampling method (1000 replicates) with a heuristic search using the tree–bisection–reconnection (TBR) as the branch-swapping algorithm. Gaps were treated as “additional state.” Chaetomys subspinosus (Olfers, 1818) and Erethizon dorsatum (Linnaeus, 1758) were used as outgroups. Most parsimonious trees were summarized as a 50% majority-rule consensus tree. We listed apomorphies and calculated the consistency indexes (CI) for each character to identify homoplasy patterns.
2.6.2 Molecular data
We used a total of 59 sequences for phylogenetic analyses: our new four sequences and 55 available erethizontid's cytochrome b sequences previously deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/) (Appendix 2). We included almost all erethizontid's sequences except the unique available sequence of Coendou pruinosus Thomas, 1905 because it is very short with only 248 nucleotides (KC463880). The alignment was performed by the Muscle algorithm in MEGA 7 software (Kumar et al., 2016). We did not trim the final alignment and treated the gaps as missing data, resulting in an alignment size of 1140 nucleotides (Alignment S1). Model selection for probabilistic analyses was carried out with JModelTest 2 (Darriba et al., 2012). The model TN93 (Tamura & Nei, 1993) with gamma distribution (+G) was selected because it had the lowest BIC (Bayesian information criterion) and AIC (Akaike information criterion) scores (see Sullivan & Joyce, 2005). Two phylogenetic analyses were performed: maximum likelihood (ML) and Bayesian inference (BI). Chaetomys subspinosus and E. dorsatum were used as outgroups for the analyses.
The maximum likelihood (ML) consensus tree was inferred from 1000 pseudoreplicates (Felsenstein, 1985) using PhyML 3.1 (Guindon et al., 2010) with an initial BioNJ tree method (Guindon & Gascuel, 2003) and NNI search algorithm with TN93 nucleotide substitution model and a discrete gamma distribution model with four categories (+G = 0.222). Estimated nucleotide frequencies are f(A) = 0.32516, f(C) = 0.28747, f(G) = 0.09416, and f(T) = 0.29320. Bayesian inference (BI1) was inferred with a GTR+G model in MrBayes 3.2 (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003; Ronquist et al., 2012) with four chains over 10 million generations sampled every 100. The first 25% of samples were discarded as burn-in to estimate consensus trees and evolutionary parameters.
2.6.3 Combined data
We performed two analyses with combined molecular and morphological datasets (Alignment S2). First, we performed a maximum parsimony (MP2) analysis with 1000 bootstrap replicates in PAUP4. Gaps were treated as "additional state." Starting tree(s) obtained via stepwise addition and the tree–bisection–reconnection (TBR) algorithm with reconnection limit of 8. Branches collapse (creating polytomies) if maximum branch length is zero.
Second, we inferred a Bayesian inference (BI2) tree based employing a partitioned model. The morphological dataset considered only variable characters and employed the parsimonious model of Lewis (2001). For the molecular data, we employed the GTR+G model with the same search parameters as described above for BI1. The analysis was performed in MrBayes v. 3.1.2 (Ronquist et al., 2012). To quantify the contribution of the morphological and molecular datasets to node support, we used the partitioned Bremer Index (Bremer, 1994; Lambkin et al., 2002).
3 RESULTS
3.1 Pelage
Here, we describe a new way of interpreting the porcupine external morphology in order to distinguish the quills of the dorsal crest from those of the rump (Figure 1a). The external morphology was described using the different quill types and their position on the body. Furthermore, we also propose a new nomenclature for porcupine quills based on the pigmentation banding pattern and functional mechanical regions (Figure 1b). The pigmented bands are enumerated from 1 to n, from proximal to distal. The band 1 (B1) is the nearest to the tegument insertion of the quill, bristle, or any modified hair of the porcupine. The upcoming bands receive the following ordinal number. The apical band is the nth band (Bn) which n is also the total number of bands. Therefore, in a three-banded quill, the basal band is the B1, the medial band B2, and the distal band B3. Mechanical regions of the quill are tripartite: (1) The bulb is the region that anchors the quill to the tegument, (2) the mucronis (Latin: a point that pricks, masculine) is the mechanical region of thinner diameter than the rest of the quill at its apex after the quill body constriction, and (3) the quill body consists of almost all its length and has a broader diameter than the bulb and the mucronis.
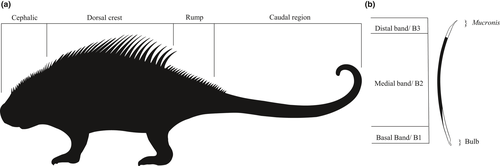
There are two types of banding pattern in erethizontid quills: The first has a darker medial band (B2) between the lighter basal (B1) and the distal (B3) bands; this is the tricolored quill banding pattern; the second type lacks a lighter distal band (B3), named the bicolored quill banding pattern, given it presents only a lighter basal band (B1) and a darker distal band (B2). The distal band (B2) of bicolored quills is considered homologous to the medial band (B2) of tricolored quills; therefore, we recommend the use of numbered band nomenclature.
3.2 Character states
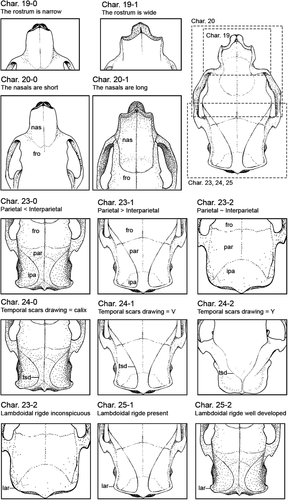
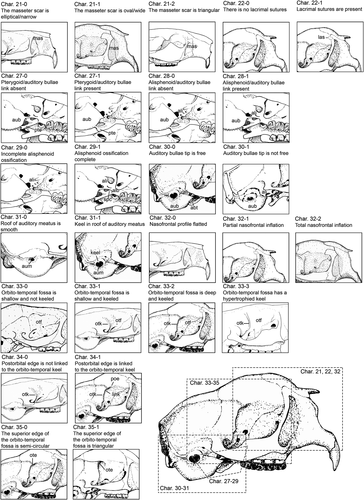
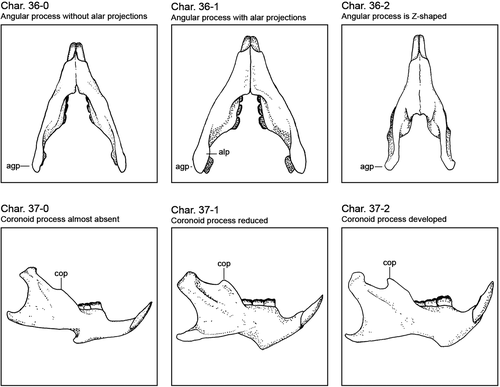
We scored 38 external and cranial characters of 12 species of erethizontids, plus the four morphotypes of the C. prehensilis complex (Table 1). The external morphological characters are based on the scheme of body regions here proposed (Figure 1). We used only species for which we have examined at least one specimen. The species with only one or/and incomplete specimens had their character states complemented with previous literature descriptions. List of characters and their states and coding follows (Figures 2-4): Char. 1—Fur covering quills on the dorsal crest: absent (0), present (1). Char. 2—Dorsal fur distribution: absent (0), present on rump (1), absent on rump (2). When present, the villous fur covers all the dorsum or is absent only on the rump. The characters 1 and 2 were considered as separated to obtain independent values of consistency and retention indices since the villous fur covering the dorsal quills was used as a diagnostic character to Sphiggurus (e.g., Woods & Kilpatrick, 2005). Char. 3—ventral pelage: soft (0), rough (1). The ventral pelage of porcupines can be composed of villous and soft fur or spiny hairs. The spiny pelage is made of hard and inflexible aristiform hairs with barbless mucronis which is absent in the soft pelage. Char. 4—Quill shape: non-wavy (0), wavy (1). Some species present quills with a wavy body, as the broomstraw spines of C. subspinosus (see Voss & Angermann, 1997 for details), while other species have curved quill bodies (Figure 1b). Char. 5—Dorsal crest composition: inflexible straight quills (0), flexible curved quills (1), bristle quills (2). Some quills are hard and inflexible with a long barbed mucronis, other ones are flexible and long with a barbless mucronis, the bristle quills present a barbless terminal filament rather than a mucronis (see Voss & da Silva, 2001 for details on bristle quills). The bands of bristle quills are treated as homologous to the bands of long quills of the dorsal crest. Char. 6—Diameter of quills or bristle quills of the dorsal crest: thin (0), thick (1). Thin <1.4 mm <thick in adult specimens. Char. 7—Tetracolored quills: absent (0), present (1). Char. 8—Tricolored quill location: posterior half of dorsum (0), head and flanks (1), all dorsum (2), almost all dorsum except rump (3). Char. 9—Bicolored quills location: head and thoracic dorsum (0), uniformly along dorsum (1), mainly on rump (2), only on rump (3). The distribution of bicolored quills could be restricted to specific body areas, densely in some areas or uniformly found in all dorsum. Char. 10—Coloration of B1 and B2 of dorsal crest quills and bristle quills: B1 whitish, B2 brownish (0), B1 slightly yellowish, B2 brownish (1), B1 strongly yellowish, B2 dark brownish (2), B1 strongly yellowish, B2 blackish (3). Char. 11—Coloration of B3 of dorsal crest: whitish (0), slightly yellowish (1), yellowish (2), orangish (3). Char. 12—B2 relative length to B1: shorter (0), about the same length (1), longer (2). Char. 13—B3 relative length to B2: shorter (0), about the same length (1), longer (2), inconspicuous (3). Char. 14—Quills on limbs: absent (0), present (1). Char. 15—Tail color type: colored (0), blackish (1). The quills of the tail can be tricolored or bicolored with a very long blackish B2. Char. 16—Tail relative length to the body: long (0), short (1). The tail is considered long if it has at least 65% of the body length. Char. 17—Incisive enamel color: orange (0), yellowish (1). Char. 18—Molar occlusion pattern viewed frontally: vertical (0), diagonal (1). Char. 19—Rostrum width: narrow (0), wide (1). Char. 20—Nasals length: short (0), long (1). The nasofrontal sutures of short nasals do not surpass the preorbital processes of frontals in dorsal view, while the nasofrontal sutures of long nasals surpass the preorbital processes in dorsal view. Char. 21—Medial masseter scar shape: elliptical and narrow (0), oval and wide (1), triangular (2). Char. 22—Lacrimal sutures: absent (0), present (1). Char. 23—Relative parietal length: shorter than interparietal (0), longer than interparietal (1), about interparietal length (2). Char. 24—Temporal scars drawing in dorsal view: calix shape (0), V-shaped (1), Y-shaped (2). Char. 25—Lambdoidal ridge: inconspicuous (0), present but weakly developed (1), strongly laterally developed (2). Char. 26—Palatal keel: absent (0), present (1). Char. 27—Pterygoid contact with auditory bullae: absent (0), present (1). Char. 28—Alisphenoid contact with auditory bullae constituting a fenestra: absent (0), present (1). Char. 29—Alisphenoid ossification: incomplete and open sphenopterygoid canal (0), complete with an alisphenoid bridge over the sphenopterygoid canal (1). Based on Voss and da Silva (2001). Char. 30—Dorsal contact between auditory bullae and alisphenoid: absent (0), present (1). Char. 31—The dorsal roof of the external auditory meatus: smooth (0), keeled (1). Based on Voss (2011). Char. 32—Nasals profile and nasofrontal inflation: flattened (0), posterior half-inflated (1), totally inflated (2). The skull dorsal region in porcupines varies greatly among the species. Some species have a flattened dorsal region without curvatures in the first two-thirds, except by a slight curvature immediately above the orbital region or with a slight inflation pronounced in the nasofrontal region. Furthermore, in some species, the posterior half of the nasal is inflated, with the anterior half-flatted. In C. baturitensis, the nasal is totally inflated. Char. 33—Orbito-temporal fossa depth: shallow (0), keeled and shallow (1), deep (2), hypertrophied keel (3). Char. 34—Postorbital edge linked to orbito-temporal keel: without link (0), linked (1). Char. 35—Shape of superior edge of orbito-temporal fossa: semi-circular (0), triangular (1). Char. 36—Angular process: narrow without alar projections (0), wide with dorsoventrally alar projections (1), Z-shaped (2). Char. 37—Coronoid process: absent (0), reduced (1), developed (2). The coronoid process is inconspicuous in some species, with only a small tip without any curvature. While a well-developed coronoid process presents a slightly curvature. The cranial and mandibular characters (Char. 19–37) are illustrated in Figures 2-4. Char. 38—Neonate pelage coloration: whitish (0), cream-colored (1), golden (2), orangish (3), brownish (4), and blackish (5). The color of the neonate's pelage is different from the adult villous pelage colors. The vivid colors observed in living neonates tend to fade after the preservation process. Therefore, the living individuals have brighter colors than the museum specimens. The codification of neonates of C. prehensilis complex, C. spinosus and C. insidiosus was based on living or recently dead and preserved specimens while the codification of C. nycthemera was based only on preserved specimens. The states of character 38 were considered constant for each species and were assessed based on 13 specimens (MNRJ 1361, MNRJ 42814, MNRJ 46935, MPEG 418, MPEG 421, MPEG 12598, MZUSP 20931, UFPB 1237, UFPB (FHM 23), UFPB (FHM 24), MNRJ 75670, MNRJ 79052, and MZUSP 25591). The states of characters 1 to 37 are exclusive of adult specimens. All characters are assumed to have unordered rather than ordered states.
