Integrative taxonomy and biogeographic affinities of the first freshwater sponge and mollusc association discovered in tropical Asia
Olga V. Aksenova ([email protected]), Yulia V. Bespalaya ([email protected]), Mikhail Y. Gofarov ([email protected]), Alexander V. Kondakov ([email protected]), Ekaterina S. Konopleva ([email protected]), Alena A. Tomilova ([email protected]), Oksana V. Travina ([email protected]), Kitti Tanmuangpak ([email protected]), Sakboworn Tumpeesuwan ([email protected]), Ilya V. Vikhrev ([email protected]), Ivan N. Bolotov ([email protected])
Abstract
Diverse associations of freshwater sponges with molluscs were recently described from the Xingu River, Amazon Basin. However, such associations in other parts of the world are almost unknown. Here, we report on the discovery of epifaunal associations of a freshwater sponge (Corvospongilla, Spongillidae) with a byssus-attaching clam (Limnoperna, Mytilidae) and a freshwater mussel (Hyriopsis, Unionidae) in tropical Asia (Mun River, Thailand). We used this association as a model system to show how the application of an integrative taxonomic approach can change modern views on the taxonomic status and biogeographic affinities of tropical invertebrates in a rather small sample. The freshwater sponge from the Mun River morphologically corresponds to the nominal taxon Corvospongilla siamensis but it is identical to Corvospongilla ultima from the Kaladan River, western Myanmar, based on the sequences of five DNA markers and the PTP species delimitation modeling. Hence, the first species is considered here to be a junior synonym of C. ultima. Conversely, the Limnoperna clam from the Mun River is found to be distant phylogenetically from the invasive golden mussel Limnoperna fortunei based on the sequences of three DNA markers. We therefore restore Limnoperna siamensis stat. rev. as a valid species. Our time-calibrated multi-locus phylogeny reveals that the split between L. siamensis and L. fortunei most likely occurred at the Miocene—Pliocene boundary. Finally, the freshwater mussel Hyriopsis khoratensis is an abundant species endemic to the Mun River. Our novel data highlight that associations of freshwater sponges with bivalves are present in Asia but were historically overlooked there and that the Mekong's sponge-mollusc association can be considered analogous to those discovered in South America.
1 INTRODUCTION
Multiple associations of various animals with sponges (phylum Porifera) were described from marine environments (e.g. Padua et al., 2013; Riberio et al., 2003; Schiaparelli et al., 2003; Skilleter et al., 2005). Conversely, such associations with freshwater sponges (order Spongillida) are poorly understood. A small body of available researches mainly reports on the composition of a few sponge-associated assemblages (Gaino et al., 2004; Gugel, 2001; Kamaltynov et al., 1993; Kharchenko et al., 1989; Konopacka & Sicinski, 1985) and describes the ecology and taxonomy of some sponge-dwelling arthropods and oligochaetes (e. g. Corallini & Gaino, 2001; Fusari et al., 2008; Gorni & Alves, 2008; Hamada et al., 2014; Steffan, 1967; Zitzler & Cai, 2006). Molluscs, though were accidentally found on spongillid sponges (Gaino et al., 2004; Old, 1932), were seldom reported as the symbionts that interact with sponges in some way.
Freshwater bivalves often coexist with sponges due to their common feeding habits. Competition for the substratum is thus the most obvious example of relationships between these animals (Lauer & Spacie, 2004; Pereira et al., 2010; Sokolova & Palatov, 2014; Volkmer-Ribeiro et al., 2010). But there are also examples of other interactions.
Specific sponge and mollusc associations were reported from Brazil (Amazon Basin) and Costa Rica. In these regions, the tiny freshwater clams Eupera cubensis (Prime, 1865) and Eupera sp. (Bivalvia: Sphaeriidae) feed, live, and reproduce inside the body of freshwater sponges (Volkmer-Ribeiro & Machado, 2009; Volkmer-Ribeiro & De Rosa-Barbosa, 1974). Some sponge specimens from the Amazon Basin were found to bear exceptionally large amounts of Eupera, that is, more than 130 clams per sponge (Volkmer-Ribeiro & De Rosa-Barbosa, 1974). These Neotropical associations can also contain freshwater bryozoans as an accessory taxon (Volkmer-Ribeiro & Machado, 2009).
In some cases, bivalves and gastropods inhabiting sandy or soft bottoms serve as the main substrate for sponges (Bonetto & Di Persia, 1975; Volkmer-Ribeiro et al., 2019). Recently, epifaunal associations of freshwater sponges with eleven bivalve and one gastropod species were described from the Xingu River, Brazil (Volkmer-Ribeiro et al., 2019). This tropical river seems to harbor the most diverse and species-rich freshwater sponge—mollusc associations globally. Three main types of these associations can be delineated as follows: IRS molluscs living inside reticulate skeleton of sponge; SGS sponges encrusted on the spire of gastropod shell; and BSA sponges encrusted on the posterior region of bivalve shell surface around siphonal area (Volkmer-Ribeiro et al., 2019: 4, Figure 2).
Three freshwater sponge species encrusting shell surface of the large cementing bivalve Etheria elliptica Lamarck, 1807 (Bivalvia: Etheriidae) were described from tropical Africa (Annandale, 1913). No remark on spatial interactions of these animals was provided, although author highlighted their close relationships by naming one of the new sponge species Spongilla aetheriae Annandale, 1913. Furthermore, Annandale (1913) specifically looked for the freshwater sponges associated with Pseudomulleria dalyi (Smith, 1898) (Bivalvia: Etheriidae), a cementing bivalve from southern India, but did not find them.
A brief review of the body of available literature presented above reveals that freshwater sponge and mollusc associations are known to occur in South America, North America, Africa, and Europe. To the best of our knowledge, none of such associations was recorded in Asia. This study (a) describes the first example of freshwater sponge and mollusc association discovered in tropical Asia (Mekong River basin, Thailand), (b) provides integrative taxonomic and evolutionary overview of sponge and mollusc taxa belonging to the Mekong's association, and (c) discusses the evolutionary origin of a secondary freshwater mollusc species involved in this association.
2 MATERIALS AND METHODS
2.1 Data sampling
In total, we morphologically examined five specimens of the freshwater sponge Corvospongilla ultima (Annandale, 1910) [=Corvospongilla siamensis Manconi & Ruengsawang, 2012 syn. nov.] (Demospongiae: Spongillidae) [two specimens were sequenced]; 18 specimens of the freshwater clam Limnoperna siamensis (Morelet, 1866) [five specimens were sequenced]; four specimens of the freshwater clam Limnoperna fortunei (Dunker, 1857) [all of which were sequenced] (Bivalvia: Mytilidae); and 28 specimens of the freshwater mussel Hyriopsis khoratensis Pfeiffer et al., 2021 (Bivalvia: Unionidae) [eight specimens were sequenced].
