The Haeckelian shortfall or the tale of the missing semaphoronts
Abstract
When faced with the daunting and exciting task of studying biodiversity, one must necessarily come to terms with a few challenging knowledge gaps, the so-called biodiversity shortfalls. Given that biodiversity is inherently multidimensional, it seems rather natural to admit that hitherto unrecognized shortfalls, on other distinct aspects of biodiversity, should be considered. Here, we introduce the Haeckelian shortfall, which has as its nexus organismal ontogeny, and refers to the relative scarcity of knowledge about the distinct semaphoronts of a substantial fraction of all known species. The Haeckelian shortfall has a profound relevance on the matter of total evidence, in the context of systematics, besides several indirect effects on the other shortfalls, as they all are intimately interconnected. The importance of studying distinct semaphoronts is crystal clear: Besides the purest and most descriptive access to the ontogeny of species (the idiographic aspect), assessing those semaphoronts will certainly promote the advancement of relevant nomothetic knowledge, contributing to an increasingly meaningful eco–evo–devo. Overcoming the Haeckelian shortfall is certainly a major challenge in our task of knowing and preserving biodiversity.
1 INTRODUCTION
One of the several definitions of biodiversity—the variety of life—referring collectively to the variation at all levels of biological organization (Gaston & Spicer, 2004), gives a proper idea of how vast this subject is. It is no surprise that the term has remained as vague as its measurement (e.g., Sarkar, 2002). What biodiversity really is and how it should be understood continues to be a matter of intense debate (Burch-Brown & Archer, 2017). Biodiversity is the cornerstone of all of evolution, ecology, biogeography, and taxonomy. In this context, one is studying biodiversity both when doing descriptive (i.e., idiographic) and predictive (i.e., nomothetic) science (Cotterill & Foissner, 2010). Yet, the knowledge of biodiversity is thwarted by two main factors: the lack of knowledge itself and the limited or even skewed construction of our own knowledge (Assis, 2018). Data scarceness and blurred snapshots of patterns and processes of biodiversity, beyond limitations of theory itself, are a byproduct of the inherent complexity of what we seek to study (see Hortal et al., 2015). Even when a simpler and meaningful proxy (e.g., species richness) is chosen, the difficulties in achieving a grand unified theory persist by the very nature of the object of investigation (Marquet et al., 2004).
Even if ignorance concerning biodiversity cannot be properly estimated, organizing and quantifying these gaps following certain aspects of biodiversity, that is, a structured rationale, is possible. Hortal et al. (2015:524) defined these knowledge shortfalls “as the gaps between realized/extant knowledge within a biological domain at a given moment in time (normally the present day).” At the time Hortal et al. (2015) presented their review, four shortfalls were recognized: (a) Linnean, the deficit on description and cataloging of species (Brown & Lomolino, 1998); (b) Wallacean, the scarcity of knowledge on species distribution (Lomolino, 2004); (c) Prestonian, the incompleteness of data on species abundance and population dynamics (Cardoso et al., 2011); and (d) Darwinian, all we do not know about the tree of life (Diniz-Filho et al., 2013). And they (Hortal et al., 2015) also presented and discussed three new shortfalls: (e) Hutchinsonian, the lack of knowledge of the scenopoetic niche of species (considering this shortfall in a wider sense than originally proposed by Cardoso et al., 2011); (f) Eltonian, the scarcity of information about species interactions; and (g) Raunkiæran, the deficit of information about species traits and their functions.
Besides the shortfalls discussed by Hortal et al. (2015), as far as we know, a single new shortfall was proposed, the “movement shortfall,” regarding the lack of knowledge on species movements, with particular reference to dispersive, irruptive, and nomadic birds (Cottee-Jones et al., 2016). But all these caveats may be even more fundamental, as the biodiversity of unexplored and unmapped environments cannot be described, analyzed, and/or conserved. This concept, the Racovitzan impediment (Ficetola et al., 2019), proposes that for some places on Earth, even the idea of shortfalls is still not applicable.