| 1 | 2 | 3 | ||
|---|---|---|---|---|
| Groups | 1234567890 | 1234567890 | 1234567890 | 12345678 |
| Chaetomys subspinosus | 0001001000 | 0B01000000 | 2021111111 | 0021021? |
| Erethizon dorsatum | 1200100212 | −1-0010100 | A01220001A | 0031A115 |
| Coendou roosmalenorum | 1200200-12 | 2021100100 | 00?010001A | 1010012? |
| Co. ichillus | 0000200-12 | 0021100100 | 10?1010010 | 0000012? |
| Co. melanurus | 1100200-12 | 211110?110 | 00?00?0010 | 10100?2? |
| Co. bicolor | 0000100-12 | −23100?110 | 10?0?00000 | 1100102? |
| Co. nycthemera | 0000100313 | C331000100 | A020011111 | 00100024 |
| Co. speratus | 0000100332 | 3000000100 | 1020100111 | 0010002? |
| Co. insidiosus N | 1100100112 | 3000000100 | 102A000111 | 00000023 |
| Co. spinosus C | 1100100312 | 3000000100 | 0020000111 | 00100023 |
| Co. spinosus S | 1100100312 | 2000000100 | 0020000111 | 00100023 |
| Co. quichua | 0010110322 | 010100111? | ?????????? | 10?????? |
| Co. prehensilis | 0010110232 | 1021000110 | AA1B1000A0 | 01210AB2 |
| Co. l. longicaudatus | 0010110222 | 0101000110 | 101BBAAAA0 | 11B0AA24 |
| Co. l. boliviensis | 00101102CB | 0011000110 | 101BBA00AA | 11A0A023 |
| Co. baturitensis | 0010110221 | 0201001111 | 100B2A0010 | 12D01A20 |
- Note: The characters were listed by 10 states per species. Polymorphic entries: A = (01), B = (12), C = (23), and D = (012). Missing data are coded by question marks.
3.3 Morphotypes
We found 16 morphotypes of erethizontids (Table 1). Two of them correspond to the outgroups (Chaetomys subspinosus and Erethizon dorsatum) used in the phylogenetic analysis of the combined morphological and molecular data. The 14 remaining morphotypes belong to the genus Coendou. Three represent the hairy dwarf porcupines of eastern Brazil: two of them consist of C. spinosus, as previously reported by Caldara Júnior and Leite (2012), and one refers to C. insidiosus. Seven morphotypes represent fully recognized species of Coendou: C. melanurus (Wagner, 1842), C. roosmalenorum, C. ichillus, C. bicolor (Tschudi, 1844), C. nycthemera (Olfers, 1818), C. speratus, and C. quichua Thomas, 1899. The following species were not included because no specimens were examined: Coendou rufescens (Gray, 1865), Coendou mexicanus (Kerr, 1792), Coendou vestitus Thomas, 1899, and C. pruinosus. We recognized the remaining four morphotypes as part of the C. prehensilis complex. We assigned each morphotype to a Latin name based on the oldest valid name and the congruent description. The four morphotypes represent three species: C. prehensilis (Linnaeus, 1758), C. baturitensis Feijó & Langguth, 2013, and Coendou longicaudatus Daudin, 1802 with two subspecies: Coendou longicaudatus longicaudatus Daudin, 1802 and Coendou longicaudatus boliviensis (Brandt, 1835). See the taxonomy account below for a full description of each taxon.
The four morphotypes of the C. prehensilis complex share some common features (Tables S1-S2). The dorsal villous pelage of adults is composed of scarce shaggy monocolored or tricolored fur, which never covers the quills (Char. 1:0 CI 0.33). The dorsal crest exhibits thick (Char. 6:1 CI 1.00), long, and flexible tricolored quills with a short barbless mucronis (Char. 5:1 CI 1.00). The tricolored quills are present on almost all the dorsum (Char. 8.2 CI 0.75). The rump is composed of short bicolored quills with barbed and relatively long mucronis. The B1 of the rump quills is usually more yellowish than quills of the other regions. The ventral pelage of adults is composed of tricolored and sometimes bicolored aristiform fur (Char. 3:1 CI 1.00). The B1 of the ventral pelage is whitish or light yellow, B2 is brownish or blackish, and B3 is usually whitish. There are quills on the limbs (Char. 14:1 CI 0.33). The tail is long and has bicolored and tricolored quills dorsally in its basal third portion (Char. 15:0 CI 1.00). They have postcranial vibrissae emerging laterally on their anterior and posterior limbs, probably with a mechanoreceptor function. These vibrissae are long, thick, and flexible with some color variation. Postcranial vibrissae are bicolored with dark B1 and yellowish B2, or tricolored with a darker pattern than tricolored quills, or monocolored black or darkish brown. The skull of adults exhibits a wide and high rostrum with total (Char. 32:2 CI 0.67) or partial nasofrontal inflation (Char. 32:1). The lambdoidal ridge is laterally evident in dorsal view (Char. 25:1–2 CI 0.88). The temporal scars are Y (Char. 24:1) or V-shaped (Char. 24:2 CI 0.97) and converge posteriorly. The maxillary teeth are pentalophodont.
The neonates of the four C. prehensilis complex morphotypes exhibit a smooth villous pelage covering all body surfaces. This pelage is monocolored and we found whitish, cream-colored, orange, and rust-brownish specimens (Char. 38 CI 0.80). The quills are thin and almost whitish with some tricolored and bicolored with inconspicuous B2. The skull of neonates has a large uninflated rostrum. Some degree of nasofrontal inflation is noticeable during the ontogeny, mainly in subadult specimens.
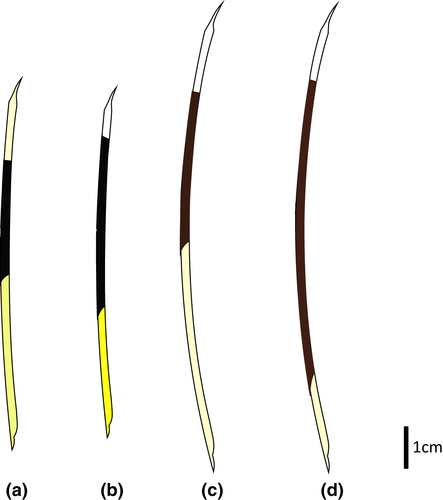
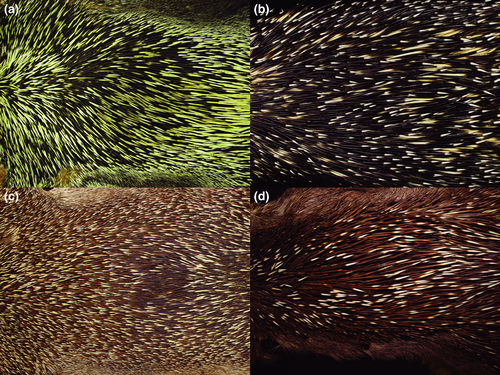
Cranially, the morphotypes of the C. prehensilis complex present similar features with exclusive character states limited to C. prehensilis and C. baturitensis (Table 2, Figure 5). The skull of C. prehensilis is smaller, while the skull of the other morphotypes exhibits a similar size (Table 3, Figure 6). Coendou baturitensis exhibits the longest nasal and the shortest parietal (Table 3). Externally, they are easily diagnosable by their quill band colors (Char. 10–13) (Figure 7) and by the distribution of each type of quill on the body and its curvature (Char. 5–9) (Table 2). The morphotypes present different color patterns based on the distribution of quill types (Figure 8).
| Character condition | C. prehensilis | C. l. longicaudatus | C. l. boliviensis | C. baturitensis |
|---|---|---|---|---|
| Size | Medium-small | Medium | Large | Medium |
| Weight (g) | 2656 (2350–2900) 3 | 3750 (3100–4200) 6 | 4056 (2330–5570) 10 | 3457 (3415–3500) 2 |
| Head and body length (mm) | 402.5 (290–480) 4 | 502.1 (450–530) 7 | 512.1 (470–570) 11 | 498.6 (460–549) 5 |
| Tail length (mm) | 386.5 (310–430) 4 | 521.5 (460–600) 10 | 522.1 (470–578) 11 | 426.6 (325–470) 5 |
| Hindfoot with claws length (mm) | 83.33 (80–85) 3 | 100.3 (87–110) 10 | 97.1 (87–105) 8 | 87.6 (80–105) 5 |
| Ear length (mm) | 10 (-) 1 | 14 (10–17) 3 | 11.5 (8–15) 9 | 27.6 (20–33) 3 |
| TL in relative HBL (%) | 97.5 (86.9–106.9) 4 | 106.9 (88.4–126.6) 7 | 102.3 (82.4–121.9) 11 | 86.5 (59.1–102.1) 5 |
| Distribution of bicolored quills (CI 0.875) | Rump agglomerated (Char. 9:3) | Uniformly distributed (Char. 9:1) | Rump agglomerated (Char. 9:3) | Uniformly distributed (Char. 9:1) |
| Length of anterior quills and Band colors | ||||
| B1 | Long and light yellowish | Medium and whitish | Long and whitish | Short and whitish |
| B2a | Short and dark brownish | Medium and dark brownish | Short and brownish | Long and brownish |
| B3 | Long and light yellowish | Short and whitish | Medium and whitish | Medium and whitish |
| Posterior quills colors | ||||
| B1 | Strongly yellowish | Yellowish | Yellowish | Light yellowish |
| B3 | Light yellowish | Whitish | Whitish | Whitish |
| Curvature of dorsal crest quills | Slightly curved | Slightly curved | Curved | Curved |
| Nasal length (CI 1.00) | Short (Char. 20:0) | Short (Char. 20:0) | Short (Char. 20:0) | Long (Char. 20:1) |
| Parietal length (CI 0.667) | Longer than interparietal (Char. 23:1) | Longer than interparietal (Char. 23:1) | Longer than interparietal (Char. 23:1) | Shorter than interparietal (Char. 23:0) |
| Lacrimal sutures (CI 1.00) | Present (Char. 22:1) or absent (Char. 22:0) | Absent (Char. 22:0) | Absent (Char. 22:0) | Absent (Char. 22:0) |
| Lambdoidal ridges (CI 0.88) | Present but weakly developed (Char. 25:1) | Present but weakly developed (Char. 25:1) or strongly laterally developed (Char. 25:2) | Present but weakly developed (Char. 25:1) or strongly laterally developed (Char. 25:2) | Strongly laterally developed (Char. 25:2) |
| Nasofrontal inflation (CI 0.667) | Partial (Char. 32:1) | Partial (Char. 32:1) | Partial (Char. 32:1) | Complete (Char. 32:2) |
| Medial masseter scar shape (CI 0.818) | Elliptical and narrow (Char. 21:0) or oval and wide (Char. 21:1) | Oval and wide (Char. 21:1) | Oval and wide (Char. 21:1) | Oval and wide (Char. 21:1) |
| Orbito-temporal fossa depth (CI 0.906) and its superior margin shape (Char. 35 CI 0.958) | Deep (Char. 33:2) and curved (Char. 35:0) | Deep or keeled shallow (Char. 33:1 or 2) and curved or triangular (Char. 35:0 or 1) | Shallow, keeled or not (Char. 33:0 or 1) and curved or triangular (Char. 35:0 or 1) | Shallow or deep, keeled or not (Char. 33:0, 1 or 2) and triangular (Char. 35:1) |
| Post-orbital edge (CI 0.50) | Linked to orbito-temporal fossa keel (Char. 34:1) | Not linked to orbito-temporal fossa keel (Char. 34:0) | Not linked to orbito-temporal fossa keel (Char. 34:0) | Not linked to orbito-temporal fossa keel (Char. 34:0) |
| Enamel color (CI 0.50) | Orangish (Char. 17:0) | Orangish (Char. 17:0) | Orangish (Char. 17:0) | Yellowish (Char. 17:1) |
| Palatal keel (CI 0.923) | Absent (Char. 26:0) | Absent or present (Char. 26:0–1) | Absent or present (Char. 26:0–1) | Absent or present (Char. 26:0–1) |
| Alisphenoid ossification (CI 1.00) | Partial or complete (Char. 29:0–1) | Partial or complete (Char. 29:0–1) | Partial or complete (Char. 29:0–1) | Complete (Char. 29:1) |
| Auditory bullae with link (CI 0.895) or dorsal contact with alisphenoid (CI 0.8) | With no link or contact (Char. 28:0; Char. 30:0) | Link present or absent (Char. 28:0–1) and no dorsal contact (Char. 30–0) | With no link (Char. 28:0) and dorsal contact can be present or not (Char. 30–1) | With no link or contact (Char. 28:0; Char. 30:0) |
| Pterygoid linked to auditory bullae (CI 0.944) | Absent (Char. 27:0) | Absent or present (Char. 27:0–1) | Absent (Char. 27:0) | Absent (Char. 27:0) |
| Dorsal roof of the external auditory meatus (CI 0.250) | Not keeled (Char. 31–0) | Keeled (Char. 31–1) | Keeled (Char. 31–1) | Keeled (Char. 31–1) |
| Coronoid process (CI 1.00) | Reduced or developed (Char. 37:1–2) | Developed (Char. 37:2) | Developed (Char. 37:2) | Developed (Char. 37:2) |
| Angular process (CI 0.958) | Narrow or wide with dorsoventrally alar projections (Char. 36:1) | Narrow or wide with dorsoventrally alar projections (Char. 36:1) | Narrow without alar projections (Char. 36:0) | Narrow or wide with dorsoventrally alar projections (Char. 36:1) |
- Note: Only adult and non-pregnant specimens were considered to obtain the body measurements. Exclusive character conditions for each morphotype are italicized.