The samples of freshwater sponge and mollusc association were collected from a deep pool (depth ca. 5 m) of the Mun River near Tha Tum town (15.3297°N, 103.6821°E) by a local free diver (Table 1 and Figure 1). The images of living specimens were taken using Canon EOS 7D digital camera (Canon Inc.) with Canon EF 100 mm f/2.8L IS USM macro lens (Canon Inc.). Samples of additional mollusc taxa were collected from rivers of Thailand, South Korea, and Vietnam, that is, H. khoratensis and L. fortunei (Table 1).
| Phylum: Class: Family | Species | Locality | Voucher No. | NCBI GenBank acc. No. of reference sequences | |||
|---|---|---|---|---|---|---|---|
| COI | 16S | 28S | ITS1 + 5.8S + ITS2 | ||||
| Porifera: Demospongiae: Spongillidae | Corvospongilla ultima (Annandale, 1910) [=Corvospongilla siamensis Manconi & Ruensawang, 2012 syn. nov.] | Sponge and mollusc association, Mun River, Mekong Basin, Thailand | RMBH Por0003 | MT299941 | N/A | MT301571 | MT301569 |
| Porifera: Demospongiae: Spongillidae | Corvospongilla ultima (Annandale, 1910) | Kaladan River, Myanmar | RMBH Por0001 | MF958996 | N/A | MF959021 | MT301568 |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Sponge and mollusc association, Mun River, Mekong Basin, Thailand | RMBH biv0459 | MT299942 | MT301562 | MT301572 | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Mun River, Mekong Basin, Thailand | RMBH biv0465_1 | MW134723 | N/A | N/A | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Mun River, Mekong Basin, Thailand | RMBH biv0465_2 | MW134724 | N/A | N/A | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Mun River, Mekong Basin, Thailand | RMBH biv0469_1 | MW256654 | N/A | N/A | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Mun River, Mekong Basin, Thailand | RMBH biv0469_2 | MW256655 | N/A | N/A | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Chi River, Mekong Basin, Thailand | RMBH biv0130_1 | KX865951 | KX865697 | KX865822 | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Chi River, Mekong Basin, Thailand | RMBH biv0130_2 | KX865952 | KX865698 | KX865823 | N/A |
| Mollusca: Bivalvia: Unionidae | Hyriopsis khoratensis Pfeiffer et al., 2021 | Chi River, Mekong Basin, Thailand | RMBH biv0130_3 | KX865953 | N/A | KX865824 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna siamensis (Morelet, 1866) stat. rev. | Sponge and mollusc association, Mun River, Mekong Basin, Thailand | RMBH biv0463_1 | MT299943 | MT301563 | MT301573 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna siamensis (Morelet, 1866) stat. rev. | Sponge and mollusc association, Mun River, Mekong Basin, Thailand | RMBH biv0463_2 | MT299944 | MT301564 | MT301574 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna siamensis (Morelet, 1866) stat. rev. | Sponge and mollusc association, Mun River, Mekong Basin, Thailand |
RMBH biv0719_1 |
MT299945 | MT301565 | MT301575 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna siamensis (Morelet, 1866) stat. rev. | Sponge and mollusc association, Mun River, Mekong Basin, Thailand |
RMBH biv0719_2 |
MT299946 | MT301566 | MT301576 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna siamensis (Morelet, 1866) stat. rev. | Sponge and mollusc association, Mun River, Mekong Basin, Thailand |
RMBH biv0719_3 |
MT299947 | MT301567 | MT301577 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna fortunei (Dunker, 1857) | Bukhan River, South Korea | RMBH biv0510 | MT408044 | MT408032 | MT408036 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna fortunei (Dunker, 1857) | Irrigation channel, Mangyeong River basin, South Korea | RMBH biv0525_1 | MT408045 | MT408033 | MT408037 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna fortunei (Dunker, 1857) | Irrigation channel, Mangyeong River basin, South Korea | RMBH biv0525_3 | MT408046 | MT408034 | MT408038 | N/A |
| Mollusca: Bivalvia: Mytilidae | Limnoperna fortunei (Dunker, 1857) | Quang Nam Province, Vietnam | RMBH biv0686_1 | MT408047 | MT408035 | MT408039 | N/A |
- N/A, not available.
2.2 Morphological studies
2.2.1 Morphological studies of freshwater sponges
Sponge fragments were investigated under light microscope (LM) and scanning electron microscope (SEM). For spicule, purification fragments of sponges were placed in potassium dichromate solution for a day, rinsed several times with water and then with ethanol. For SEM, spicules and gemmules were mounted on stubs, coated with gold, and investigated under Tescan Vega TS5130MM and Tescan Vega-II XMU (Tescan Orsay Holding). For LM, clean spicules were mounted in Canada balsam and studied under an Olympus CX21 microscope (Olympus). Measurements were carried out with an ocular micrometer or the ATLAS software; five gemmules and 50 spicules of each spicule type were analyzed.
2.2.2 Morphological studies of freshwater molluscs
Samples of the freshwater byssus-attaching clam (Mytilidae) were studied morphologically based on the approaches of Morton and Dinesen (2010), Morton (2015), and Morton et al. (2020) using a Leica M165C microscope (Leica). We examined several qualitative morphological characters as follows: the external appearance of the shell, anterior and posterior adductor muscle scars, anterior and posterior byssal retractor muscle scars, ctenidial attachment muscle scars, outer demibranch, outer and inner labial palps, exhalant siphon, and inhalant aperture. Samples of the freshwater mussel (Unionidae) were examined using the approaches of Bolotov, Vikhrev, et al. (2017), Bolotov, Pfeiffer, et al. (2018), Bolotov, Konopleva, et al. (2019); Bolotov, Konopleva, et al. (2020) and Pfeiffer et al. (2021). Morphological features of the shell were studied as follows: general shell shape, outlines of the dorsal, ventral, anterior, and posterior margins, umbo position, shape of anterior and posterior adductor muscle scars, and hinge plate with pseudocardinal and lateral teeth.
2.3 Molecular analyses
2.3.1 DNA analyses of freshwater sponges
Total genomic DNA was extracted from 96% ethanol-preserved sponge samples using the NucleoSpin® Tissue Kit (Macherey-Nagel GmbH & Co. KG), according to the manufacturer's protocol. Partial sequences of the mitochondrial cytochrome c oxidase subunit I gene (COI; amplicon length 707 bp), and of the nuclear ribosomal RNA (rRNA) genes: the 28S rRNA gene (28S; amplicon length 817 bp) as well as a region comprising two internal transcribed spacers ITS1 and ITS2 and the 5.8S rRNA gene lying in-between them (hereafter abbreviated ITS1 + 5.8S + ITS2; amplicon length 735 bp) were obtained using appropriate PCR primers (Table 2). For the amplification, we applied marker-specific PCR programs as follows: (a) COI: 95°C (4 min), 28–33 repeats at 95°C (50 s), 48°C (50 s), 72°C (50 s), and 72°C (5 min); (b) 28S: 95°C (4 min), 26–30 repeats at 95°C (50 s), 56°C (50 s), 72°C (50 s), and 72°C (5 min); and (c) ITS1 + 5.8S + ITS2: 95°C (4 min), 35 repeats at 95°C (50 s), 49°C (50 s), 72°C (50 s), and 72°C (5 min). Forward and reverse sequence reactions were executed on an ABI PRISM® 3730 DNA analyzer (Thermo Fisher Scientific Inc.) with the ABI PRISM® BigDye™ Terminator v. 3.1 reagents kit. The sequences were inspected visually with BioEdit v. 7.2.562 (https://bioedit.software.informer.com/).
| Gene fragment | Primer's name | Direction | Sequence (5′–3′) | Reference |
|---|---|---|---|---|
| COI | LoboF1 | Forward | kbtchacaaaycayaargayathgg | Lobo et al. (2013) |
| LoboR1 | Reverse | taaacytcwggrtgwccraaraayca | ||
| 16S | 16Sar | Forward | cgcctgtttatcaaaaacat | Palumbi (1996) |
| 16Sbr | Reverse | ccggtctgaactcagatcacgt | ||
| 28S | C1 | Forward | acccgctgaatttaagcat | Jovelin & Justine (2001) |
| D2 | Reverse | tccgtgtttcaagacgg | ||
| ITS1 + 5.8S + ITS2 | ITS1F | Forward | taacaaggtttccgtaggtg | White et al. (1996) |
| ITS4 | Reverse | tcctccgcttattgatatgc |
2.3.2 DNA analyses of freshwater molluscs
Total genomic DNA was extracted from 96% ethanol-preserved tissue samples as described above. PCR primers listed in Table 2 were used to amplify the following marker sequences: partial sequences of the mitochondrial COI (amplicon length 707 bp) and 16S rRNA (16S; amplicon length 532, 538, and 539 bp) genes, and the nuclear 28S gene (amplicon length 807 and 826 bp). These markers were sequenced following the protocol of Bolotov, Kondakov, et al. (2017) and Bolotov, Vikhrev, et al. (2017). The PCR programs for amplification of the COI and 28S fragments were as those described for sponges (see above). The 16S gene was amplified using the following program: 95°C (4 min), 25–27 repeats at 95°C (50 s), 48°C (50 s), 72°C (50 s), and 72°C (5 min). The sequence generating and inspection followed those applied for sponges (see above).