If one concedes that sampling biases are a characteristic of biodiversity studies (e.g., Ribeiro et al., 2016), it is quite obvious to realize that, besides being scale-dependent, all those shortfalls are at least taxonomically and geographically structured. Some taxa are preferred to be studied (e.g., Jeschke et al., 2012; Titley et al., 2017), and some places are much more inventoried than others (e.g., Oliveira et al., 2016; Sastre & Lobo, 2009). Even though such effects are more obvious in Linnean and Wallacean shortfalls, the others are also greatly hindered by these biases (see Hortal et al., 2015). Therefore, possible attempts of data correction for Linnean and Wallacean shortfalls based on taxonomically restricted studies should not be generalized (Vale & Jenkins, 2012).
In the “Future issues” section of their paper, Hortal et al. (2015:541) state that “new ways of representing and communicating uncertainty should be developed to raise awareness of the certainties behind the uncertainty and the extent of current ignorance on biodiversity patterns and processes.” Considering the multidimensional nature of biodiversity itself (e.g., Burch-Brown & Archer, 2017), it seems rather obvious to consider that new shortfalls, on other distinct aspects of biodiversity, need to be proposed. We here introduce the Haeckelian shortfall, having the ontogeny of individuals as a biodiversity aspect, and referring to the lack of knowledge about the distinct semaphoronts of the individuals that instantiate the species. This shortfall is named after the German naturalist and Professor of Zoology Ernst Heinrich Philipp August Haeckel (1834–1919), for all his influential contribution to several areas of biology, philosophy, ethics, and arts (see Kutschera et al., 2019), and especially for his contributions to comparative embryology. Haeckel is commonly remembered by the Biogenetic Law and recapitulation (e.g., Richardson & Keuck, 2002), besides fraud accusations in embryo illustrations (see Richards, 2009). Nevertheless, as stated by Watts et al. (2019:681), "this image does not do justice to the true impact his work has had in sciences." We also believe that “Haeckelian” is able to synthesize in a single name studies on ontogeny, ecology, and evolution.
2 A FEW WORDS ABOUT SEMAPHORONTS
Given that the semaphoront concept is central to the Haeckelian shortfall, a brief discussion of this term is presented below in order to clarify how it is being considered here. Several comprehensive revisions and discussions, dealing both with the philosophical, theoretical, and methodological aspects of this subject, can be found elsewhere (e.g., Havstad et al., 2015). Here, we are not aiming at presenting a more detailed discussion of this concept.
The importance of the semaphoront concept was brought forth in the seminal Phylogenetic Systematics, where Hennig (1966) presented the basis of phylogenetic systematics. He dedicated several pages to the great diversity of ontogenetic processes in nature, further concluding that semaphoronts should be regarded as the fundamental unit of systematics (Sharma et al., 2017). When interpreting the organism as a system of causal interactions, in his own words, Hennig used the semaphoront concept as a way to obtain a character-bearer that represents the organism at distinct stages of its life cycle (Havstad et al., 2015; Rieppel, 2007). The semaphoront might be considered as a space–time slice through the set of events that constitute the fluid organizational system that is the organism (Rieppel, 2003). The semaphoront is a causal hypothesis account, by way of ontogeny, of the characters of individuals at a given time in their life history relative to the characters observed at some other time, for example, “embryo,” “larva,” “adult” (Fitzhugh, 2006). As noted by Havstad et al. (2015), in this view of the semaphoront as a processual system, the series of semaphoronts that represent the organism at different stages of its life cycle is causally integrated and interconnected. In this way, as pointed out by Rieppel (2003), the systematist recovers the organism through the logical reconstruction from semaphoronts, which produces a semaphoront complex. These semaphoronts then combine to form the ontogeny of a male and female, which, in turn, would mate to generate the next individual (Archibald, 2014).