- a B2 of posterior quills has the same color tones of anterior quills.
| Measurements | C. prehensilis | C. l. longicaudatus | C. l. boliviensis | C. baturitensis |
|---|---|---|---|---|
| CIL | 86.2 ± 5.5 (75.7–94.4) 14 | 92.9 ± 4.9 (81.6–101.6) 18 | 94.1 ± 3 (89.1–99.4) 19 | 94.3 ± 4.3 (89.9–103.3) 12 |
| LD | 23.5 ± 2.7 (18.3–27.7) 15 | 24.7 ± 2.4 (20–28.1) 18 | 25.7 ± 1.4 (23.8–28.9) 19 | 25.7 ± 1.9 (22.2–29.9) 13 |
| LIF | 7.5 ± 1.61 (5.1–9.8) 15 | 8.4 ± 1.7 (5.8–11.7) 18 | 8.3 ± 1.1 (7.0–10.7) 18 | 7.4 ± 1.7 (4.2–9.6) 13 |
| BIF | 4.2 ± 0.5 (3.4–5) 15 | 4.8 ± 0.7 (3.9–6.1) 18 | 4.9 ± 0.6 (3.6–6) 18 | 3.6 ± 0.5 (2.7–4.4) 13 |
| MTR | 19.4 ± 0.8 (18.2–20.6) 14 | 20.4 ± 1 (18.8–22.1) 17 | 20.7 ± 1.1 (18.8–22) 18 | 21.3 ± 0.9 (19.2–22.7) 11 |
| LM | 14.5 ± 0.5 (13.7–15.3) 14 | 15.3 ± 0.6 (14.5–16.6) 17 | 15.4 ± 0.9 (13.1–16.4) 18 | 15.7 ± 0.7 (13.9–16.5) 11 |
| BP4 | 5.3 ± 0.4 (4.7–5.9) 15 | 5.9 ± 0.4 (5.2–7) 17 | 5.8 ± 0.3 (5.2–6.2) 18 | 5.9 ± 0.4 (5.2–6.5) 13 |
| BM1 | 5.4 ± 0.31 (4.9–5.7) 15 | 5.6 ± 0.3 (5–6.3) 17 | 5.5 ±.03 (5–5.9) 18 | 5.5 ± 0.3 (5.1–6.1) 12 |
| APB | 5.2 ± 0.61 (4.1–6.1) 15 | 6.7 ± 1 (3.5–8) 18 | 6.1 ± 0.7 (4.7–7.2) 19 | 5.3 ± 0.8 (3.7–6.6) 13 |
| PPB | 8.3 ± 0.6 (6.9–8.9) 14 | 9.8 ± 0.9 (7.7–11.6) 18 | 9.3 ± 1 (6.8–10.8) 19 | 9.2 ± 0.8 (8.1–10.5) 11 |
| PZB | 51.3 ± 1.9 (48.1–54.8) 14 | 55.5 ± 2.8 (51.1–61.2) 18 | 55.4 ± 2 (51.1–58.8) 18 | 53.6 ± 1.6 (51.0–55.9) 13 |
| HIF | 13.1 ± 1.5 (10.5–14.9) 15 | 14.7 ± 2.2 (9.8–17.4) 18 | 14.5 ± 1.1 (12.1–16.4) 19 | 14.8 ± 1.4 (12.9–16.5) 13 |
| ZL | 36.6 ± 3.73 (31.1–41.6) 15 | 40.4 ± 3.5 (36.2–49.8) 18 | 37.3 ± 2.4 (32.5–43.1) 19 | 36.0 ± 2.4 (33.0–41.3) 13 |
| LN | 32.93 ± 4.4 (26.24–40.59) 12 | 31.6 ± 5.3 (20.6–38.9) 10 | 33.9 ± 3 (28.8–38.5) 16 | 41.3 ± 3.8 (36.3–48.7) 11 |
| BNA | 22.54 ± 1.48 (20.13–25.54) 15 | 22.9 ± 1.2 (20.9–24.9) 17 | 24.0 ± 1.2 (22.4–26.5) 18 | 23.8 ± 1.2 (21.5–25.6) 13 |
| BB | 36.52 ± 1.47 (32.55–38.52) 15 | 39.3 ± 1.8 (35.8–42.4) 18 | 39.7 ± 1.8 (37.2–44.4) 19 | 39.0 ± 0.9 (37.6–40.7) 13 |
| DI | 4.39 ± 0.2 (4.06–4.68) 15 | 4.6 ± 0.5 (4–6) 18 | 4.4 ± 0.3 (4.1–5) 17 | 4.5 ± 0.3 (4.0–5.2) 12 |
| BIT | 6.95 ± 0.84 (5.82–8.43) 15 | 7.1 ± 0.5 (6.3–7.8) 17 | 6.9 ± 0.5 (6.1–7.7) 16 | 7.5 ± 0.3 (7.1–8.0) 11 |
| AHR | 27.12 ± 1.92 (22.66–30.05) 15 | 27.8 ± 1.8 (25.3–30.7) 16 | 28.4 ± 1.6 (25.6–32.4) 19 | 30.7 ± 2.1 (27.7–33.5) 13 |
| PHR | 34.11 ± 3.46 (27.4–42.25) 15 | 37.7 ± 6.7 (22.4–46.9) 18 | 40.4 ± 2.9 (35.9–45.9) 19 | 43.0 ± 3.9 (37.9–50.0) 13 |
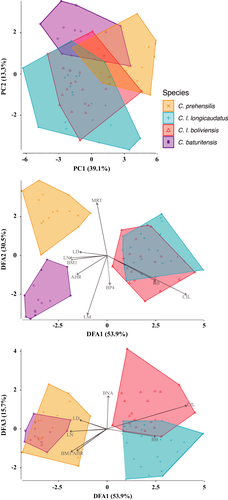
By comparing cranial measurements across the morphotypes, the first two principal components (together explained 52.4% of the variation) show that the three species of C. prehensilis complex occupy different morphospaces (Figure 6). The two subspecies of C. longicaudatus shows a marked overlap. The four taxa, however, can be clearly separated in the discriminant analysis (Figure 6). The correct classification percentage among the three species after leave-one-out cross-validation is 93% (C. prehensilis: 81.2%, C. longicaudatus: 97.7%, C. baturitensis: 92.3%).
The separation of C. prehensilis from the other two species along the DFA2 axis is mainly related to its smaller size, longer maxillary toothrow, narrower P4, and narrower braincase (Figure 6, Table S3). Coendou baturitensis is clearly differentiated from C. longicaudatus along the DFA1 axis. The former has longer nasals, larger M1, higher rostrum, and narrower zygomatic breadth in relation to C. longicaudatus (Table 3, Figure 6). In addition, the two subspecies of C. longicaudatus cluster apart along the DFA3, where C. l. longicaudatus show longer zygomatic length, deeper upper incisors, shorter upper molars row, a narrower nasal aperture, and a shorter diastema when compared to C. l. boliviensis (Table 3, Figure S1). Interestingly, we found the two subspecies of C. longicaudatus tend to have longer tails (averaging about 102.3% of HBL for C. l. boliviensis, and 106.3% for C. l. longicaudatus) than C. baturitensis (average 86.5% of HBL) and C. prehensilis (average 97%) (Table 2).
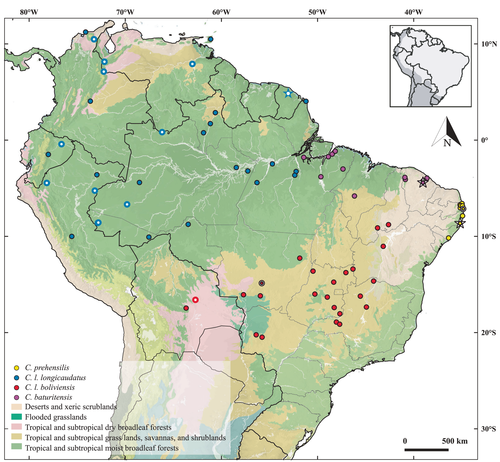
The localities of the specimens for each morphotype of the C. prehensilis complex delimit allopatric distributions for the four morphotypes (Figure 9). The Figure 9 considers geographic information of specimens examined morphologically and also the sequenced specimens with congruent morphotypes.
3.4 Phylogenetic relationships
The maximum parsimony tree obtained from morphological data (MP1) analysis has 30 parsimony informative characters. The consensus tree has length 124 (min 62, max 161), consistency index (CI) 0.50, and a retention index (RI) of 0.37. Eight morphological characters are uninformative (autapomorphies) and 29 are informative. The MP1 consensus tree shows the four morphotypes of C. prehensilis with Coendou quichua in a polytomy, C. roosmalenorum and C. melanurus are grouped together, and another polytomy comprised of the morphotypes of the hairy dwarf porcupines of eastern Brazil, C. insidiosus and C. spinosus (Figure S2). See Table S1 for the transformation list.
The maximum likelihood (ML) and Bayesian inference (BI1) analyses of the cytochrome b generated consensus trees with the same topology (Figure S3). The genus Coendou has three well-supported clades of species with a topology similar to the trees obtained by Voss et al. (2013). Most Coendou species show well-supported monophyletic groups except C. spinosus which is paraphyletic (Figure S3). The C. prehensilis complex is recovered as a monophyletic clade with high support (BS 96, PP 100) composed of three main lineages. The first divergence separates the neotype of C. prehensilis and one individual from João Pessoa, Paraíba (Northeastern Brazil), from all other species of the complex. The second divergence separates three individuals from the Baturité range, the type locality of C. baturitensis, from a clade encompassing all other C. prehensilis complex individuals from central and western Amazon and Cerrado biomes. This latter clade includes individuals of C. longicaudatus longicaudatus and C. longicaudatus boliviensis. These three main clades are well supported in both ML and BI. Specimens of C. l. boliviensis are monophyletic (BS 97, PP 100) within a polytomy of C. longicaudatus lineage. Therefore, C. l. longicaudatus is a paraphyletic clade.
The trees obtained by maximum parsimony (MP2) and Bayesian inference (BI2) with combined morphological and molecular datasets show fully congruent topologies with those inferred from the molecular dataset, ML and BI1 trees (Figure 10); however, they showed a stronger resolution than the molecular dataset alone. Almost all species presented posterior probability values (PP) of 100 and bootstrap support (BS) values above 90%. The MP2 tree has a length of 1364 (min. 795, max. 4186), a CI of 0.583 and a RI of 0.832. A total of 694 characters are constant, 123 characters are uninformative (autapomorphies) and 360 are informative. Partitioned Bremer indices show that almost all clades have congruent morphological and molecular signals under parsimony except for the genus Coendou and the C. prehensilis complex, in which signal conflict is detected (Figure 10).