2.4 Phylogenetic analyses, divergence dating, and biogeographic reconstructions
2.4.1 Phylogenetic analyses and species delimitation modeling of freshwater sponges
It was shown that the mitochondrial DNA of demosponges is characterized by slow substitution rates (Kenny & Itskovich, 2020; Wörheide et al., 2012). Hence, phylogenetic reconstructions based on nuclear markers share better resolution compared with those calculated using mitochondrial genes such as the COI (Meixner et al., 2007). In the present study, two nuclear sequence datasets of Porifera were compiled for phylogenetic analyses: (a) one-marker dataset with 28S (N = 69 haplotype sequences, including two outgroup taxa) (Table S1 and Alignment S1); and (b) three-marker dataset with ITS1 + 5.8S + ITS2 (N = 139 haplotype sequences, including two outgroup taxa; Table S2 and Alignment S2). For the dataset 1, 68 additional 28S haplotypes were collected from published sources using GenBank (Borchiellini et al., 2004; Erpenbeck et al., 2011; Gazave et al., 2010; Idan et al., 2018; Leal et al., 2016; Morrow et al., 2012, 2019; Nichols, 2005; Plotkin et al., 2017; Redmond et al., 2011; Thacker et al., 2013; Wiens et al., 2009; Zhou et al., 2019) (Table S1). In its turn, altogether 137 additional haplotype sequences were sampled from GenBank for the nuclear dataset 2 (Addis & Peterson, 2005; Bukshuk & Maikova, 2020; Itskovich et al., 2008, 2013, 2017; Karlep et al., 2013; Maikova et al., 2010; Sokolova et al., 2020; Wiens et al., 2009) (Table S2).
The sequence datasets were aligned by MUSCLE algorithm of MEGA7 (Kumar et al., 2016) with the lengths as follows: 813 bp of 28S, and 1138 bp of ITS1 + 5.8S + ITS2 fragment. The 28S alignment was additionally processed through the GBlocks Server v. 0.91b (Talavera & Castresana, 2007) to exclude hypervariable positions. The final length of 28S alignment after GBlocks treatment was 389 bp (48% of the original 813 bp).
The maximum likelihood 28S and ITS1 + 5.8S + ITS2 phylogenies were reconstructed using IQ-TREE v. 1.6.11 (Nguyen et al., 2015) with an automatic detection of the best evolutionary model via an online resource of the Center for Integrative Bioinformatics Vienna, Austria (Trifinopoulos et al., 2016). The node support values were estimated using an ultrafast bootstrap algorithm (Hoang et al., 2018).
To delimit the Molecular Operational Taxonomic Units (MOTUs) probably corresponding to biological species, an automatic species delimitation approach was applied. The species delimitation modeling was performed with the Poisson Tree Process (PTP) algorithm via the PTP web service (http://mptp.h-its.org) (Kapli et al., 2017). This method was found to be more appropriate for other slowly evolving animal groups such as freshwater mussels (Bolotov, Konopleva, et al., 2020) and leeches (Bolotov, Klass, et al., 2019). As an input tree, we used the consensus IQ-TREE phylogeny reconstructed based on the nuclear dataset 2 (ITS1 + 5.8S + ITS2; see above). Outgroup taxa were removed using an appropriate option of the PTP server. The final PTP species delimitation model largely aligned with the recent species-level systematics of freshwater sponges (see section 3), and, hence, we concluded that it is a reliable approach to delimit the MOTUs in our dataset.
Genetic differences between available haplotypes of each gene fragment were estimated separately using uncorrected p-distances of MEGA7 (Kumar et al., 2016). The COI gene fragment of 660 bp (Table 1) was used only to calculate p-distance between the haplotypes of C. siamensis and C. ultima.
2.4.2 Phylogenetic analyses, divergence dating, and ancestral environment reconstructions of freshwater byssus-attaching clam (Mytilidae)
The multi-locus sequence dataset (COI + 16S + 28S; total length 1901 bp) of the family Mytilidae contained 16 haplotypes including one outgroup taxon (Table 1, Table S3 and Alignment S3). Among them, 12 haplotypes were obtained from published sources (Hardivillier et al., 2006; Lee et al., 2019; Lorion et al., 2010, 2013; Morton & Dinesen, 2010; Morton et al., 2020; Ozawa et al., 2017; Robicheau et al., 2017; Sharma et al., 2013; Sun et al., 2017) via GenBank (Table S3). The single-gene alignments were processed by MUSCLE algorithm of MEGA7 (Kumar et al., 2016). The 16S and 28S alignments were checked using the Gblocks Server v. 0.91b (Talavera & Castresana, 2007) to exclude hypervariable positions. The final length of alignments was as follows: 659 bp of COI, 469 bp of 16S (70% of the original 670 bp), and 773 bp of 28S (98% of the original 789 bp) fragments. These single-gene alignments were combined to a three-locus alignment using FaBox v. 1.5 (Villesen, 2007). This alignment was used to reconstruct a Bayesian phylogeny with BEAST v. 2.6 (Bouckaert et al., 2019). A lognormal relaxed clock algorithm with the Yule speciation process was applied as the tree priors (Drummond et al., 2006, 2012; Drummond & Rambaut, 2007). The HKY + G evolutionary model was assigned to each partition (see Bolotov, Kondakov, et al., 2017; Bolotov, Vikhrev, et al., 2017 for detail). To calibrate the phylogeny, we used an external COI substitution rate (range = 0.067–0.095% per Myr) that was obtained from marine ark clams (Bivalvia: Arcidae) based on pairs of sister taxa separated by the Isthmus of Panama (Marko, 2002: the rate inferred from calibration 1). The substitution rate was implemented only to the COI partition. The phylogenies were reconstructed at the San Diego Supercomputer Center through the CIPRES Science Gateway (Miller et al., 2010). Three replicate BEAST searches were conducted, each with 5 × 107 generations and a tree sampling every 1000th generation. The log files were checked by eyes with Tracer v. 1.7 for an assessment of the MCMC chains convergence and the effective sample size (ESS) of characters (Rambaut et al., 2018). The tree sets inferred from independent runs were compiled with LogCombiner v. 1.8.4 (Drummond et al., 2012) using a 10% burn-in. The ESS values for all characters in the combined tree set were found as >1000. The maximum clade credibility tree was constructed from the post-burn-in trees using TreeAnnotator v. 1.8.4 (Drummond et al., 2012).
Ancestral habitat patterns of the Mytilidae were reconstructed using RASP v. 3.2 (Yu et al., 2015). The set of time-calibrated binary trees that were combined from the three runs of BEAST v. 2.6 (see above) was used for analyses. As a condensed tree, we used our maximum clade credibility tree (see above). The outgroup taxa were removed from the tree set using the RASP's appropriate option. Ancestral habitat types were coded as follows: (a) marine, (b) freshwater, (ab) marine to freshwater (euryhaline). The Bayesian MCMC analysis was performed with default settings and 500,000 generations.