When considering the semaphoront concept, the fact that Willi Hennig was an entomologist was likely influential in the development of his views, given that he would be intimately familiar with the radical morphological changes undergone by some insects from larva to pupa to adult (Archibald, 2014). As insects undergo complex metamorphism (i.e., the differences in form between ontogenetically related semaphoronts; Hennig, 1966), the use of the semaphoront concept seems even more appropriate (e.g., Assis, 2016). As suggested by Rieppel (2003), it is even possible to equate Hennig's idea of semaphoront with that of an ontogenetic stage in the life cycle of organisms. We could take a few steps further to find a general definition of semaphoront that seems quite adequate for our purpose: A semaphoront can be understood as “any time-limited life-stage of any organism” (Reif, 2005). Even if in Hennig's original proposal a semaphoront should rely on a theoretically infinitely small period of the life of an individual/organism (Hennig, 1966), we end up dealing with developmental stages as clusters of characters in a developmental sequence that are arbitrarily forced by the observer to be simultaneous (Richardson & Keuck, 2002). That is, the semaphoronts that we study have an implicit subjective scope.
When considering specifically the shortfall we propose here, we have in mind the ontogenetic stages that are clearly discrete (e.g., eggs, larvae in general, pupae, and adults). But of course these definitions, both regarding ontogenetic and terminological matters, are not always so simple (e.g., see discussions in Ceh et al., 2015; Rice, 1980). But neither an in-depth discussion regarding how semaphoronts are defined in specific groups (e.g., Miranda et al., 2010) nor terminological matters on the ontogeny of some groups (e.g., the use of instar, stadium, stage, stase in arthropods; see, e.g., Andre & Jocque, 1986; Fink, 1983) will be presented here. We will try to keep the focus on the problems that the lack of semaphoronts may cause in the study of biodiversity.
We will not discuss females and males as distinct semaphoronts, nor the definition of each sex (including those intricate cases as protandric and protogynous hermaphrodites, e.g., Lowry & Stoddart, 1986), yet we recognize that they might often be an important aspect of the shortfall. Here, we propose the Haeckelian shortfall as also including our ignorance about conspecific females and males, which precludes even the association of these semaphoronts under a given species proposal (discussed below).
3 THE HAECKELIAN SHORTFALL AND THE PLEA FOR TOTAL EVIDENCE
Hennig himself considered the semaphoront, a specific period of the life history of a given organism, as the fundamental unit of systematics (Sharma et al., 2017). In this context, the lack of some of those slices will certainly undermine the reconstruction of both individuals and species. The Haeckelian shortfall is at the heart of the total evidence approach. A simple and relevant definition of total evidence was presented by Rieppel (2005:81): “in systematics, the total evidence is the sum of all character statements available at the time.” Since its debut in systematic (see Kluge, 1989), the concept of total evidence was extensively debated in its philosophic sense (e.g., Kluge, 1998 contra Miyamoto & Fitch, 1995; Rieppel, 2004). But here, when dealing with total evidence, we are not aiming at discussing questions concerning data partitioning, congruence, how datasets are analyzed, etc. We consider total evidence simply as the greatest amount of characters that one can obtain based on all semaphoronts of the individuals of a given taxon/species.
Besides representing a quantitative gain of characters, the inclusion of different semaphoronts may also represent a substantial qualitative gain of information, mainly for morphology-based systematics, since different developmental stages may represent distinct evolutionary scenarios. Often, what happens in systematics is a mere recycling of existing data (Jenner, 2001), with the same known semaphoronts being considered. Coding characters for specific life stages may bias tree reconstruction (Blanke et al., 2015), and considering structures that are particular to specific ontogenetic stages has the potential to enrich datasets and improve phylogenetic resolution (Sharma et al., 2017). Despite potential conflict, analyses based on immature and adult data may improve tree resolution and node support (Meier & Lim, 2009). But, in most cases, only adults have been used as semaphoronts for the reconstruction of phylogenies, despite the rich source of additional characters provided by larvae and other developmental stages (Dahms, 2000). However, one cannot dismiss that existing evidence suggests that the informativeness of certain stages in phylogenetic analyses may be taxon-specific (discussed in Sharma et al., 2017).