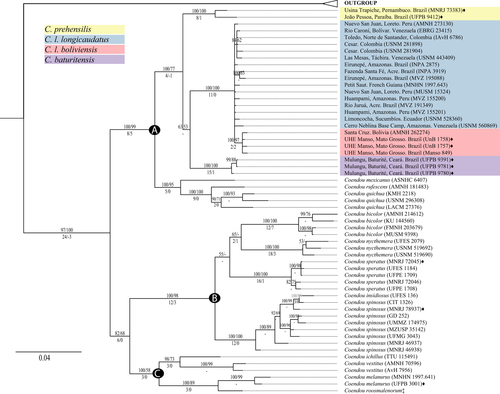
All individuals attributed to C. prehensilis and C. baturitensis were consistently recovered as monophyletic allopatric lineages and therefore considered as valid species. The individuals of the C. prehensilis complex from central western Amazon and Cerrado biomes are, respectively, referred to Coendou longicaudatus longicaudatus and C. longicaudatus boliviensis. The subspecies classification is based on the fact they are easily diagnosable externally, show cranial morphometric differences, occur in different biomes, but are not reciprocally monophyletic. Therefore, we recognize three species as part of the C. prehensilis complex: C. prehensilis (Linnaeus, 1758), C. baturitensis Feijó & Langguth, 2013, and Coendou longicaudatus Daudin, 1802 with two subspecies.
4 DISCUSSION
4.1 Taxonomic assessment
Our comprehensive study of the C. prehensilis complex, combining morphological, morphometric, and molecular datasets from a large part of its distribution, allowed us to clarify the taxonomy of this widespread Neotropical group. Our main findings include the recognition of three species previously held within C. prehensilis, confirming the specific status of C. baturitensis and revalidating Coendou longicaudatus. The inclusion of C. baturitensis individuals in the phylogenetic analyses and an additional sequence of C. prehensilis from northeastern Brazil were decisive for our new classification. It is noteworthy that no previous systematic revision of the C. prehensilis complex examined animals from the Atlantic Forest, Cerrado, Chaco, Caatinga, and Amazonian biomes altogether, allowing the identification of the autapomorphies, synapomorphies, and combination of diagnostic characters here presented. Indeed, prior to the designation of the neotype of C. prehensilis (Leite et al., 2011), no taxonomic study had examined porcupines from the Brazilian Northeastern region. Most of the studies limited their analyses to specimens from Amazonia Forest, Bolivian Chaco, or Central Brazil. Therefore, the authors had used either longicaudatus or boliviensis specimens as representative of the morphological characteristics of C. prehensilis to compare with other Coendou morphotypes, in order to describe new species or to synonymize taxa. Leite et al. (2011) and Feijó and Langguth (2013) were the first to compare and describe specimens of C. prehensilis complex from northeastern Brazil. It is also noteworthy that at least three aspects of the systematics of the Coendou are still unclear: (1) the precise phylogenetic position of C. roosmalenorum, for which no DNA sequence data are available; (2) the relationship between C. insidiosus and C. spinosus, which seem to be not reciprocally monophyletic (as also noted in Mendes Pontes et al., 2013); and (3) the relationships within the C. longicaudatus subspecies, which requires the analysis of additional molecular markers for an improved resolution.
4.2 Subgeneric classification of Coendou
Traditionally, the species of Coendou were divided into two genera (or subgenera): Sphiggurus F. Cuvier, 1823 and Coendou Lacépedè, 1799 (Allen, 1904; Cabrera, 1961; Ellerman, 1940; Feijó & Langguth, 2013; Husson, 1978; Tate, 1935). This classification was based on the nasofrontal sinus inflation and/or on the length of the dorsal fur (Bonvicino et al., 2002, 2008; Feijó & Langguth, 2013; Voss, 2011, 2015; Woods & Kilpatrick, 2005). Nevertheless, these characters were considered insufficient to distinguish the two (sub)genera (Emmons, 1997; Handley & Pine, 1992; Voss, 2011) and the dorsal fur was recovered as a homoplastic trait (Voss et al., 2013), leading recent authors to abandon the subgeneric classification in Coendou (Voss, 2015; but see Feijó & Langguth, 2013). In this study, we recovered three well-supported lineages within Coendou that show distinct morphological traits (Figure 7). A similar topology was found by Voss et al. (2013), but their third lineage (our clade C in Figure 10) had weak support. Additionally, for Voss et al. (2013), the three lineages were not morphologically diagnosable. Here, we advocate for the subgeneric classification in Coendou given that each of the three main clades has unique morphological synapomorphies and was consistently recovered as monophyletic in our analyses.
The subgeneric groups are designated by their oldest available names for the genus nominal taxa. Group A represents the subgenus Coendou, based on C. prehensilis, the nominotypical taxon, and is composed of the Andean and Trans-Andean porcupines: Coendou (Coendou) prehensilis, C. (Co.) longicaudatus, C. (Co.) baturitensis, C. (Co.) quichua, C. (Co.) mexicanus, and C. (Co.) rufescens. This group has the following putative synapomorphies: the quills are thick, the dorsal crest is composed of flexible quills, the ventral pelage is rough, and the tail exhibits tricolored and bicolored quills. No specimens of C. (Co.) mexicanus and Coendou (Co.) rufescens were examined. Group B represents the subgenus Sphiggurus, based on Sphiggure spinosa F. Cuvier, 1823 (its original spelling, but maintained as Sphiggurus due to its prevailing subsequent usage as in Voss, 2011:6), and it is composed of five species: Coendou (Sphiggurus) insidiosus, C. (S.) spinosus, C. (S.) nycthemera, C. (S.) bicolor, and C. (S.) speratus. These species possess thin quills, the dorsal crest is composed of flexible tricolored or bicolored quills, soft ventral pelage, and the tail exhibits tricolored and bicolored quills. There are four additional valid generic names for porcupine species: Sinetheres F. Cuvier, 1823, Cercolabes Brandt, 1835, Echinoprocta, Gray, 1865, and Cryptosphingurus Miranda Ribeiro, 1936. However, their type species are grouped in the two former subgenera. Therefore, the group C has no subgeneric name available as determined by the articles 42–44 of the International Code of Zoological Nomenclature, so we propose the name Caaporamys subgen. nov. (Caaporã’s Rat, masculine. Zoobank's act DC888480-8EAF-4C0A-9AC1-2BF4B3ED8590). Caaporã (or Caipora) is an Amazonian indigenous deity, guardian of the animals; the name means “who lives in the forest” in Tupi-Guarani languages, the most widespread indigenous linguistic family of Brazil. Caaporamys comprises the smallest species of Coendou and is equivalent to the vestitus group of Voss and Silva (2001). It is composed of five species: Coendou (Caaporamys) melanurus designated as type species, C. (Ca.) vestitus, C. (Ca.) pruinosus, C. (Ca.) ichillus, and C. (Ca.) roosmalenorum. These species have thin quills, the dorsal crest is composed of tricolored bristle quills, soft ventral pelage, and the tail has an overall blackish color because of its bicolored quills with a long and blackish B2.
4.3 Quill banding color pattern as an important taxonomic tool for porcupines
The quills are an important taxonomic character in the family Erethizontidae (Handley & Pine, 1992; Leite et al., 2011; Mendes Pontes et al., 2013; Voss & Angermann, 1997; Voss & da Silva, 2001). Although there are numerous studies describing the functional morphology of the erethizontid quills (e.g., Cho et al., 2012; Vincent & Owers, 1986; Yang et al., 2013), the distinction of functional quill types are not widely used as taxonomic characters. The quills of erethizontids have a cortex compounded of juxtaposed scales, which are the barbs in the mucronis region (e.g., Chernova, 2002; Cho et al., 2012; Vincent & Owers, 1986). The diversity of function of quill types in Coendou species likely relates to distinct defensive strategies. For example, the long tricolored quills have barbless mucronis while short bicolored quills have barbed mucronis. The barbed mucronis represents an anchoring system in the tegument of a potential aggressor (Chernova, 2002; Cho et al., 2012; Vincent & Owers, 1986), what makes the quills commonly found in agonistic encounters with domestic dogs (e.g., Johnson et al., 2006; Rangel & Neiva, 2013) and sometimes lethal to large predators as cougars, Puma concolor (as reported by Elbroch et al., 2016). The fact that the long tribanded quills are barbless suggests the importance of the preservation of these quills when a porcupine is attacked, with potential trade-offs between the cost of loss and protection types. In our study, we show that the distribution of long tricolored and short bicolored quills along the body is useful to differentiate species and subspecies in Coendou and might also reflect distinct evolutionary strategies of porcupines’ lineages.
Previous taxonomic studies on quill morphology of porcupines applied a divergent nomenclature from the functional trichology studies. The term ‘tip’ refers to the nth band in taxonomic works, referring to the region homogeneously colored at the distal part of the hair (e.g., Leite et al., 2011; Mendes Pontes et al., 2013), while the same term refers to mucronis in functional trichology descriptions (e.g., Chernova, 2002; Cho et al., 2012; Vincent & Owers, 1986). The term ‘tip’ has also been used to refer to the nth band and the mucronis in the same work (e.g., Voss & da Silva, 2001). The confusion of the term “tip”, in this case, does not make clear whether the mucronis region is equivalent to band n. Moreover, the name “tip” does not represent the mechanical function performed by mucronis. Therefore, our nomenclature aims to highlight the mechanical and functional differences among the regions of quills.
The color tones and length of each band and its position in the bicolored and tricolored quills define the color pattern of the porcupine. The color patterns appear to be conservative in the species of the C. prehensilis complex as well as almost all of the examined species of erethizontid, except for the C. insidiosus/C. spinosus complex (Caldara Júnior & Leite, 2012). Therefore, our study shows that the location and banding pattern of each type of quill on the porcupine dorsum have proven to be of high value as diagnostic characters. Furthermore, the coloration of the porcupine quills is aposematic, an ecological trait (Inbar & Lev-Yadun, 2005; Stankowich & Campbell, 2016), which reinforces its importance in porcupine biology. We thus encourage future taxonomic studies on porcupines to explore the color tones and distribution of the quill types. Additionally, this trait can be easily assessed in live animals or on sylvatic and domesticated predators, which can be a useful tool for species identification in the field or occurrence confirmation.
4.4 Biogeography of Neotropical forested biomes
Because of their herbivorous diet, arboreal habits, and medium size, porcupines are strongly associated with forested ecosystems. The distribution of the four taxa of the C. prehensilis complex suggests that the species occur in both dry and moist forests, as well as in forests ecosystems within savannahs, for example, in the Cerrado biome. Occupation of these ecosystems is emblematic in C. baturitensis that occurs both in the eastern Amazon forests and in mid-altitude moist forest remnants within the Caatinga biome, generally grouped under the term brejos de altitudes or moist altitude forests (Moro et al., 2015), what reinforces previously known past connections between the Amazon and the Atlantic forests (Costa, 2003; Vanzolini & Williams, 1981), the two major tropical moist forest biomes of the Americas. Moreover, the brejos de altitude fauna is usually composed of endemic species of forest dwellers. Some species found in brejos de altitude have a disjunct distribution with their populations or sister species in the eastern portion of the Amazon, including reptiles (e.g., genera Atractus, Enyalius, Pseustes), anurans (e.g., genus Adelophryne), birds (e.g., Pipra fasciicauda and Selenidera gouldii), and mammals (e.g., Makalata, Proechimys roberti) (Borges-Nojosa, 2007; Girão et al., 2007; Leite & Loss, 2015; Loebmann & Haddad, 2010). This pattern is also found in other species from the northern Atlantic forest Lowlands as in the howler monkey Alouatta belzebul and the kinkajou Potos flavus, or at the generic and specific level in other small mammals (Costa et al., 2000).