2.4.3 Phylogenetic analyses of freshwater mussel (Unionidae)
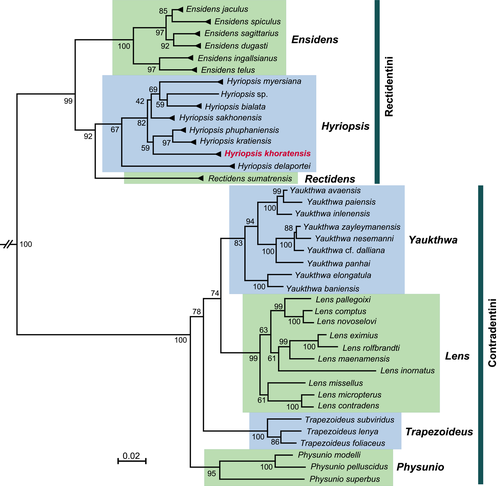
The multi-locus phylogeny of Unionidae (three codons of COI + 16S + 28S) was based on 86 in-group haplotypes of Contradentini and Rectidentini (Table S4 and Alignment S4). Four other representatives of the subfamily Gonideinae, that is, Gonidea angulata, Leguminaia wheatleyi, Lamprotula leaii, and Potomida littoralis, were used as outgroup (Table S4). To compile this dataset, altogether 83 published haplotype sequences of freshwater mussels were sampled from GenBank (Bolotov, Kondakov, et al., 2017; Bolotov, Konopleva, et al., 2020; Bolotov, Konopleva, et al., 2019; Bolotov, Vikhrev, et al., 2017; Graf, 2002; Graf & Cummings, 2006; Jeratthitikul et al., 2019; Konopleva, Bolotov, et al., 2019; Lopes Lima et al., 2017; Muanta et al., 2019; Pfeiffer & Graf, 2015; Pfeiffer et al., 2021; Zieritz et al., 2016). The sequences were initially aligned through the MUSCLE algorithm in MEGA7 (Kumar et al., 2016) and joined into combined nucleotide sequence alignments through an online FASTA sequence toolbox (FaBox 1.5) (Villesen, 2007). Maximum likelihood phylogenetic analysis was performed using the server for IQ-TREE (W-IQ-TREE) with GTR + G evolutional model (Trifinopoulos et al., 2016) and ultrafast bootstrap algorithm (UFBoot) with 5,000 replicates (Hoang et al., 2018).
3 RESULTS
3.1 Discovery of the Mekong's freshwater sponge-mollusc association
In the Mun River, we found an epifaunal association of a freshwater sponge (Corvospongilla, Spongillidae) and two species of freshwater bivalve molluscs (Figures 1-4). The freshwater mussel species (Hyriopsis, Unionidae) serves as the substrate for a sponge, while the byssus-attaching clam (Limnoperna, Mytilidae) mostly uses the sponge's crust surface as a substrate for its attachment (Figure 2a). The freshwater mussel individuals seem to be negatively threatened by the sponge encrustation that hampers opening of the shell and, hence, in some cases, may reduce water intake by the mussel due to partly blocked siphons (Figure 2b).

3.2 Taxonomic position of the freshwater sponge species
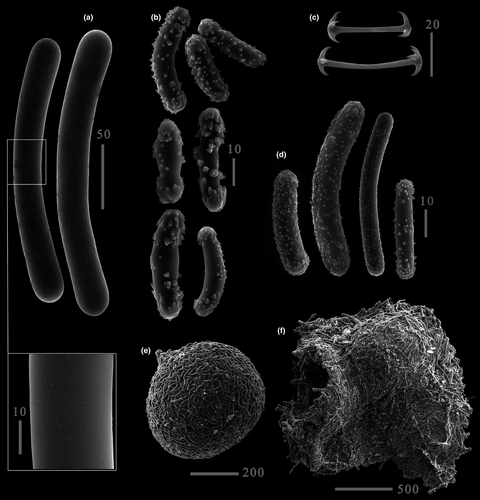
Based on morphological survey, the sponges collected in the Mun River in Thailand belong to C. siamensis Manconi & Ruengsawang, 2012. Our collection site is situated within the same basin but only 150 km away from its original type locality, the Pong River. Furthermore, we found that C. siamensis from the Mun River is nearly identical to C. ultima from the Kaladan River in Myanmar based on sequences of five molecular markers, that is, the COI (no substitutions; p-distance = 0%), 28S (no substitutions; p-distance = 0%), and ITS1 + 5.8S + ITS2 (one substitution; p-distance = 0.2%) fragments. These values are within the range of intraspecific variation in freshwater sponges (e.g., Addis & Peterson, 2005).
Our 28S phylogeny indicated that this species takes a distant position in the order Spongillida being a sister lineage to a few other available taxa in this group (Figure 5). The same pattern was found on the basis of the ITS1 + 5.8S + ITS2 phylogeny (Figure 6). These findings indicated that Corvospongilla is a valid and phylogenetically distant genus of freshwater sponges. Whether this genus is a member of the Spongillidae as it was thought until recently based on morphological data or belongs to other family, cannot be understood from our phylogenies. Both the 28S and ITS1 + 5.8S + ITS2 consensus phylogenetic trees clearly demonstrated that the family Spongillidae in its current understanding is a polyphyletic group (Figures 5 and 6). Members of smaller families such as Lubomirskiidae, Metaniidae, Metschnikowiidae, and Potamolepidae were found to be subclades/lineages within the Spongillidae.
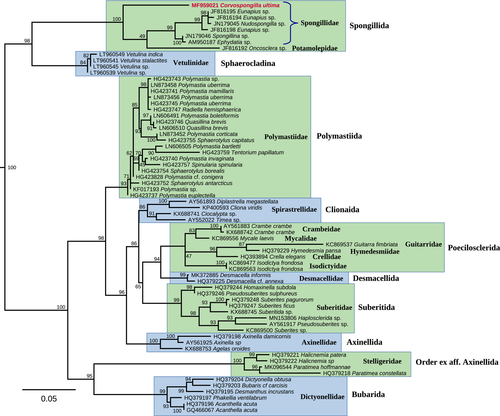

Our PTP species delimitation modeling returned a number of MOTUs, most of which correspond well to certain species such as Spongilla lacustris (Linnaeus, 1759), Ephydatia fluviatilis (Linnaeus, 1759), Ephydatia muelleri (Lieberkühn, 1856), and Radiospongilla cerebellata (Bowerbank, 1863) (Figure 6). Conversely, C. siamensis from the Mun River and C. ultima from the Kaladan River were recorded within a single MOTU with a high probability value (p < 0.001). The lack of clear genetic differences between these nominal taxa together with the results of our species delimitation modeling strongly supported the conclusion that C. siamensis and C. ultima are conspecific. Furthermore, the range of this species (i.e., C. ultima with its junior synonym C. siamensis syn. nov.) is broader than it was assumed earlier and extends from India through Myanmar to the Mekong River Basin.
3.3 Taxonomic position and evolutionary biogeography of the byssus-attaching clam
The byssus-attaching clam from the sponge and mollusc association from the Mun River is a member of the genus Limnoperna Rochebrune, 1882 (Figure 4). This genus was thought to contain several marine taxa but one widespread freshwater species, that is, the golden mussel L. fortunei (see section 4 and section 5 for detail and references). Conversely, our samples from the Mun River were found to be a sister species-level lineage to L. fortunei (Figure 7). Based on available taxonomic names, this species should be considered L. siamensis stat. rev. that was described from the Mekong Basin (see section 4 for detail).
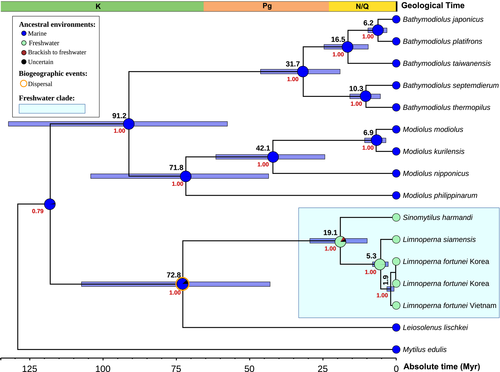
Our multi-locus time-calibrated phylogeny revealed that Limnoperna is a sister clade to Sinomytilus Thiele, 1934, other freshwater mytilid genus from tropical Asia (Figure 7). The origin of the most recent common ancestor (MRCA) of these clades was placed in the Early Miocene (mean age of the crown group = 19.1 Ma, 95% HPD 9.9–29.5 Ma). The ancestral habitat reconstruction indicated that this MRCA was a freshwater species (probability = 83.5%; Figure 7). Hence, freshwater Mytilidae in Asia represents a monophyletic group originated via a single colonization event from marine to freshwater environment followed by a subsequent radiation in continental water bodies. The divergence event between L. siamensis and L. fortunei most likely occurred at the Miocene—Pliocene boundary (mean age = 5.3 Ma, 95% HPD 2.7–8.1 Ma). The MRCA of these species was almost certainly a freshwater mollusc (probability = 99.3%).