The connection between Haeckelian and Darwinian shortfalls (Diniz-Filho et al., 2013) is obvious. The main direct effect is that the lack of some semaphoronts will preclude more data-comprehensive phylogenies, mainly in a morphology-based context. One could argue that we have already overcome any “amount of information” problem, as huge datasets (e.g., from phylogenomics), unimaginable until recently, are able to be built and analyzed. Nevertheless, a more subtle relation between these shortfalls, which even passes by the Linnean shortfall, should be considered. Even if the same genotype is found in different semaphoronts, the difficulty of identifying some specific semaphoronts, besides associating them with the semaphoronts already known, considerably hampers a reliable recognition of the species (with possible impacts the quality of these big data phylogenies). Therefore, even the latest phylogenetic techniques still rely on the few known semaphoronts, which could restrict the number of species (at least named species) to be analyzed.
Even if we consider that the reliability of phylogenies cannot be evaluated only by the number of included taxa (Assis, 2018), taxonomic completeness is a target for overcoming the Darwinian shortfall (Assis, 2018; Diniz-Filho et al., 2013; Hortal et al., 2015). Some techniques present a rapid, efficient, and cheap way of matching semaphoronts under the same species hypothesis (e.g., Yeo et al., 2018), but the inconvenient truth is that we are not able to safely identify and pair an infinity of semaphoronts, so that the Haeckelian shortfall could be considered as a serious challenge to increase the quality of the existing phylogenies.
4 INTERCONNECTIONS BETWEEN THE HAECKELIAN AND THE OTHER SHORTFALLS
The effects of the Haeckelian shortfall go far beyond the simple questions related to total evidence in a phylogenetic context, as all biodiversity shortfalls are interconnected to varying degrees, according to spatial, temporal, and taxonomic coverage (Hortal et al., 2015). A summary of the relationships and effects of a given shortfall on the others was presented by Hortal et al., 2015:533). Following the same rationale, we believe the Haeckelian shortfall would strongly affect all the others, including pronounced effects on the intrinsic shortfalls, that is, Eltonian (distinct semaphoronts could interact with different organisms), Hutchinsonian (the scenopoetic conditions may be radically different for various semaphoronts, e.g., a mayfly nymph and an adult, a tadpole and an adult frog), and Raunkiaerean (traits and functions may change as a response to the deep changes in the life history of organisms). The Haeckelian shortfall also regards a dimension of our ignorance on the basic entities, in the same way as the Linnean (see Hortal et al., 2015). But we argue that the first is not a simple dimension of the latter as, far beyond of species description and recognition, the Haeckelian shortfall gathers all we do not know about the ontogeny itself (Figure 1).