The available information about the species limits in the C. prehensilis complex suggests they are allopatric and loosely associated with particular biomes and ecoregions. However, sympatry among species from distinct subgenera seems to be common in Neotropical porcupines (as reported by Gregory et al., 2015; Menezes et al., 2020; Ramírez-Chaves et al., 2016, 2019). For example, C. (Co.) baturitensis is sympatric with Coendou (Sphiggurus) nycthemera in the eastern amazon forest, and C. (Co.) l. boliviensis exists in sympatry with Coendou (Sphiggurus) spinosus in the transition zone between Atlantic Forest and Cerrado in southeastern Brazil (see the examined specimens in Supporting Information). The distribution of C. (Co.) l. longicaudatus overlaps with almost all Amazonian porcupine species, with documented sympatry with Coendou (Sphiggurus) bicolor and Coendou (Caaporamys) ichillus (Menezes et al., 2020). C. (Co.) prehensilis occurs in sympatry with Coendou (Sphiggurus) speratus in the Pernambuco Endemism Centre (PEC) (Feijó & Langguth, 2013; Mendes Pontes et al., 2013). This ecoregion is one of the most important Brazilian biodiversity hotspots and encompasses a coastal block of Atlantic Forest between the states of Alagoas and Rio Grande do Norte. The area represents less than 5% of the original Brazilian Atlantic rainforest, but it is home to more than 60% of all the bird species and 8% of the vascular plants related in this biome (Tabarelli & Roda, 2005). In addition to C. prehensilis and C. speratus, there are other mammal species endemic to PEC such as Sapajus flavius, Hylaeamys oniscus, and Sylvilagus brasiliensis (Garbino et al., 2018; Mendes Pontes et al., 2016).
4.5 Conservation
Our new taxonomic arrangement will likely cause profound changes in the evaluation of the conservation status of the whole group. Coendou prehensilis is considered as least concern by international (Marinho-Filho & Emmons, 2016) and Brazilian (ICMBio, 2018) red lists due to its previous wide distribution and presumed large population. Our study, however, shows that this species is restricted to the Pernambuco Endemism Centre (Figure 9), which, despite its impressive ecological importance, has lost 94.6% of its original forest cover mainly due to sugarcane plantation over the last 500 years (Mendes Pontes et al., 2016; Ribeiro et al., 2009). This habitat loss is reflected by a severe defaunation, resulting in the local extinction of seven species of medium and large-sized mammals, which represents 20.6% of the original richness of this group (Garbino et al., 2018). It is relevant to point out that the other endemic mammal species from PEC are threatened: the blonde capuchin Sapajus flavius, the tapeti Sylvilagus brasiliensis, and the congeneric species Coendou (S.) speratus are considered as endangered by the IUCN (Roach & Mendes Pontes, 2020; Ruedas & Smith, 2019; Valença Montenegro et al., 2020) and the Brazilian Red List (ICMBio, 2018). In addition to endemism, S. flavius and C. speratus share similar habits, habitats, and threats with C. prehensilis. Therefore, it is likely to expect an increase in conservation concerns for the Brazilian Porcupine. Among the 11 localities here recorded for C. prehensilis, nine are from the Atlantic Forest of Paraíba state, where most of the specimens were collected in the protected area Guaribas Biological Reserve (Feijó et al., 2016). This area could be prioritized to develop biological and ecological research regarding this species and to promote conservation actions.
The conservation status of C. baturitensis, currently classified as data deficient (ICMBio, 2018; Roach, 2016), also requires a re-evaluation. In the northeastern portion of Brazil, this species is distributed in or close to forested remnants (brejos de altitude) inserted in the Caatinga biome and that have faced accentuated deforestation, overexploitation of natural resources, introduction of invasive species, pollution of water resources, and real estate speculation (Fernandes-Ferreira et al., 2015; de Oliveira et al., 2007; Tabarelli et al., 2010). The Amazonian populations are in one of the most threatened regions of this biome, the Eastern Amazon. This region faces high rates of deforestation caused by agriculture, livestock, logging, and hydroelectric plants (Lees et al., 2016; Silva Junior et al., 2020; Vieira et al., 2008) including the Belo Monte Dam located in the Xingu River, the west limit of C. baturitensis distribution. In addition, the species is hunted mainly for food purposes throughout its range. In the Baturité range, for example, the hunting of this porcupine increases during the mango harvest, one of its favorite diet items according to local interviewees. Luckily, in some localities, there is a consumption taboo associated with C. baturitensis. The folk knowledge claims that the consumption of its meat can lead to bad luck (Alves et al., 2016).
The ample distribution of Coendou longicaudatus and its presence in several protected areas are certainly positive factors for its conservation status. Nevertheless, there are several threats associated with this species that are gradually and worryingly increasing. Deforestation in the Brazilian Amazon, Cerrado, and Pantanal has sharply risen in the last years. Since 2013, official deforestation rates have been on an upward trend, worsening in the last two years. In 2019, the vegetational loss increased 34% when compared to 2018. The Brazilian Amazon, for example, lost 11,088 km2 of forested area, the highest rate in the decade (Escobar, 2020; Silva Junior et al., 2021). Cerrado is one of the most impacted biomes in the world, with over 20,400 km2 of native vegetation suppressed between 2017 and 2019 (Assis et al., 2019). Over 22,000 fires were recorded in Pantanal in 2020, the highest rate in its entire history and which surpasses the records of 2019, 2018, and 2017 together (INPE, 2021). The increased investment in livestock and soy production as well as the loosening of environmental laws are the main reasons for the documented loss in these biomes. Other causes include logging, mining, construction of dams, global climate changes, and the continuous use of fires for traditional and non-traditional communities for agricultural purposes (Fearnside et al., 2013; Pivello, 2011; Silva Junior et al., 2021; Sonter et al., 2017; Trigueiro et al., 2020). In addition, hunting of Coendou spp. for food use is also documented in these areas (Baía Júnior et al., 2010).
It is important to note that it is a major challenge to adequately estimate the impact of the threats discussed above on the populations of the C. prehensilis complex and therefore of all species and subspecies recognized in this study. Except for some restricted ecological records (e.g., Griffiths et al., 2020; Ramírez-Chaves et al., 2020), there is practically no research focused on their natural history such as reproduction, diet, population density, and home range of this group. Thus, it is urgent to conduct studies to fill these gaps in order to establish accurate assessments and effective conservation strategies.
5 TAXONOMIC ACCOUNT

Type material
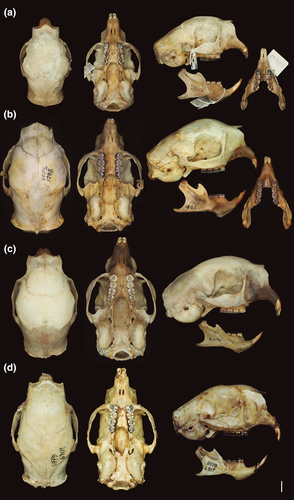
MNRJ 73383, neotype designated by Leite et al. (2011). Adult male collected by Antônio Rossano Mendes Pontes (field number ARMP 63) on May 12, 2009. The specimen consists of a well-preserved skin, skull, and mandible (Figure 2a). The cyt b sequence of the neotype is archived in GenBank with accession number HM462243.
Type locality
“Asia, America meridionalis” (Linnaeus, 1758), posteriorly fixed to “Pernambuco” by Thomas (1911). The neotype was collected in Mata Xanguá, Usina Trapiche, municipality of Sirinhaém, state of Pernambuco, Brazil, coordinates 8°38′50″S 35°10′15″W, elevation 100 m (Leite et al., 2011).
Etymology
prehensilis refers to the characteristic prehensile tail of the erethizontid porcupines.
Diagnosis
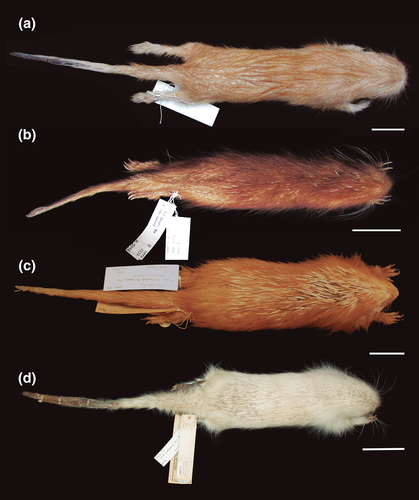
Small-medium-sized porcupine (Table 2). Coendou prehensilis presents the dorsal crest composed mainly of tricolored quills and the rump of bicolored quills (Figure 11a). The brown villous pelage, sometimes tricolored, never covers the quills in adults. The B1 of tricolored and bicolored quills is light yellowish on the cephalic portion and strongly yellowish on the rump, while intermediary yellow tones in the dorsal crest. The B2 is blackish and short compared to B1 and B3 in tricolored quills. The B3 of tricolored quills is white yellowish and sometimes half of B2 length (Figure 7a). The general body color pattern is light yellowish with dark brownish dashes, due to the predominance of tricolored quills with long B3 (Figure 8a). The tricolored quills of the dorsal crest are shorter than 10 cm and are slightly curved. Among the four taxa of C. prehensilis complex, the skull of C. prehensilis (Figure 5a) is the smallest and shows the lowest rostrum (Table 3). The nasals have only the posterior half-inflated and the nasofrontal suture is not close to the postorbital processes in dorsal view. The interparietal is short and triangular shaped and the fronto-parietal and parietal–interparietal sutures are distant in dorsal view. Some specimens of C. prehensilis (e.g., MZUSP 8456, UFPB 6762, UFPB 9516, UFPB 9790) exhibit the lacrimal sutures not completely obliterated in adults, a unique character among Coendou. The lacrimal sutures are not visible even in neonates of other species. The neonates of C. prehensilis have cream-colored pelage (Figure 12a). The neonates are born with thin white quills on their dorsum covered by villous furs. The body sides and paws have a lighter cream-colored fur pattern than the dorsum. The skin of the tail and paws are dark grayish.
Distribution
Coendou prehensilis is restricted to the north portion of the Atlantic Forest, where its south limit is the right margin of the São Francisco River, region known as the Pernambuco Endemism Centre (Figure 9). Examined specimens were collected in three Brazilian States: Paraíba (cities of Cabedelo, João Pessoa, Mamanguape, Sapé, Rio Tinto and Mataraca), Pernambuco (Igarassu and Sinharém), and Alagoas (Penedo, Murici).
Remarks
Coendou prehensilis is the type species of the genus Coendou Lacépède, 1799. Except for two studies (Feijó & Langguth, 2013; Leite et al., 2011), all previous papers referring to “Coendou prehensilis” actually describe and present data from Coendou longicaudatus (Menezes et al., 2020; Moraes-Santos, 1997; Ramírez-Chaves et al., 2020; Roberts et al., 1985) and even C. baturitensis (as in Moraes-Santos, 1997). Therefore, natural history information about this species is virtually unknown.
- Coendou (Coendou) longicaudatus Daudin, 1802
- Long-Tailed Porcupine
Type
No type specimen available.
Type locality
Cayenne, French Guiana.
Etymology
Reference to the long tail of ‘Le Coendou’ of Buffon (1789, p. 305): ‘Le Coendou à longue queue’ (the long-tailed coendou).
Diagnosis
Largest species of genus Coendou with a long tail (Table 2). The nasals have only the posterior half-inflated. The lacrimal sutures are ever obliterated, even in neonate specimens. See under subspecies for detailed diagnosis.
Distribution
This species is widely distributed in the Amazonia and Cerrado biomes, with two confirmed records for the Bolivian Chaco.
See more under subspecies.
Subspecies
Type
No type specimen available.
Type locality
Cayenne, French Guiana.
Etymology
Reference to the long tail of ‘Le Coendou’ of Buffon (1789, p. 305): ‘Le Coendou à longue queue’ (the long-tailed coendou).
Diagnosis
The Amazonian long-tailed porcupine shows a dark brown with short white dashes general color pattern in dorsal view (Figure 11b). This general color pattern is due to the presence of some bicolored quills among the tricolored quills of the dorsal crest and short whitish B3 of the tricolored quills. The B2 of tricolored quills is dark brown and comprises about half of the total quill length (Figure 7b). The B3 is most whitish and sometimes light yellowish around the rump. The B1 of anterior quills is light yellowish and the posterior quills exhibit a B1 very yellowish, mainly on the rump (Figure 8b). The dorsal crest tricolored quills are shorter than 10 cm and slightly curved. Cranially, the nasofrontal suture is not close to the postorbital processes in dorsal view (Figure 5b). The interparietal is short and triangular shaped in dorsal view. The auditory bullae can be linked to the alisphenoid. The neonate of this subspecies exhibits rust-brown long soft fur (Figure 12b), delicate bicolored quills with a gradient in which its basal third is whitish and the two distal thirds are rust-brown. Most of the quills are dirty white colored distributed on the entire dorsal surface, without any differentiation in color bands. In addition, rare tricolored quills can be found on the basal portion of the tail. These tricolored quills have dirty whitish B1 and B3 with a very short blackish B2.