The Limnoperna + Sinomytilus clade was recovered as a sister group to the endolithic species Leiosolenus lischkei Huber, 2010 [=Lithophagus curtus Lischke, 1874 (unavailable name, secondary homonym)] (Figure 7).
3.4 Taxonomic position of the freshwater mussel species
The freshwater mussel species serving as a substrate for the Mekong's epifaunal association of a freshwater sponge and byssus-attaching clam belongs to the genus Hyriopsis Conrad, 1853 (Figure 8). Based on sequences of three DNA markers (COI, 16S, and 28S), our samples must be considered H. khoratensis, a recently described species endemic to the Mun River basin (Pfeiffer et al., 2021).
4 TAXONOMIC ACCOUNT
4.1 Family Spongillidae Gray, 1867 (Porifera: Demospongiae: Spongillida)
- Type species: Spongilla loricata Weltner, 1895
- =Spongilla ultima Annandale (1910): 31; type locality: “a tank near Cape Comorin, the southernmost point of the Indian Peninsula” [a water tank near Cape Comorin, approx. 8.0805°N, 77.5512°E, Kanyakumari, Tamil Nadu, South India].
- =Corvospongilla ultima var. spinosa Annandale (1912): 390; type locality: “a stream at Taloshi on the eastern watershed of the Western Ghats <…> Koyna valley, Satara district, Bombay Presidency (2000 ft)” [Venna River at Taloshi, approx. 17.8828°N, 73.7491°E, Krishna River Basin, Satara District, Maharashtra, India].
- =Corvospongilla siamensis Manconi & Ruengsawang in Ruenswang et al. (2012) syn. nov.: 49; type locality: Ban Huai Sai, 16.7723°N, 102.7134°E, Pong River, Lower Mekong Basin, Thailand.
- =Corvospongilla ultima Annandale (1912): 384; Annandale (1918): 213; Gee (1930–1931): 51; Gee (1931–1932): 27; Penney (1960): 38; Penney and Racek (1968): 59–60; Soota (1991): 54; Jakhalekar and Ghate (2016): 507.
- =Corvospongilla ultima var. spinosa Gee (1930–1931): 49; Gee (1931–1932): 27; Penney (1960): 38.
Material examined. THAILAND: Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.3297°N, 103.6821°E, deep pool with clay and stony bottom substrate, depth ca. 5 m, March 07, 2018, 3 specimens, local collector leg. MYANMAR: Kaladan River, 21.0094°N, 92.9813° E, submerged siltstone rocks with boreholes, 01.05.2015, 2 specimens, Bolotov, Vikhrev, Aksenova, Konopleva, & local villagers leg.
Re-description. Growth form from thick crusts to massive roundish cushion on shells or rocks. Color light brown, yellowish, greenish. Consistency extremely hard, stony, firm. Basal spongin plate present. Surface flat or notably irregular and conulose, with conules supported by short ascending pauci- to multispicular tracts. Skeleton as an alveolar (isotropic) network of megascleres with paucispicular polygonal meshes and ascending tracts supporting conules. Megascleres oxeas or strongyles (201–306 × 17–29.2 μm) covered with single to moderately abundant microspines, hardly seen even under SEM. Microscleres micropseudobirotules with smooth bent shaft (25–48 × 2–2.5 μm) bearing distal smooth pseudorotules with 4–8 hooks. Gemmules large (up to 1000 μm), hemispherical, sole or grouped, strictly adhering to the substratum. Pneumatic layer of fixed gemmules reduced. Gemmular cage easily detachable, constituted by megascleres and smaller strongyles of various length (12–162 μm) covered with spines or tubercles. The cage forms a dark brown mat extending to 1–5 gemmules. Gemmuloscleres short strongyles with bent spines (30–55.2 × 6.2–22.5 μm). Foramen single, lateral, porus tube prominent (about 60 × 40 μm).
Distribution. India (Annandale, 1910, 1912; Jakhalekar & Ghate, 2016; Soota, 1991), Myanmar (Bolotov, Aksenova, et al., 2018), and Thailand (Ruengsawang et al., 2012; this study). Inhabits solid substrata in rivers, ponds, and reservoirs.
Comments. The description of morphology and measurements given here is based not only on the original material, but also on samples studied by other authors. Measurements of spicules for specimens from India, Myanmar, and Thailand are presented in Table 3.
| Nominal taxon | Locality | Megascleres: min–mean–max length × min–mean–max width, µm | Microscleres: min–mean–max length, µm | Strongyles of gemmular cage: max length × max width, µm | Gemmuloscleres min–mean–max length × min–mean–max width, µm | Reference |
|---|---|---|---|---|---|---|
| Corvospongilla siamensis syn. nov. | Pong River, Thailand | 126-N/A−245 × 13–26 | 19-N/A−51 | 190 × 27 | 32-N/A−50 × 7-N/A−14 | Ruengsawang et al. (2012) |
| Corvospongilla ultima | Mun River, Thailand | 201–224.85–260 × 25.25–22.5–29.25 | 25–38.52–48 | 162.5 × 17 | 30–42.2–55.25 × 6.25–8.34–22.5 | This study |
| Corvospongilla ultima | several waterbodies of Maharashtra, India | 228–263–306 × 17–18–21 | 21–26–35 | 124 × 18 | 25–41–54 × 5–7.5–11 | Jakhalekar & Ghate (2016) |
| Corvospongilla ultima | Kaladan River, Myanmar | 217–242–275 × 20–28.5–23.5 | 27.5–37.5–45 | 130 × 19 | 30.5–43.5–50 × 6–7.3–8.8 | Bolotov, Aksenova, et al. (2018) |
- N/A, not available.
We have compared a sponge found in the Mun River to another sponge previously found in the Kaladan River, Myanmar (Bolotov, Aksenova, et al., 2018). Morphological analysis showed that these two sponges are distinct according to the current taxonomic system (Manconi & Pronzato, 2019; Ruengsawang et al., 2012). The sponge from the Mun River was identified as C. siamensis due to microspined strongyles as megascleres, pseudobirotules as microscleres, and spined strongyles as gemmuloscleres. The sponge from Myanmar was identified as C. ultima due to microspined oxeas as megascleres, pseudobirotules as microscleres, and spined strongyles as gemmuloscleres (Annandale, 1910; Bolotov, Aksenova, et al., 2018; Jakhalekar & Ghate, 2016; Manconi & Pronzato, 2004). Both sponges highly fit the original and consequent descriptions. Identification of these species was also confirmed by the location of the sampling sites. Corvospongilla siamensis from the Mun River was found relatively close to the type locality of this nominal species, which is also situated within the Mun Basin (Ruengsawang et al., 2012). In its turn, our sample of C. ultima was collected from a costal river of the Bay of Bengal in northwestern Myanmar housing a derivative of the Indian fauna (Bolotov, Aksenova, et al., 2018). This finding aligns with the Indian origin of the type specimen (Annandale, 1910).
A notable difference between these two sponges occurs in the shape of megascleres: C. ultima has oxeas (see suppl. figure 3 in Bolotov, Aksenova, et al., 2018), while C. siamensis has strongyles. However, our comprehensive genetic study revealed that the sponges share identical sequences of the COI and 28S fragments. Furthermore, these two samples share a single nucleotide substitution in the ITS1 + 5.8S + ITS2 region (uncorrected p-distance = 0.2%). The DNA sequence analysis and the PTP species delimitation model unambiguously indicate that the samples from Myanmar and Thailand belong to a single species and that C. siamensis should be considered as a junior synonym of C. ultima.
4.2 Family Mytilidae (Mollusca: Bivalvia: Mytilida)
- Type species: Dreissena siamensis Morelet, 1866
- =Dreissena siamensis Morelet (1866): 167; type locality: “in regno siamensi, haud procul ab urbe Battambang” [near the city of Battambang, approx. 13.1°N, 103.2°E, Mekong Basin, Cambodia (formerly a part of the Kingdom of Siam)].