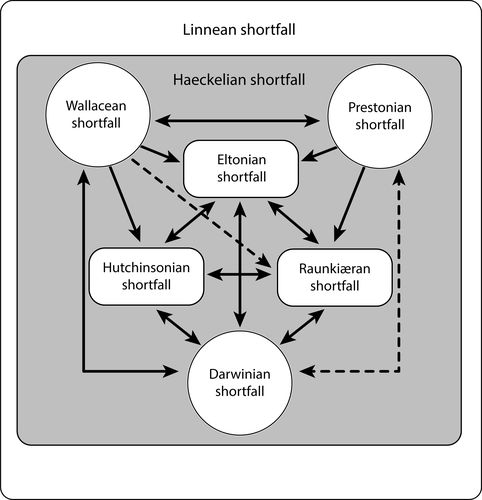
4.1 Linnean and Darwinian shortfalls
Overcoming the Haeckelian shortfall also obviously depends on the recognition and description of species, so that it is greatly impacted by the Linnean shortfall. But it is clear that a two-way effect exists, given that the lack of access to semaphoronts may preclude the proposition of meaningful species hypothesis (i.e., decisions likely to alleviate our ignorance on biodiversity). In short, taxonomy is concerned with taxa and nomenclature with their names (de Queiroz, 2006). So, we could consider the question on two fronts: The taxonomic issue, as recognizing a species, at least a meaningful species, would benefit from knowledge of distinct semaphoronts; and the nomenclatural issue, as if the name is associated with a determined semaphoront, a proper association among them is critical for the correct application of names. As Orton (1955:76) stated long ago, “judgment in taxonomy and evolution should be based on a unified summary of the data obtainable from the entire developmental pattern rather than on any single stage.” It is obvious that following the ontogenetic development of the individuals of a given species, recognizing each ontogenetic stage is a herculean task. Most times it is necessary to make decisions based on a few (or even a single) semaphoront, and although it is often possible to make satisfactory decisions in these scenarios (e.g., a given semaphoront is very different from the same semaphoront of the individuals of other species), sometimes we have to pay the price: the impossibility of identifying a specimen, since names are given more often or even exclusively to a single semaphoront (i.e., male adults in the case of insects); or the erection of incomparable taxa and potential synonyms (i.e., when clearly related species are described based on distinct semaphoronts).
Even if the association of semaphoronts (including male and female, see above) is still competently done in studies where the morphology of reared organisms is analyzed (e.g., Archangelsky et al., 2017; Fuhrmann et al., 2019; Kluge, 2018; Morón, 2017; Soldán & Godunko, 2013; Tormos et al., 2014), in many cases morphology alone appeared to be unable to associate males with females, and immature stages with adults, mainly for the drastic differences between the sexes and the life stages (Zhang & Weirauch, 2011). Moreover, unlike most other taxonomic characters, sequence polymorphisms are maintained throughout the life cycle (Gattolliat & Monaghan, 2010). This way, molecular techniques have proven to be an important ally in associating distinct semaphoronts (e.g., Elías-Gutiérrez et al., 2019; Jiruskova & Bocak, 2015; Miller et al., 2005; Salles et al., 2016; Wei & Ren, 2019; Yeo et al., 2018).
An interesting example of how knowing and associating semaphoronts, that is, removing the Haeckelian shortfall, may spur taxonomy (both regarding the knowledge of biodiversity per se but also the application of names), was presented by Zhang and Weirauch (2011). They faced a taxonomic problem of a group of assassin bugs (Hemiptera: Reduviidae): The species of two monotypic genera, Bekilya Villiers, 1949 and Hovacoris Villiers, 1964, were described from macropterous males, whereas two species of a third genus, Mutillocoris Villiers, 1964, were described based on brachypterous females. Using both morphological and molecular techniques, they found that the species of Mutillocoris correspond in fact to the females of the first two genera. They also found that the type species of Mutillocoris is a Bekilya and, as an important taxonomic rearrangement, they proposed the synonymy of these two genera. As a further corollary, six new species were recognized and described, and the Darwinian shortfall was not set aside, as they also proposed a phylogeny for the group.
The Haeckelian shortfall has also direct impacts on the recognition of suprageneric groups. A monotypic mayfly genus, Coryphorus Peters, 1981 (Ephemeroptera: Coryphoridae), whose description was based only on nymphs (Peters, 1981), was placed at various arrangements around two mayfly families, while only this semaphoront was known. When eggs and winged stages (male and females imagoes, male subimago) of Coryphorus aquilus Peters, 1981 were collected, and associated with the previously known nymphs, the authors (Molineri et al., 2001) realized a series of autapomorphies that led them to propose a new family, Coryphoridae.