Distribution
This taxon is widely distributed in the Amazon Forest, east of the Andes (Figure 9). The examined specimens are limited to the west bank of the Xingu River.
Remarks
Type
BMNH 47.11.22.6. It consists of a skin and skull collected by Mr. Bridges.
Type locality
“Bolivia”, probably near Santa Cruz de la Sierra, Bolivia (suggested by Voss, 2011).
Etymology
The specific epithet refers to the country of origin of the type specimens, Bolivia.
Diagnosis
This subspecies is the largest and heaviest South American porcupine (Figure 11c). It shows a brown and white mixed general color pattern (Figure 8c). The dorsal crest is composed mostly of tricolored quills with rare or no bicolored quills. The B3 of tricolored quills is whitish and relatively long (when compared with the B3 of C. l. longicaudatus) (Figure 7c). The B2 is shorter than B1 and has a lighter brown color than the B2 of C. prehensilis and C. l. longicaudatus. The B1 is generally whitish but yellowish in the rump. The tricolored quills in the dorsal crest are longer than 10 cm and very curved. The nasal is short and the parietal is long. Some individuals exhibit the auditory bullae dorsally in contact with the alisphenoid forming a foramen. Cranially, C. l. boliviensis has a shorter zygomatic length, larger upper molars row, and wider nasal aperture in comparison with C. l. longicaudatus. The villous pelage of neonate of C. l. boliviensis completely covers the quills. The neonate pelage is orangish and the quills are thin and mostly white (Figure 12c). The subadults show a light brownish villous pelage mixed with the dorsal quills; this pelage is conspicuous on the rump region. The lacrimal sutures are not visible in any development stage. The skull of the neonate has a large uninflated rostrum. No diagnostic feature is present on neonate skulls.
Distribution
This subspecies seems to be strictly associated with forested vegetation in the diagonal open/dry formations of South America. Our records include individuals from both Bolivian Chaco and Brazilian Cerrado (Figure 9). We did not find any evidence of this taxon in the Caatinga biome and the Atlantic Forest at the right bank of the São Francisco River.
Remarks
Cercolabes (Synetheres) platycentrotus Brandt, 1835, was also applied to individuals from central Brazil (Miranda Ribeiro, 1936; Tate, 1935; Waterhouse, 1848) and it is an older name than C. boliviensis (Gray, 1850) and would have priority. However, Brandt (1835) considers Cercolabes platycentrotus very similar to Cercolabes prehensilis, the only difference is the longitudinal grooves in the quills of C. platycentrotus, which Voss (2011) considered an artifact of preservation or a developmental pathology of the holotype. Because of that uninformative description, the fading color of the quills of the holotype, and the lack of a place of origin of C. platycentrotus, the name was treated as nomen dubium by Voss (2011), with which we agree.
Type material
The holotype (UFPB 6809) is an adult specimen collected by H. Fernandes–Ferreira on August 21, 2012 (Figure S4). It consists of a skull and mandible with all teeth (Figure 5d). The paratype (MNRJ 34504) consists of a skull with all teeth and a flat skin of an adult female collected by Alfonso Mota on January 19, 1954.
Type locality
The holotype was collected in the Community Sítio Barreiros, municipality of Aratuba, Ceará state, Brazil. This site is part of the Baturité range.
Etymology
The specific epithet refers to the locality of origin of the type specimens, Baturité range.
Diagnosis
The Baturité’s Porcupine is a medium to large-sized species (Table 2, Figure 11d). The dorsal crest is composed of a homogeneous mixture of long tricolored and bicolored quills. The B2 is light brown, the same brown tone presented in the B2 of most specimens of C. l. boliviensis. The B2 is longer than B1 in tricolored and bicolored quills. The B3 is the same length or slightly longer than B1 in the long tricolored quills (Figure 7d). In short tricolored quills, the B3 is sometimes shorter than B1 or about the same length. The long B2 of tricolored quills and the abundant short bicolored quills present in the dorsal crest give this species an overall brown background color pattern with some whitish dashes (Figure 8d). Adults of C. baturitensis present tricolored quills longer than 10 cm and very curved. The ventral surface has a general color pattern mixed between brownish and grayish tones, with some individuals with strong rust-brownish tones. The nasofrontal inflation is complete and the rostrum is the highest among the other species of the complex (Figure 5d). The nasofrontal suture is located near to the postorbital process in dorsal view. The interparietal is wide and the frontoparietal and parietal–interparietal sutures are close in dorsal view. The lacrimal sutures are never evident in any ontogenetic stage. The neonate of C. baturitensis has a white woolly pelage that covers the dorsum, with no visible quills (Figure 12d). Its belly has a whitish or light brownish pelage. The juveniles of this species have white villous pelage with furs uniformly spread over the dorsum among the quills. This white pelage disappears with maturation and is completely absent in adults. During the development, the density of villous fur of the ventral surface reduces and is replaced by the hard-spiny pelage of adults. The dark gray tail has few ontogenetic variations. The most remarkable variation is the increase of quill density during maturation. The tegument of snout, limb, and tail present the same general coloration in neonates, juveniles, subadults, and adults. The lacrimal sutures are not visible in neonate skulls.
Distribution
The species occurs in eastern Amazon (Pará and Maranhão states) and montane forested enclaves (Brejos de altitude) in Ceará state in Northeastern Brazil. This species appears restricted to forested areas. It was previously known only for the Baturité Range (its type locality). Here, we register the presence of C. baturitensis in new localities of Ceará, Maranhão, and Pará states (Figure 9). These new localities increase in about 1460 km westward from the previously known distribution of the species. We confirmed the presence of C. baturitensis through collected quills in Pacatuba, Ceará. The presence of C. baturitensis for the Ibiapaba region, Ceará, was confirmed by the capture of one living specimen from Ubajara which was released with some quills collected (UFPB 9392) and by a neonate from São Benedito (MNRJ 75670) (Figure 12d). This neonate was anteriorly used only to document the presence of the genus in the Ibiapaba Range (Feijó & Langguth, 2013).
Remarks
Voss (2015) considered C. baturitensis synonymy of C. prehensilis, arguing it falls within the variation of the widespread C. prehensilis, opinion followed by Gutiérrez and Marinho-Filho (2017). However, none of these authors have examined individuals of C. baturitensis. Here, we presented additional morphological diagnostic traits and confirmed the monophyly of the species.
Moojen (1952, p. 104) placed the type locality of Cercolabes tricolor Gray (1850) to Igarapé-Açu, a municipality in the east portion of the Pará State, within the distribution of C. baturitensis here documented. However, the actual collection locality of C. tricolor is unknown (Voss, 2011) and Moojen (1952) when describing “C. tricolor” provides characters of C. (S.) nycthemera, a sympatric species with C. baturitensis in Pará.
ACKNOWLEDGMENTS
We thank Alexandra Bezerra and José de Sousa e Silva Jr (MPEG), Jader Marinho-Filho (UnB), Juliana Gualda and Luís Fábio Silveira (MZUSP), Diego Astúa (UFPE), João Alves de Oliveira (MNRJ), Robert Voss and Eileen Westwig (AMNH), and Bruce D. Patterson (FMNH) for their assistance with the specimens preserved under their supervision. We are very grateful to Leandro Rodrigues and Roberto Otoch for their support by collecting samples of living specimens. We would like to thank Jamille Martins Forte, Maria das Graças Lima Coêlho, Renata Pinheiro Chaves, and Prof. Dr. Rodrigo Maggioni of Labomar-UFC for the sequencing of porcupine specimens and Dr. Edvar Monteiro Júnior for the help provided during the process of extracting DNA from specimens. Special thanks to the photographers Marco Antonio de Freitas, Carlos A. Aya, João Marcos Rosa, and Sanjay Veiga who provided the pictures of the porcupine specimens in nature and Thais Kubik Martins who sent the pictures of the neonate specimens during the pandemic. We are grateful to Alexei V. Abramov for providing photographs and information on the type of Cercolabes platycentrotus. During the development of this study, Fernando Heberson Menezes was supported by a scholarship provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Anderson Feijó was supported by CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Sandwich Scholarship (Grant Number 01129/2015-9) at the FMNH, AMNH Grants Program (Collection Study), Visiting Scholarship from the Field Museum of Natural History, by the Chinese Academy of Sciences President's International Fellowship Initiative (grants numbers. 2018PB0040/2021PB0021), and by the second Tibetan Plateau Scientific Expedition and Research Program (2019QZKK0402/2019QZKK0501).
APPENDIX 1
Examined Specimens of Erethizontidae
†Sequenced specimens
Chaetomys subspinosus
BRAZIL Bahia: MNRJ 11202, MNRJ 11459, MNRJ 11460, MNRJ 11461, MNRJ 11462, MNRJ 11463, MNRJ 11464, MNRJ 11465, MNRJ 50682, MNRJ 81959, MNRJ 81960, MNRJ 81961, MNRJ 9680—Ilhéus (14°48′S, 39°02′W). MNRJ 46250—Nova Viçosa, Helvecia 1 (17°53′S, 39°23′W). Espírito Santo: MNRJ 81127—Aracruz, Rodovia Aracruz–Comboios, Km 6 (19°49′S, 40°15′W). MNRJ 8278—São José River (no GPS information). Minas Gerais: MNRJ 50683—Serra do Caparaó (20°27′S, 41°50′W). Unknown localities: MNRJ 34503, MNRJ 81958, MNRJ 81962.
Erethizon dorsatum
UNITED STATES OF AMERICA New Hampshire: MNRJ 81956—Carroll Municipality (43°52′N, 71°12′W). Unknown localities: MNRJ 898.
Coendou baturitensis
BRAZIL Ceará: UFPB 6809—Aratuba, Comunidade Barreiros (4°25′S, 39°1′W). UFPE 2387—Baturité. UFPB 9390, UFPB 9391†, UFPB 9780†, UFPB 9781†—Mulungu, Maciço de Baturité (4°18′S, 39°0′W). UFPB 9518—Pacatuba, Trilha do Letreiro, RPPN Sítio Monte Alegre (3°57′14.35″S, 38°37′23.29″W). MNRJ 34504—Pacoti (4°13′S, 38°55′W). MNRJ 75670—São Benedito, Cinta da Solidade (4°02′S, 40°50′W). UFPB 9392—Ubajara, Igreja de Ubajara (3°52°16″S, 40º54′58″W). Maranhão: MPEG 20214—Grajaú, Rod. Transmaranhão, Km 36, Faz. Sr. Adoam. (5°48′27.24″, 46°9′27.23″W). Pará: MPEG 38334—Abaetetuba (1°43′S, 48°52′W). MPEG 418, MPEG 421, MPEG 6623, MPEG 8853, MPEG 9137, MPEG 11837—Belém, Jardim Zoobotânico do Museu Paraense Emílio Goeldi (1°27′S, 48°28′W). MPEG 38854—Melgaço, FLONA Caxiuanã (1°47′S, 51°26′W). MPEG 30679—Paragominas (3°0′S, 47°21′W). MZUSP 13486—Santo Antônio do Tauá (01°8′S, 48°09′W). MPEG 8872, MPEG 11876, MPEG 11899, MPEG 12316, MPEG 12598—Tucuruí (3°48′S, 49°39′W).
Coendou bicolor
BRAZIL Amazonas: MPEG 24574, MPEG 37122—Uarini, Reserva de Desenvolvimento Sustentável Mamirauá (2°12′S, 65°42′W).
Coendou ichillus
BRAZIL Amazonas: MZUSP 11465—Japurá (1°49′ S, 66°35′ W). ECUADOR Pastaza: AMNH 126171, Pastaza River (No other information available).
Coendou insidiosus
BRAZIL Bahia: MNRJ 1361—Feira de Santana (13°14′S, 39°00′W). MNRJ 11210, MNRJ 11211, MNRJ 11466, MNRJ 11467, MNRJ 68199—Ilhéus (14°48′S, 39°02′W). MNRJ 55527—Porto Seguro (16°26′S, 39°04′W). Espírito Santo: MNRJ 8277 –São José, Rio São José (19°17′S, 40°08′W). MNRJ 68200 (the exact locality is unknown).