- =Limnoperna lemeslei Rochebrune (1881): 104; type locality: “Grand lac de Rham-Penh, Mekong” [Lake Tonlé Sap, approx. 12.9°N, 104.1°E, Mekong Basin, Cambodia].
- =?Limnoperna depressa Brandt and Temcharoen (1971): 115; type locality: “Mekong branch at Muang Sene, Khong Island, Laos” [Muong Saen Nua, approx. 14.0970°N, 105.7861°E, Khong Island, Champasak Province, Laos].
- =?Limnoperna supoti Brandt (1974): 256; type locality: “Kaek River in Sopa Falls, 80 km E of Pitsanuloke” [Kaeng Sopa Waterfalls, 16.8716°N, 100.8351°E, Wang Thong (=Khek) River, Chao Phraya Basin, Phitsanulok Province, Thailand].
Material examined. THAILAND: Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.3297°N, 103.6821°E, deep pool with clay and stony bottom substrate, depth ca. 5 m, attached to freshwater mussel and sponge crusts, 07 March 2018, 5 specimens [RMBH biv0463, including biv0463_1 and biv0463_2 being sequenced], local collector leg.; Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.2966°N, 103.5965°E, attached to petrified wood and hard clay fragments, 09.iii.2018, 13 specimens [RMBH biv0719, including biv0719_1, biv0719_2, and biv0719_3 being sequenced], local collector leg.
Diagnosis. Apparently, there are no reliable diagnostic features to separate L. siamensis from L. fortunei based on morphological characters. However, L. siamensis differs from its sister species by 36 and 21 fixed nucleotide substitutions in the COI and 16S gene fragments, respectively. The diagnostic substitutions in the COI gene fragment are as follows: 17 G, 23 A, 29 A, 77 G, 83 G, 98 G, 101 C, 122 A, 137 C, 161 G, 191 A, 206 G, 207 C, 227 C, 230 A, 260 T, 284 G, 296 A, 302 A, 317 G, 332 A, 350 G, 371 C, 374 G, 422 A, 431 T, 437 A, 473 A, 479 G, 494 A, 503 A, 518 G, 549 T, 563 G, 582 C, and 638 T. The diagnostic substitutions in the 16S gene fragment are as follows: 44 A, 48 G, 108 G, 114 T, 138 C, 227 A, 233 G, 242 T, 248 A, 249 C, 250 G, 255 A, 280 G, 296 G, 301 Del, 303 T, 321 A, 336 G, 343 T, 361 G, and 408 T. The mean uncorrected p-distances are 6.6% and 4.0% for the COI and 16S gene fragments, respectively. Intraspecific differences in the 28S gene fragment of L. siamensis and L. fortunei were not recorded. Finally, these species share strictly allopatric distribution that can also be used as a supplementary feature for species-level diagnostics, although the rapid human-mediated range expansion of L. fortunei may change this pattern in the near future.
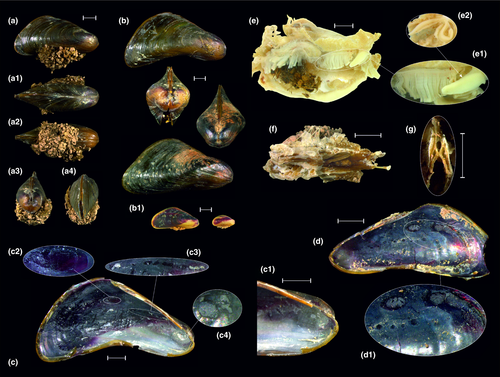
Re-description. The shell length of adult individuals up to 20 mm, the shell height up to 10 mm (larger specimens can probably be found). The shell shape typical for Mytilidae, rounded-triangular, elongated wedge-shaped, pointed anteriorly, and rounded posteriorly, with almost straight or concave ventral margin and obtuse keel on dorsal side (Figure 4a,b). In ventral margin, there is narrow chink for the byssus. The shell is attached to the substratum by an array of byssal threads (Figure 4-8). The umbo is placed anteriorly. The ligament internal and relatively long, situated between the hinge plates but visible externally. The hinge plate narrow, without teeth. The outer surface of the shell of adult individuals dark brown to black, with olive-green lines between coarse growth lines (Figure 4a,b). The shell of small individuals yellowish-white on the ventral margin and umbo, and burgundy or reddish-brown on the dorsal aspect (Figure 4-8). Internally, the shell of L. siamensis nacreous. The internal shell layer slightly lustrous, darker postero-dorsally, and lighter antero-ventrally. The nacre purple or gray-blue above and white below the keel at the anterior margin (Figure 4c,d). The shell varies somewhat in size, shape, and coloration. The internal architecture of the shell is illustrated in Figure 4c–c4,d–d1. Apical septum absent. Large posterior adductor muscle scar and two byssal retractor muscle scars present (Figure 4-8). There is also a small antero-ventral anterior adductor muscle scar and an anterior pedal retractor muscle scar underneath the ligament (Figure 4c1,c4), and ctenidial attachment muscle scars (Figures 4c3,d). The valves thin and brittle. The mantle is fused on the dorsal side and between the exhalant siphon and the inhalant aperture (Figure 4g). The ctenidial-labial palp junction typical of the Mytiloidea in general (Figure 4-8). Foot finger-shaped, with byssus.
Distribution. Mekong Basin and, probably, some adjacent freshwater systems such as the Chao Phraya River. Numerous occurrences of L. fortunei from the Mekong Basin (e.g., Morton & Dinesen, 2010; Ng et al., 2020) belong to L. siamensis.
Comments. Earlier authors listed L. siamensis and several other taxa described from the Mekong and Chao Phraya as valid species (e.g., Brandt, 1974). Conversely, Morton (1973) assumed that L. siamensis could be synonymous with L. fortunei based on the similarity of conchological and anatomical characters. The conspecificity of these two taxa was widely accepted until recently (Morton et al., 2020; Ng et al., 2020), with a plethora of names listed as synonyms of a single widespread species, L. fortunei (e.g., Morton, 2015; Morton & Dinesen, 2010). Here, we revise the status of the two species and propose L. siamensis stat. rev.
Two peculiar nominal species of Limnoperna were described from rapids and waterfalls, that is, L. depressa Brandt & Temcharoen, 1971 from the Mekong River in Laos, and L. supoti Brandt, 1974 from the Chao Phraya Basin in Thailand (Brandt, 1974; Brandt & Temcharoen, 1971). Interestingly, the latter species was described as a dwarf clam that exclusively inhabits eroded tips of Brotia shells (Gastropoda: Pachychilidae) (Brandt, 1974). Here, we tentatively assign both L. supoti and L. depressa to be probable synonyms of L. siamensis, although these remarkable “white-water” populations need future DNA-based studies of topotypes.
The nominal taxon Modiola taprobanensis Preston, 1915 [type locality: Ceylon] cannot be linked to the freshwater Mytilidae of Southeast Asia (i.e., Limnoperna and Sinomytilus taxa) as several authors assumed (Beu, 2006: 191) because it was described from marine waters of Sri Lanka (Preston, 1915). Furthermore, this nominal taxon was recorded only once since its original description, that is, from the saline Ennur backwater, Madras [currently, the Ennore Creek, 13.2313°N, 80.3239°E, Chennai, Tamil Nadu, South India] (Preston, 1916: 35). There are no doubts that it is a saltwater mytilid bivalve, which is nothing to do with Limnoperna.
4.3 Family Unionidae (Mollusca: Bivalvia: Unionida)
Genus Hyriopsis Conrad, 1853
Type species: Unio delphinus Gruner, 1841 [=Hyriopsis bialatus Simpson, 1900]
- =Hyriopsis sp.2 sensu Bolotov, Kondakov, et al. (2017): 4, figure 2; Bolotov, Vikhrev, et al. (2017): 3, Figure 1a.