Regarding the Darwinian shortfall, using distinct semaphoronts for proposing phylogenetic hypotheses brings the obvious gain that those hypotheses would rely on something closer to the goal of total evidence. Additionally, a new scenario for testing current hypotheses would become apparent: With the inclusion of new semaphoronts in the data set, either those hypotheses would become stronger (including here stronger support, i.e., bootstrap and Bremer, for the monophyly of groups), or the existing hypotheses should be revisited. Empirical evidence exist for both cases, as analyses considering distinct semaphoronts may have a broad correspondence with previous phylogenetic hypothesis (e.g., Badano et al., 2017; Miranda et al., 2014), even with higher support (e.g., Alarie & Bilton, 2005; Pugener et al., 2003), or introduce alternative clades (e.g., Lawrence et al., 2011; Straka & Bogusch, 2007).
Exploring distinct semaphoronts could also be very useful considering that some semaphoronts are regarded to carry much more phylogenetic information, as they could be subjected to distinct selective pressures. An interesting example was presented by Lee et al. (2007) who have studied psephenid beetles (Coleoptera: Psephenidae). The first question, where the Haeckelian shortfall already appears, is that the identification of those beetles relies on adult stages, although their larvae are more easily found, and are both long-lived and common in streams (Lee et al., 2007). Moreover, the authors also comment that data from immatures are prone to be more phylogenetically informative, as these semaphoronts are subject to more selective pressures for inhabiting various habitats, whereas adults, the short-living semaphoront, are responsible only for reproduction (Lee et al., 2007). Thus, there is a semaphoront on which taxonomy is based, and other semaphoronts (mainly the larvae) regarded to encompass much more of the life history of the biological group. Correctly associating them would certainly ensure a much more comprehensive progress in understanding the phylogeny of the group, since the terminals would be accordingly named, regardless of the selected semaphoront, and immatures, regarded to provide more relevant phylogenetic information (Lee et al., 2007), could be safely included in the analysis. As Lee et al. (2007:502) argue, “if associated with adults, larval characters would contribute significantly to phylogenetic studies.” As a picture of the Haeckelian shortfall, however, the authors regret that “unfortunately, few species have had larval-adult associations, especially for those from the Oriental Region” (Lee et al., 2007:502).
The understanding that some semaphoronts are more useful than others in reconstructing phylogenies is suggested for some particular groups. Two illustrative examples involve dragonflies (Insecta: Odonata) and some plesiotypic anurans (Lissamphibia: Anura). In the case of dragonflies, some results indicate that imaginal characters, wing venation in particular, are often prone to homoplasy, whereas larval characters are regarded to be much more informative, especially for deep-level systematic studies (Fleck et al., 2008). In the same direction, Pugener et al. (2003) state that the derived morphology of adults of the “archaeobatrachians” (some plesiotypic anurans) does not carry an adequate phylogenetic signal, so that relying on the larval morphology is crucial for recovering the evolutionary history. By adopting an approach based on both semaphoronts, they were able to propose a hypothesis assumed to resolve the long-lasting incongruence between the phylogenies derived from characters of adults or larvae. They also stressed that in analyses conducted separately for data from adults and larvae, the higher support values were found for groups revealed by larval characters.
In short, the Haeckelian shortfall is a serious impediment to our knowledge of phylogenies. Phylogenetic inference using phenotypic data has been hindered by the scarcity of information on different ontogenetic stages (e.g., Haas, 2003). As noted by Lawrence et al. (2011:72) when studying the phylogeny of beetles, based both on adult and larval characters, the position of some clades in places other than those expected by current phylogenetic concepts may be also related to the “lack of knowledge concerning females or larvae” (i.e., the crude Haeckelian shortfall).
4.2 Wallacean and Prestonian shortfalls
Regarding the other two extrinsic shortfalls (sensu Hortal et al., 2015), not knowing some semaphoronts may hinder the analysis of geographical distribution and abundance of species, particularly in cases where the taxonomy of the group and/or ecological data are based on a single or few semaphoronts. When new semaphoronts become available, the delimitation of the distribution of a given taxon can be done much more accurately (e.g., Argañaraz & Rubio, 2011; Magalhães et al., 2017), so that the Wallacean shortfall can be more properly addressed. A very pertinent example of such gain, especially when thinking about groups for which studies are traditionally based on a single semaphoront, can be found in Cranston and Tang’s (2018:41) statement about the distribution of Skusella subvittata (Skuse, 1889) (Diptera, Chironomidae): “a wider distribution of S. subvittata in Australia and Asia is revealed by extensive pupal exuviae sampled from drift.”