Coendou longicaudatus longicaudatus
BRAZIL Acre: MZUSP 7345—Iquíri River (the exact location is unknown). Amazonas: MNRJ 30481, MNRJ 30490—Barreirinha, Ariaú River, right bank of Negro River (3°12′S, 57°15′W). MPEG 41760—Jutaí, Roscujubim (4°24′S, 68°31′W). MZUSP 5040—Silves (2°51′S, 58°27′W). Pará: MZUSP 20931—Altamira, Cachoeira do Espelho, Xingu River (3°39′S, 52°22′W). MZUSP 25232—Barreira, Tapajós River (no information about this locality could be recovered). MNRJ 7657—Belterra, Santarém (2°28′S, 54°42′W). MZUSP 5042—Bravo (no information about this locality could be recovered). MZUSP 25230—Uruá, Parque Nacional da Amazônia (4°2′N, 51°12′W). MNRJ 2671—Villa Braga, Rio Tapajós (4°25′S, 56°18′W). Rondônia: UFPB 1205, UFPB 1206, UFPB 1207, UFPB 1208, UFPB 1129, UFPB 1230, UFPB 1231, UFPB 1232, UFPB 1233, UFPB 1237, UFPB 1279, UFPB 1280, UFPB 1282, UFPB 1374—Candeias do Jamari, UHE Samuel, Jamari River (8°45′1″S, 63°27′20″W). Roraima: FMNH 20031—Boa Vista, Rio Branco (2°51′N, 60°37′4W). MNRJ 70735—Caracaraí, PARNA Viruá (1°43′N, 61°09′W). MZUSP 13683—Catrimani River (the exact location is unknown). Unknown localities: MPEG 22481, MPEG 22482. COLOMBIA Magdalena: AMNH 15460—Santa Marta, Bonda (11°14′N, 74°7′W). Meta: FMNH 87897, AMNH 136311—Villavicencio (4°3′N, 73°37′W). ECUADOR Pastaza: Puyo, FMNH 43290—Rio Capihuara (1°29′S, 78° 0′W). PERU Maynas: FMNH 86915, FMNH 86916, FMNH 86917—Santa Cecilia (3°36′S, 72°57′W). Pasco: FMNH 41205—Pozuzo (10° 0′S, 75°33′W). TRINIDAD AND TOBAGO Trinidad: FMNH 61862, FMNH 61863—Mount Harris (10°29′N, 61° 6′W).
Coendou longicaudatus boliviensis
BOLIVIA Santa Cruz: FMNH 21396—Buena vista (17°27′S, 63°40′W). BRAZIL Bahia: FMNH 21709—Barra (11°2′S, 43°9′W). Goiás: MNRJ 4923, MNRJ 4924, MNRJ 4925, MNRJ 4936, MNRJ 4937, MNRJ 4938, MNRJ 34186—Anápolis (16°19′S, 48°59′W). MZUSP 4271, MZUSP 4272, MZUSP 4273—Cana Brava (13°37′S, 50°28′W). UnB 2802, UnB 2803—Catalão, SETAS UHE Serra do Facão (18°02′46″S, 47°40′30″W). MZUSP 6984—GO–70 (the exact locality is unknown). MNRJ 50162—Minaçu, UHE Serra da Mesa (13°53′52,2″S, 48°19′02″W). MNRJ 2679—Nova Roma, Rio Paraná (13°46′S, 47°03′W). MNRJ 2672—São Domingos (13°24′S, 46°19′W). MNRJ 2670—São Miguel River (unknown). MNRJ 2665 (The exact locality is unknown). Minas Gerais: UFPB 9410, UFPB 9411—Indianópolis, UHE Miranda (18°54′33″S, 48°02′31″W). MNRJ 29080—Matias Cardoso, Santa Idália Farm (14°39′S, 44°10′W). UFPB 1616, UFPB 1617—Nova Ponte, UHE Nova Ponte, Araguari River (19°07′S, 47°41′W). MZUSP 3115—Pirapora (17°21′S, 44°55′W). MNRJ 2681—Urucuia, Urucuia River (16°13′S, 45°33′W). Mato Grosso: MNRJ 64213, MNRJ 64569—Barão de Melgaço, RPPN SESC Pantanal (16°11′S, 55°57′W). MNRJ 930—Cárceres, Jauru River (16°04′S, 57°42′W). MZUSP 32335, UnB 1756, UnB 1757†, UnB 1758†, UnB 1759—Chapada dos Guimarães, UHE Manso (14°52′S, 55°48′W). MZUSP 6983—Cocalinho, Lago Dumbá Grande (14°29′26.89″S, 50°59′8.16″W). MZUSP 6357—Palmeiras (12°16′S, 51°51′W). MNRJ 252 (the exact locality is unknown). Mato Grosso do Sul: MNRJ 65561—Aquidauana, Foz do Rio Negro (20°29′S, 55°46′W). MNRJ 81806—Miranda (20°14′S, 56°23′W). MZUSP 1859—Porto Faia (no information about this locality could be recovered). Piauí: MZUSP (ARP 132)—Guaribas, PARNA Serra das Confusões (9°07′S, 43°45′W). UFPB 9589—São Raimundo Nonato, PARNA Serra da Capivara, Caldeirão do Gato (8°47′S, 42°37′W).
Coendou melanurus
BRAZIL Roraima: UFPB 3001†—São José da Baliza, UHE Jatapu, Left Bank of Japatu River (0°48′06″S, 59°16′59″W).
Coendou nycthemera
BRAZIL Pará: MPEG 6622, MPEG 6625, MPEG 6627, MPEG 6628, MPEG 8784, PEG 22451, MPEG 22478, MPEG 22479—Belém, Jardim Zoobotânico do Museu Paraense Emílio Goeldi (1°27′S, 48°28′W). MNRJ 7652, MNRJ 7653, MNRJ 7654, MNRJ 81956—Belterra, Santarém (2°28′S, 54°42′W). MZUSP 5031, MZUSP 5034, MZUSP 5036, MZUSP 5039—Cometá (2°14′S, 49°30′W). MNRJ 4913, MNRJ 4914, MNRJ 4915, MNRJ 4916, MNRJ 4917, MNRJ 4918, MNRJ 4919, MNRJ 4920, MNRJ 4921, MNRJ 72043—Curralinho (1°48′S, 49°48′W). MPEG 24191, MPEG 24192—Igarapé–Miri (1°58′S, 48°57′W). MPEG 38377—Juruti, Porto da ALCOA (2°9′S, 56° 5′W). MZUSP 25591—Santa Teresinha, BR–010 Km 87–94 (1°18′S, 47°43″W). MPEG 12496—Tucuruí (3°48′S, 49°39′W).
Coendou prehensilis
BRAZIL Alagoas: MZUSP 7531—Penedo, Manimbu (10°10′S, 38°22′W). Paraíba: UFPB 931, UFPB 932, UFPB 9412†, UFPB 9789—João Pessoa (7°09′S, 34°51′W). MZUSP 8456—Mamanguape, Uruba (6°50′S, 35°08′W). UFPB 10896—SEMA 1, REBio Guaribas (6°41′29.54″S, 35°7′25.63″W), UFPB 7299, UFPB 9516, UFPB 9790, UFPB 10689—Mamanguape, SEMA 2, REBio Guaribas (6°42′S, 35°10′W). UFPB 7301—Mataraca, Millenium Miner (6°35′S, 34°58′W). UFPB 9517, UFPB 9785, UFPB 9787, UFPB (FHM 30)—Santa Rita, RPPN Engenho Gargaú (6°59′52″S, 34°57′30″W). UFPB 6762—Sapé, RPPN Pacatuba (7°02′S, 35°09′W). UFPB 9791—Rio Tinto, Mata do Oiteiro (69°50′15.6″S, 34°55′21.2″W). UFPB 9590, UFPB 9592, UFPB (FHM 23)—Rio Tinto, SEMA 3, REBio Guaribas (6°48′S, 35°5′W). Pernambuco: UFPB 10688—Aldeia dos Camarás, Camaragibe (7°56′03.6″S 35°01′11.1″W). UFPE 1770—Igarassu (7°52′18.67″S, 34°54′46.02″W). MNRJ 73383†—Sinharém, Usina Trapiche, Mata Gindai (8°38′50″S, 35°10′15″W). Unknown localities: UFPB s/n 1.
Coendou quichua
COLOMBIA Cundinamarca: AMNH 73679—San Juan de Rioseco (4°50′N, 74°37′W). ECUADOR Esmeraldas: AMNH 33242—Esmeraldas (0°58′N, 79°39′W). Pichincha: AMNH 46539—Quito (0°10′S, 78°28′W).
Coendou speratus
BRAZIL Alagoas: MNRJ 81600—Viçosa (9°21′S, 36°13′W). Pernambuco: MNRJ 72045†—Sinharém, Usina Trapiche, Mata Gindai (8°38′50″S, 35°10′15″W).
Coendou spinosus
BRAZIL Espirito Santo: MNRJ 78937†—Santa Tereza, Várzea Alegre, Fazenda Dora Francisca Loss (19°49′S, 40°40′W). Minas Gerais: MNRJ 1365—Viçosa (20°45′S, 42°52′W). MNRJ 69781—Além Paraíba, Fazenda Cachoeirão (21°51′S, 42°42′W). UFPB 1618, UFPB 2014, UFPB 2999, UFPB 3000, UFPB 3002, UFPB 3003, UFPB 3004—Nova Ponte, UHE Nova Ponte, Araguari River (19°07′S, 47°41′W). Rio de Janeiro: MNRJ 19327—Petrópolis (22°30′S, 43°10′W). MNRJ 59613—Carmo, Providência Farm (21°36′S, 42°36′W). MNRJ 75961—Sumidouro, Vale do Encanto (22°02′S, 42°40′W). MNRJ 7260—Teresópolis (22°25′S, 42°56′W). São Paulo: MZUSP 1177—Franca (20°31′S, 47°25′W). MZUSP 327—Osasco (23°31′S, 46°48′W). UFPB 9593—São João da Boa Vista, Sítio Mamonal, Pico do Gavião (21º58′S, 46°47′W). MZUSP 2342—São Paulo (23°38′S, 46°37′W). MZUSP 1816, MZUSP 1817, MZUSP 1819—Ubatuba (23°25′S, 45°05′W). Santa Catarina: MPEG 22198, MPEG 22227—Campos Novos, Rio Marombas (27°29′S, 51°12′W). MPEG 22225—Concórdia, B. Jacutinga (27°14′S, 52° 1′W). Unknown localities: MZUSP (w/number)
Acronym of Collections of Mammals of the sequenced specimens
AMNH—American Museum of Natural History, New York, USA.
ASNHC—Angelo State Natural History Collection, San Angelo, USA.
EBRG—Estación Biológica de Rancho Grande, Maracay, Venezuela.
FMNH—Field Museum of Natural History, Chicago, USA.
IAvH—Instituto Alexander von Humboldt, Bogota, Colombia.
INPA—Instituto Nacional de Pesquisas da Amazônica, Manaus, Brazil.
KU—Biodiversity Research Center da Universidade do Kansas, Lawrence, USA.
LACM—Los Angeles Country Museum, Los Angeles, USA.
MCNU—Museu de Ciências Naturais da Universidade Luterana do Brasil, Canoas, Brazil.
MNHN—Muséum National d’Histoire Naturelle, Paris, França.
MHNLS—Museo de Historia Natural La Salle, Caracas, Venezuela.
MNRJ—Museu Nacional, Rio de Janeiro, Brazil.
MUSM—Museo de Historia Natural de La Universidad Nacional Mayor de San Marcos, Lima, Peru.
MVZ—Museum of Vertebrate Zoology da Universidade da Califórnia, Berkeley, USA.
MZUSP—Museu de Zoologia da Universidade de São Paulo, São Paulo, Brazil.
TTU—Museum of Texas Tech University, Lubbock, USA.
UFES—Coleção da Universidade Federal do Espírito Santo, Vitória, Brazil.
UFMG—Coleção da Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
UFPB—Coleção da Universidade Federal da Paraíba, João Pessoa, Brazil.
UFPE—Coleção da Universidade Federal de Pernambuco, Recife, Brazil.
UnB—Coleção da Universidade de Brasília, Destrito Federal, Brazil
UMMZ—University of Michigan Museum of Zoology, Ann Arbor, USA.
USNM—National Museum of Natural History, Washington, USA.
ZINAS—Zoological Institute of the Russian Academy of Sciences, St. Petersburg, Russia.
Manso—Mammals Collected in Manso Hydroelectric Dam, Chapada dos Guimarães, Mato Grosso, Brazil.
FHM—Field Number of F.H. Menezes.
MNFS—Field Number of M.N.F. da Silva (see Lara et al., 1996).