- =Hyriopsis gracilis sensu Do et al. (2018) [identification error; non Haas, 1910]: 8–9; Konopleva, Pfeiffer, et al. (2019): 2, Figure 1; Bolotov, Konopleva, et al. (2020): 3, Figure 1.
- =Hyriopsis khoratensis Pfeiffer et al. (2021): 119.
Figure 2.
Material examined. THAILAND: Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.3297°N, 103.6821°E, deep pool with clay and stony bottom substrate, depth ca. 5 m, 07.iii.2018, one specimen [RMBH biv0459, being sequenced], local collector leg.; Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.3575°N, 103.6637°E, 07.iii.2018, eight specimens [RMBH biv0465, including biv0465_1 and biv0465_2 being sequenced], local collector leg.; Surin Province, Mekong River Basin, Mun River near Tha Tum town, 15.2966°N, 103.5965°E, 07.iii.2018, five specimens [RMBH biv0469, including biv0469_1 and biv0469_2 being sequenced], local collector leg.; Maha Sarakham Province, Mekong Basin, Chi River, 16.2158°N, 103.2526°E, 11.iv.2014, 14 specimens [RMBH biv0130, including biv0130_1, biv0130_2, and biv0130_3 being sequenced], Bolotov and Vikhrev leg.
Diagnosis. Conchologically, H. khoratensis can be distinguished from Hyriopsis bialata by having less elongate and more ovate shell (Pfeiffer et al., 2021). Both these species share a large, triangular dorsal wing.
Distribution. Endemic to the Mun River watershed within the Khorat Plateau (Pfeiffer et al., 2021).
Comments. In earlier revisions, Hyriopsis samples collected from the Mun and Chi rivers in Thailand were listed as H. bialata Simpson, 1900, which was thought to be widespread throughout Southeast Asia (e.g., Brandt, 1974). Bolotov, Kondakov, et al. (2017) and Bolotov, Vikhrev, et al. (2017) found that a separate Hyriopsis species inhabits the Chi River and that this species is distinct phylogenetically from the true H. bialata Simpson, 1900 from Malaysia. A year later, Do et al. (2018) suggested that the name H. gracilis Haas, 1910 could be used for the Mekong's species, which was historically identified as H. bialatus Simpson, 1900. Finally, Pfeiffer et al. (2021) resolved this taxonomic puzzle. These authors revised the entire genus Hyriopsis based on a comprehensive DNA sequence dataset and described H. khoratensis as a species new to science (Pfeiffer et al., 2021).
5 DISCUSSION
5.1 Sponge-mollusc associations in fresh waters of Asia and South America
To the best of our knowledge, we report on the first discovery of a freshwater sponge-mollusc association in Asia. Furthermore, the association described here is the only example discovered by us during a long-term survey of freshwater bivalves throughout tropical and temperate Asia in 2011–2020 (Myanmar, Laos, Thailand, Vietnam, Malaysia, Korea, and the Russian Far East) (Bolotov, Aksenova, et al., 2018; Bolotov, Klass, et al., 2019; Bolotov, Kondakov, et al., 2017, 2020; Bolotov, Pfeiffer, et al., 2018; Bolotov et al., 2014; Bolotov, Vikhrev, et al., 2017; Konopleva, Pfeiffer, et al., 2019). At first glance, it could be assumed that such associations are not so common in the region.
Conversely, our data can be biased by sampling approaches because we primarily collected samples by wading and snorkeling from smaller water bodies and shallow places of larger rivers and lakes (e.g., Bolotov, Konopleva, et al., 2020). Volkmer-Ribeiro et al. (2019) noted that their team was able to collect large series of freshwater sponge-mollusc associations from the Xingu River by bottom trawling and surface-supplied diving at depth 5–30 m, and that these associations were not discovered there based on traditional sampling approaches. Our examples from the Mun River were collected by a local free diver in a deep pool with stony bottom, while we did not find such associations in shallower places of the river examined ourselves.
Generally, the freshwater sponge-mollusc associations discovered in the Xingu River also comprise a freshwater sponge (Spongillidae), a freshwater mussel (Hyriidae), and a byssus-attaching clam (Dreissenidae) (Volkmer-Ribeiro et al., 2019). Hence, the Mekong's association can be considered analogous to those discovered in South America. Based on the classification scheme of Volkmer-Ribeiro et al. (2019), the Mekong's association belongs to the BSA type, that is, sponges encrusted on the posterior region of bivalve shell surface around siphonal area.
5.2 Note on Corvospongilla taxonomy: does a spicule shape reflect species limits?
These findings are not surprising given the intricate taxonomy of Corvospongilla. The genus comprises 19 species to date (Van Soest et al., 2020). It can be distinguished from other taxa by having a specific combination of spicule types: microscleres shaped as pseudobirotules and gemmuloscleres shaped as stout spiny strongyles or oxeas (Manconi & Pronzato, 2002, 2004). The species-level concept of this genus is based exclusively on morphological features, some of which are quite inconspicuous. For example, Corvospongilla micramphidiscoides Weltner, 1913 and Corvospongilla victoriae Annandale, 1914 share similar spicules (Manconi & Pronzato, 2004, 2009), and their taxonomic status as separate species is supported only by different proportion of oxeas in the megasclere composition.
The ability of widely used morphological traits to mark true species borders should be carefully checked. For instance, C. siamensis differs from Corvospongilla burmanica (Kirkpatrick, 1908) only by the presence of microspines on the megasclere surface (Ruengsawang et al., 2012), while Corvospongilla loricata (Weltner, 1895) differs from C. burmanica by the presence of spiny megascleres supplementing smooth strongyles (Kirkpatrick, 1908; Manconi & Pronzato, 2004). Currently, the usability of megasclere microspines for Corvospongilla taxonomy can be questioned, since the majority of holotype descriptions lack SEM photographs. These spines can be extremely small and totally invisible under LM, as those in a C. ultima specimen described here (Figure 3), or hardly visible, as those in a specimen from Myanmar (Bolotov, Aksenova, et al., 2018). Moreover, in Spongillida the number of spines often varies from spicule to spicule, for example, in E. fluviatilis (Manconi & Pronzato, 2002), and from sponge to sponge, for example, in Spongilla alba Carter, 1849 (our unpublished data) or Metschnikowia tuberculata Grimm, 1877 (Sokolova et al., 2020). Many spicules should be meticulously checked under SEM to be sure that none of the spines was missed.
The general shape of megascleres is also considered informative in current taxonomy of the genus (Manconi & Pronzato, 2004). Thus, C. ultima has sharpened spicules (oxeas), while C. siamensis has blunt ones (strongyles) (Annandale, 1910; Ruengsawang et al., 2012). Shape of megascleres in freshwater sponges is, however, not as stable as one would like. For example, Rezvoj (1925) described a new species, Eunapius rotundacuta Rezvoj, 1925, based on the megasclere strongyles that were unusual for the known representatives of the genus. Later, more specimens were investigated revealing different strongyles-to-oxeas ratio in certain individuals, and this taxon was reconsidered as a subspecies of Eunapius carteri (Bowerbank, 1863) (Rezvoj, 1926). Same unstable strongyles-to-oxeas ratio was observed in specimens of S. alba living in adjacent waterbodies but having identical DNA sequences (our unpublished data). Furthermore, many Corvospongilla representatives comprise an additional megasclere type besides the main type (Manconi & Pronzato, 2004, 2019), which also impedes understanding of the character's reliability. Finally, the developmental stage of a spicule also influences its tips shape. Megasclere shape seems therefore not so useful in species-level identification. Our genetic analysis suggests its total inconsistency at least within Corvospongilla, since a sponge with exclusive oxeas has been proved to have identical DNA sequences to a sponge with exclusive strongyles.