The Haeckelian shortfall may lead to equally strong effects also on the understanding of abundances. Some semaphoronts are simply ignored or discarded both at the time of collecting or sorting (Yeo et al., 2018), which leads to the very pertinent statement of Yeo and colleagues that, regarding insects, “the commonness-of-rarity problem is partially caused by not assessing diversity based on all life-history stages” (Yeo et al., 2018:679). Estimates of the abundance of species, even in most applied contexts (e.g., pest control or biomonitoring), can be substantially different when distinct semaphoronts are considered (e.g., Buss & Salles, 2007; Park et al., 2009), which becomes even more problematic in cases where the responses of a given semaphoront are assumed to represent the species as a whole.
4.3 Intrinsic shortfalls
The scenopoetic niches of species, the focus of the Hutchinsonian shortfall, can be quite different when considering ontogeny. As an example, the variation in the cold tolerance between larval and adult stages may be emphasized (e.g., Bouchard et al., 2006; Clough et al., 1990). Detailed knowledge of the natural history of species becomes even more important when organisms present more complex life cycles, different life stages occupy distinct niches, and respond to environmental variables at varying scales (Taboada et al., 2013). Regarding specifically conservation approaches, the efficiency of actions is directly related to understanding the environmental tolerances of organisms across their life cycles (Turlure et al., 2009). We should critically evaluate the effects that disregarding niche variation across the ontogeny has on niche modeling and conservation programs (Taboada et al., 2013).
Constructing a link between the Haeckelian and the Eltonian shortfalls would be quite complicated if organisms interact with the same species during their entire ontogeny. But this is certainly not the case. Both when considering, in a trophic context, different stages of ontogeny in a broader (i.e., including age and body size increase) or a more strict way (easily identifiable stages), one can easily perceive that the idea of ontogenetic niche (Werner & Gilliam, 1984; see also Hammerschlag-Peyer et al., 2011) is very appropriate. In a paper where an idea of an ontogenetic perspective of community ecology is defended by detailed arguments, Nakazawa (2015:347) comments that “community ecology has traditionally assumed that species are composed of identical individuals with invariant traits and ignored the potentially important ecological roles of ontogenetic niche shifts.” Outstanding examples of this variation exist, as in the relation between hawkmoths of the genera Manduca Hübner, 1807 (Lepidoptera, Sphingidae) and Datura wrightii Regel (Solanaceae), where, while adult moths forage for nectar and oviposit on the leaves, larvae feed upon the same plant (Alarcón et al., 2008; Bronstein et al., 2009). Other remarkable examples regard holometabolous insects whose larvae and adults house entirely different symbionts in response to habitat changes and diet of these semaphoronts (Hammer & Moran, 2019).
As we also consider here the limited knowledge on males or females as a facet of the Haeckelian shortfall, there are cases in which the semaphoronts present pronounced distinct interactions, as in orchid bees where only the males seek aromatic compounds on plants, mainly orchids (see, e.g., Dressler, 1982). Ontogenetic shifts may couple agonistic and mutualistic networks without new species being necessarily included (Picot et al., 2019; Yang & Rudolf, 2009), and those shifts could even justify considering different life stages as distinct nodes in food webs (Olson, 1996). But, as a lot of information on semaphoronts is still lacking, the Haeckelian shortfall haunts us also that way.