APPENDIX 2
| GenBank accession number | Species | Voucher |
Sequence size (bp) |
Locality | Source |
|---|---|---|---|---|---|
| KY784123 | Coendou baturitensis | UFPB 9391 | 827 | Mulungu, Ceará, Brazil | This work |
| KY784124 | Coendou baturitensis | UFPB 9780 | 1140 | Mulungu, Ceará, Brazil | This work |
| KY784125 | Coendou baturitensis | UFPB 9781 | 1140 | Mulungu, Ceará, Brazil | This work |
| KC463857 | Coendou bicolor | AMNH 214612 | 682 | Rio Mamoré, Beni, Bolivia | Voss et al., 2013 |
| KC463858 | Coendou bicolor | KU 144560 | 1140 | Cajamarca, Peru | Voss et al., 2013 |
| KC463859 | Coendou bicolor | MUSM 9398 | 1140 | Madre de Dios, Peru | Voss et al., 2013 |
| KC463860 | Coendou bicolor | FMNH 203679 | 1140 | San Martím, Peru | Voss et al., 2013 |
| KC463861 | Coendou ichillus | TTU 115491 | 1027 | Loreto, Peru | Voss et al., 2013 |
| KC261591 | Coendou insidiosus | UFES 136 | 801 | Nova Viçosa, Bahia, Brazil | Mendes Pontes et al., 2013 |
| NC_021387 | Coendou insidiosus a | – | d | – | Voloch et al. 2013 |
| AF411581 | Coendou longicaudatus boliviensis | UnB 1757 (Manso 212) | 1132 | UHE Manso, Mato Grosso, Brazil | Bonvicino et al., 2002 |
| AF411582 | Coendou longicaudatus boliviensis | UnB 1758 (Manso 138) | 1113 | UHE Manso, Mato Grosso, Brazil | Bonvicino et al., 2002 |
| AF411584 | Coendou longicaudatus boliviensis | Manso 849 | 1123 | UHE Manso, Mato Grosso, Brazil | Bonvicino et al., 2002 |
| KC463873 | Coendou longicaudatus boliviensis | AMNH 262274 | 1140 | San Ramon, Bolivia | Voss et al., 2013 |
| U34851 | Coendou longicaudatus longicaudatus | INPA 3919 (MNFS 1016) | 1140 | Fazenda Santa Fé, Acre, Brazil | Lara, Patton, and da Silva 1996 |
| U34852 | Coendou longicaudatus longicaudatus | MVZ 195088 (MNFS 439) | 1140 | Eirunepé, Amazonas, Brazil | Lara et al. 1996 |
| KC463866 | Coendou longicaudatus longicaudatus | USNM 560869 | 802 | Cerro Neblina Base Camp, Amazonas, Venezuela | Voss et al., 2013 |
| KC463867 | Coendou longicaudatus longicaudatus | AMNH 273130 | 762 | Nuevo San Juan, Loreto, Peru | Voss et al., 2013 |
| KC463868 | Coendou longicaudatus longicaudatus | MUSM 15324 | 762 | Nuevo San Juan, Loreto, Peru | Voss et al., 2013 |
| KC463869 | Coendou longicaudatus longicaudatus | MVZ 155200 | 768 | Huampami, Amazonas, Peru | Voss et al., 2013 |
| KC463870 | Coendou longicaudatus longicaudatus | MVZ 155201 | 772 | Huampami, Amazonas, Peru | Voss et al., 2013 |
| KC463871 | Coendou longicaudatus longicaudatus | MVZ 191349 | 772 | Rio Juruá, Ocidente, Acre, Brazil | Voss et al., 2013 |
| KC463872 | Coendou longicaudatus longicaudatus | INPA 2875 | 788 | Eirunepé, Amazonas, Brazil | Voss et al., 2013 |
| KC463874 | Coendou longicaudatus longicaudatus | MNHN 1997.643 | 795 | Petit Saut, French Guiana | Voss et al., 2013 |
| KC463875 | Coendou longicaudatus longicaudatus | EBRG 23415 | 798 | Río Caroní, Bolívar, Venezuela | Voss et al., 2013 |
| KC463876 | Coendou longicaudatus longicaudatus | USNM 281898 | 802 | Valledupar, Cesar, Colombia | Voss et al., 2013 |
| KC463877 | Coendou longicaudatus longicaudatus | USNM 281904 | 678 | Valledupar, Cesar, Colombia | Voss et al., 2013 |
| KC463878 | Coendou longicaudatus longicaudatus | USNM 443409 | 802 | Las Mesas, Táchira, Venezuela | Voss et al., 2013 |
| KC463879 | Coendou longicaudatus longicaudatus | USNM 528360 | 1140 | Limoncocha, Sucumbíos, Ecuador | Voss et al., 2013 |
| MG775435 | Coendou longicaudatus longicaudatus | IAvH 123 | 1140 | Toledo, Norte de Santander, Colombia | Torres-Martínez et al., 2019 |
| AF411583 | Coendou melanurus | UFPB 3001 | 786 | São João da Baliza, Roraima, Brazil | Bonvicino et al., 2002 |
| KC463862 | Coendou melanurus | MNHN 1997.641 | 1140 | Barragem Petit Saut, French Guiana | Voss et al., 2013 |
| KC463863 | Coendou mexicanus | ASNHC 6407 | 1140 | Campeche, Mexico | Voss et al., 2013 |
| KC261597 | Coendou nycthemera | UFES 2079 | 801 | UHE Estreito, Tocantins, Brazil | Mendes Pontes et al., 2013 |
| KC463864 | Coendou nycthemera | USNM 519690 | 795 | Ilha de Marajó, Pará, Brazil | Voss et al., 2013 |
| KC463865 | Coendou nycthemera | USNM 519692 | 794 | Ilha de Marajó, Pará, Brazil | Voss et al., 2013 |
| KY784126 | Coendou prehensilis | UFPB 9412 | 1119 | João Pessoa, Paraíba, Brazil | This work |
| HM462243 | Coendou prehensilis | MNRJ 73383b | 801 | Usina Trapiche, Pernambuco, Brazil | Leite et al., 2011 |
| KC463880 | Coendou pruinosus | MHNLS 7692 | 259e | Zulia, Venezuela | Voss et al., 2013 |
| KC463881 | Coendou quichua | KMH 2218 | 1114 | Cotopaxi, Ecuador | Voss et al., 2013 |
| KC463882 | Coendou quichua | LACM 27376 | 1140 | Cesar, Colombia | Voss et al., 2013 |
| KC463883 | Coendou quichua | USNM 296308 | 682 | Zona Canal, Panama | Voss et al., 2013 |
| KC463884 | Coendou rufescens | AMNH 181483 | 814 | Cauca, Colombia | Voss et al., 2013 |
| KC261592 | Coendou speratus | UFPE 1708 | 801 | Usina Trapiche, Pernambuco, Brazil | Mendes Pontes et al., 2013 |
| KC261593 | Coendou speratus | UFPE 1709 | 801 | Usina Trapiche, Pernambuco, Brazil | Mendes Pontes et al., 2013 |
| KC261594 | Coendou speratus | MNRJ 72046 | 801 | Usina Trapiche, Pernambuco, Brazil | Mendes Pontes et al., 2013 |
| KC261595 | Coendou speratus | MNRJ 72045c | 801 | Usina Trapiche, Pernambuco, Brazil | Mendes Pontes et al., 2013 |
| KC261596 | Coendou speratus | UFES 1184 | 801 | Usina Trapiche, Pernambuco, Brazil | Mendes Pontes et al., 2013 |
| KC463885 | Coendou spinosus | UNMZ (GD 252) | 1112 | Itapúa, Paraguay | Voss et al., 2013 |
| KC463886 | Coendou spinosus | UMMZ 174975 | 1112 | Caazapá, Paraguay | Voss et al., 2013 |
| KC463887 | Coendou spinosus | UFMG 3043 | 1140 | Sorocaba, São Paulo, Brazil | Voss et al., 2013 |
| AF407277 | Coendou spinosus | MNRJ 46938 | 1071 | Rio das Ostras, Rio de Janeiro, Brazil | Bonvicino et al., 2002 |
| AF411580 | Coendou spinosus | MNRJ 46937 | 1106 | Sumidouro, Rio de Janeiro, Brazil | Bonvicino et al., 2002 |
| EU544661 | Coendou spinosus | CIT1326 | 1140 | UHE Rosal, Espírito Santo, Brazil | Vilela et al. 2009 |
| EU544662 | Coendou spinosus | MZUSP 35142 | 1140 | Biritiba Mirim, São Paulo, Brazil | Vilela et al. 2009 |
| JX312693 | Coendou spinosus a | MNRJ 78937 | d | Santa Tereza, Espírito Santo, Brazil | Voloch et al. 2013 |
| KC463888 | Coendou vestitus | AMNH 70596 | 682 | Cundinamarca, Colombia | Voss et al., 2013 |
| MG383643 | Coendou vestitus | IAvH 7956 | 1121 | Villa de Leyva, Colombia | Ramírez-Chaves et al., 2019 |
| FJ357428 | Erethizon dorsatum | – | 1140 | – | Vilela et al. 2009 |
| KC463889 | Erethizon dorsatum | USNM 568658 | 1140 | – | Voss et al., 2013 |
| EU544660 | Chaetomys subspinosus | MCNU 918 | 1140 | Salvador, Bahia, Brazil | Vilela et al. 2009 |
- a The sequences from Voloch et al. (2013): NC_021387 has no information about its collection locality and the identification of MNRJ 78937 (JX312693) was corrected after examination of the specimen (only skin available). Both sequences are identical, probably due duplicate in GenBank. The sequence NC_021387 is not cited in the work of Voloch et al. (2013), what reinforces the hypothesis of duplicate.
- b Neotype of Coendou prehensilis.
- c Holotype of Coendou speratus.
- d The authors sequenced the complete mitochondrial genome, but we used only 1140 bp of the cytochrome b gene.
- e Not used due small sequence size.
References of the cytochrome b sequences
Bonvicino, C. R., Penna-Firme, V., & Braggio, E. (2002). Molecular and Karyologic Evidence of the Taxonomic Status of Coendou and Sphiggurus (Rodentia: Hystricognathi). Journal of Mammalogy, 83(4), 1071–1076.
Lara, M. C., Patton, J. L., & da Silva, M. N. F. (1996). The simultaneous diversification of South American echimyid rodents (Hystricognathi) based on complete cytochrome b sequences. Molecular Phylogenetics and Evolution, 5(2), 403–413. https://doi.org/10.1006/mpev.1996.0035
Mendes Pontes, A. R., Gadelha, J. R., Melo, É. R. A., de Sá, F. B., Loss, A. C., Caldara Jr, V., Costa, L. P., & Leite, Y. L. R. (2013). A new species of porcupine, genus Coendou (Rodentia: Erethizontidae) from the Atlantic forest of northeastern Brazil. Zootaxa, 3636(3), 421. https://doi.org/10.11646/zootaxa.3636.3.2
Ramírez-Chaves, H. E., Torres-Martínez, M. M., Noguera-Urbano, E. A., Passos, F. C., & Colmenares-Pinzón, J. E. (2019). State of knowledge and potential distribution of the Colombian endemic brown hairy dwarf porcupine Coendou vestitus (Rodentia). Mammalian Biology, 99, 1–11. https://doi.org/10.1016/j.mambio.2019.09.012
Torres-Martínez, M. M., Ramírez-Chaves, H. E., Noguera-Urbano, E. A., Colmenares-Pinzón, J. E., Passos, F. C., & García, J. (2019). On the distribution of the Brazilian porcupine Coendou prehensilis (Erethizontidae) in Colombia. Mammalia, 83(3), 290–297. https://doi.org/10.1515/mammalia-2018-0043
Vilela, R. V., Machado, T., Ventura, K., Fagundes, V., Silva, M. J. de J., & Yonenaga-Yassuda, Y. (2009). The taxonomic status of the endangered thin-spined porcupine, Chaetomys subspinosus (Olfers, 1818), based on molecular and karyologic data. BMC Evolutionary Biology, 9(29), 1–17. https://doi.org/10.1186/1471-2148-9-29
Voloch, C. M., Vilela, J. F., Loss-Oliveira, L., & Schrago, C. G. (2013). Phylogeny and chronology of the major lineages of New World hystricognath rodents: insights on the biogeography of the Eocene/Oligocene arrival of mammals in South America. BMC Research Notes, 6, 160. https://doi.org/10.1186/1756-0500-6-160
Voss, R. S., Hubbard, C., & Jansa, S. A. (2013). Phylogenetic relationships of New World porcupines (Rodentia, Erethizontidae): implications for taxonomy, morphological evolution, and biogeography. American Museum Novitates, 3769, 1–36. https://doi.org/10.1206/3769.2