The structure of gemmuloscleres, being a relevant diagnostic character in other freshwater sponges, shows some stability in Corvospongilla species (Manconi & Pronzato, 2004, 2009, 2019; Ruengsawang et al., 2012). Gemuloscleres are represented by spiny strongyles in the majority of species (10 species), but five species have also spiny oxeas or oxeas-strongyloxeas: Corvospongilla caunteri Annandale, 1911, Corvospongilla lemurensis Manconi & Pronzato, 2019, Corvospongilla thysi (Brien, 1968), Corvospongilla seckti Bonetto & Ezcurra de Drago, 1966, and Corvospongilla mesopotamica Manconi & Pronzato, 2004. Two species, that is, Corvospongilla novaeterrae (Potts, 1886) and Corvospongilla bhavnagarensis Soota, Pattanayak & Safena, 1984, have oxeas as the only gemmuloscleres. Gemmules are absent in the type material of Corvospongilla sodenia Brien, 1969 and Corvospongilla zambesiana (Kirkpatrick, 1906) (see Manconi & Pronzato, 2004), which decreases the credibility of these species.
Microscleres seem to be the most stable character within Corvospongilla. Pseudobirotules are rare within freshwater sponges but always occur in Corvospongilla taxa (Manconi & Pronzato, 2002). Three species, that is, C. mesopotamica, C. victoriae, and C. micramphidiscoides, possess spiny oxeas or strongyles as microscleres in addition to the pseudobirotules, but this feature was not stressed in the current classification scheme.
Spicule complement of the gemmular cage in Corvospongilla usually includes megascleres, gemmuloscleres, and plenty of spicules of intermediate types (Manconi & Pronzato, 2004; Ruengsawang et al., 2012; Jakhalekar & Ghate, 2016; Bolotov, Aksenova, et al., 2018: suppl. figure 3 AND figure 4e2). In identification keys, these spicules do not attract much attention (Manconi & Pronzato, 2004, 2016; Soota, 1991), and some of species descriptions lack information on the cage composition.
Finally, our results call into question the taxonomic potential of the morphological trait “shape of megascleres” in Corvospongilla. We obviously need genetic data to reveal which morphological traits are informative in every genus of freshwater sponges. It is also important to describe spicules from more specimens when reporting on new findings, since extremely little data on natural variability of this group exist.
5.3 Taxonomy and evolutionary biogeography of the freshwater Mytilidae
Morton et al. (2020) revealed that the septate Sinomytilus harmandi and the aseptate L. fortunei are closely related taxa and that the MRCA of these two species colonized Asian freshwaters relatively recently. Our statistical modeling results align with these earlier findings. In particular, our time-calibrated phylogeny suggests a Miocene age for the MRCA of Sinomytilus and Limnoperna (mean age = 19.1 Ma, 95% HPD 9.9–29.5 Ma), while the ancestral habitat reconstruction indicates that this ancestral mollusc most likely was a freshwater species (probability = 83.5%). The origin of Novaculina Benson, 1830 (Bivalvia: Pharidae), another secondary freshwater genus from Asia, was also placed in the Miocene, with the MRCA being a freshwater taxon (Bolotov, Vikhrev, et al., 2018).
It was suggested that Limnoperna and Sinomytilus could be placed in a separate subfamily, the Limnoperninae Scarlato & Starobogatov, 1979 (Lee et al., 2019; Morton et al., 2020). Since the DNA-based subfamily-level classification scheme of the Mytilidae is still not developed, we would agree with this preliminary placement. The Limnoperna + Sinomytilus clade sisters to the endolithic Leiosolenus (Lee et al., 2019 [as “Lithophaga”]; Morton et al., 2020; this study), the latter genus could be transferred from the Lithophaginae Adams & Adams, 1857 to Limnoperninae. If Sinomytilus comprises freshwater taxa only (Brandt, 1974; Morton & Dinesen, 2010), the genus Limnoperna appears to harbor a plethora of fossil and recent marine species (e.g., Beu, 2006: 191). Although molecular sequences of these saltwater species are not available, our phylogenetic reconstruction raises some doubts that they can belong to the freshwater Limnoperna + Sinomytilus clade, which seem to colonize fresh waters since the Early Miocene.
The golden mussel L. fortunei attracts the full attention of scientists and stakeholders as an invasive species rapidly spreading in South America, Japan, and Hong Kong, and causing large economic and environmental impacts (Morton, 1973, 2015; Pessotto & Nogueira, 2018; Ricciardi, 1998). Although a number of molecular studies were already focused on this species, including the whole genome sequencing (De Paula et al., 2020; Uliano-Silva et al., 2018), any DNA sequence data on its populations from the Mekong Basin were not available. It was thought that the genus Limnoperna mostly contains brackish and marine taxa but one freshwater species, L. fortunei, which is widespread from the Mekong Basin to China (Beu, 2006; Graf, 2013; Morton, 1973, 2015; Morton & Dinesen, 2010; Morton et al., 2020). Hence, the presence of two vicariate species of Limnoperna, that is, L. siamensis in the Mekong Basin and L. fortunei in East Asia, is an unexpected finding of the present study that agrees with available data on distribution of other freshwater animals. The allopatric ranges of the razor clam species Novaculina siamensis Morlet, 1889 (Mekong, Bang Pakong, and Pa Sak rivers) and Novaculina chinensis Liu & Zhang, 1979 (Yangtze River) seem to reflect the same biogeographic pattern (Bolotov, Vikhrev, et al., 2018). It was shown that freshwater mussels assemblages of the Sundaland (Mekong, Chao Phraya, Mae Klong and the drainages of the Malay Peninsula and Greater Sunda Islands) and East Asian (rivers east and north of the Mekong Basin up to the Russian Far East) subregions are clearly different even at the subfamily and tribe levels (Bolotov, Pfeiffer, et al., 2018). Three subfamilies, that is, Parreysiinae, Gonideinae (with large radiations of the Contradentini, Rectidentini, and Pseudodontini), and the monotypic Modellnaiinae, prevail in the Sundaland Subregion, while the Unioninae and Gonideinae (with Lamprotulini, Gonideini, and Chamberlainiini) form the basis of the East Asian fauna (Bolotov, Konopleva, et al., 2020; Bolotov, Pfeiffer, et al., 2018). However, one member of the East Asian Unioninae is known to occur in the Mekong Basin, that is, Cristaria cf. plicata (Leach, 1814) (Bolotov, Pfeiffer, et al., 2018; Zieritz et al., 2018). Among freshwater fish assemblage, Carassius praecipuus Kottelat, 2017 was described from central Laos as the unique Mekong's representative of this East Asian genus (Kottelat, 2017a). Several other fish taxa of East Asian affinities such as Vanmanenia orcicampus Kottelat, 2017 and Rhinogobius milleri Chen & Kottelat, 2003 are known to occur in the Mekong's tributaries in Laos (Kottelat, 2017a,2017b). In summary, there were several faunal exchanges between the Mekong and East Asian rivers, most likely via ancient stream captures. The timing of these freshwater connections and subsequent vicariance events needs future studies using time-calibrated phylogenies of various freshwater taxa. As for the L. fortunei—L. siamensis species pair, it indicates a divergence event at the Miocene—Pliocene boundary (mean age = 5.3 Ma).
ACKNOWLEDGEMENTS
We are grateful to two anonymous reviewers for their valuable comments on earlier version of our work. This study was partly supported by the Russian Ministry of Science and Higher Education (project No. AAAA-A17-117033010132-2 to Y.V.B. and O.V.A., and No. 0793-2020-0005 to I.N.B., A.V.K., and I.V.V.), and the Ministry of Education and Science of the Arkhangelsk Region (project No. 07-2020a to O.V.T.). The molecular analyses of freshwater molluscs were supported by the Russian Science Foundation (project No. 21-17-00126 to I.N.B. and E.S.K.). The work of A.M.S. was conducted under IDB RAS GBRP No. 0088-2021-0020. The research was partially done using equipment of the Core Centrum of Institute of Developmental Biology RAS. The SEM study was performed at Electron Microscopy Centre of A. N. Severtsov Institute of Ecology and Evolution of the Russian Academy of Science and Borissak Palaeontological Institute of the Russian Academy of Science. We thank R. Rakitov for assistance during SEM studies.
CONFLICT OF INTEREST
The authors declare no conflict of interest.