In order to address the effects of the Haeckelian shortfall on the Raunkiaeran shortfall, the plea of Hortal et al. (2015:529) seems particularly relevant: “a major characteristic of the Raunkiaeran shortfall is that the traits that are generally measured are often the most simple, rather than the most ‘functional’.” We could simply append “in the most known, collected and/or available semaphoront” at the end of the original sentence, since it is undeniable that a very strong semaphoront bias exists in functional ecology and evolution. Even when more than one semaphoront is known (e.g., adults and larvae), measuring traits in some of them (e.g., soft-bodied and microscopic larvae) is much more challenging. The tradition of studying some particular semaphoronts (e.g., the taxonomic/ ecological tradition of studying male orchid bees or nymphs of baetid mayflies) can make things even worse. Studying traits of a given semaphoront may significantly help to illuminate the theory mainly constructed from the study of traits of other semaphoronts (e.g., Luiz et al., 2013), or even support the understanding of patterns occurring at distinct moments of the ontogeny, including interactions with other species (e.g., Dingeldein & White, 2016).
When defining a trait, Violle et al. (2007:884), suggested that traits should be used “at the level of the individual only.” Moreover, in their definition, “no information external to the individual (environmental factors) or at any other level of organization (population, community or ecosystem) is required to define a trait.” One can therefore notice that any issue related to trait variation over ontogeny is certainly consistent with this definition. We should also consider that there must necessarily be an ontological connection between traits of a precedent semaphoront and the fitness of the following ones (for insects, see, e.g., Nguyen et al., 2019 and references therein). After all, traits in one given life-history stage are rarely able to evolve without impacting other stages (Marshall & Morgan, 2011). It is important to realize, for instance, which of the adult traits that are able to affect its fitness are shaped by stressors affecting the larvae (Campero et al., 2008). Finally, as also suggested by Hortal et al. (2015:533), “knowledge about abiotic and biotic components of the niche and the functional traits of each species are also tightly linked.” This way, the impact of the Haeckelian shortfall over the Raunkiaerean is very much related to what was presented for the Hutchinsonian and the Eltonian shortfalls.
5 THE HAECKELIAN SHORTFALL AND DNA-BASED SYSTEMATICS
Despite some caveats, the DNA revolution brought a tremendous opportunity for resolving problems in taxonomy and systematics (Kapli et al., 2020). Studies previously restricted to morphological approaches started including molecular information, as sequencing and PCR technologies became available in the late 1970s (see Young & Gillung, 2020). Current technologies allow the production and availability of an amount of data that was unthinkable a few years ago (e.g., Kapli et al., 2020). But some shortcuts (see, e.g., Seberg et al., 2003) cannot be taken, and other data sets, mainly morphological data, were and still are essential for taxonomy and systematics (e.g., Beutel & Kristensen, 2012). Molecular phylogenetics and phylogenomics are powerful tools for mitigating biodiversity shortfalls (mainly Linnean, Darwinian and also the Haeckelian) but, in short, no matter how immense the amount of molecular information is, it will not be able to answer all we wonder and need to know about biodiversity and evolution. A comprehensive integration of characters from distinct semaphoronts with DNA sequence data seems pivotal to resolve the phylogeny of most taxa (see, e.g., Badano et al., 2017). After all, even if the DNA-based taxonomy and systematics achieve incredible progress, we draw attention to two important bottlenecks that would only be overcome with studies comprising an even more integrative approach, considering distinct semaphoronts.
Even though we have access to comprehensive and robust molecular phylogenies, the inference of evolutionary histories depends on fine-scale, semaphoront-rich morphological databases (and also behavioral, biochemical, etc.). The explanatory power behind phylogenetic trees is only feasible if morphological data are combined, so that the evolutionary changes become evident (Schmidt-Rhaesa, 2009). As stated by Peters et al. (2014:1), “the combination of well-resolved phylogenies obtained by phylogenomic analyses and well-documented extensive morphological datasets is an appropriate basis for reconstructing complex morphological transformations and for the inference of evolutionary histories.” In the same direction, Simonsen et al. (2012:307) commented that “understanding morphology is essential for understanding and evaluating the evolutionary scenarios phylogenetic trees are supposed to illustrate.”
The metazoan phylogeny itself properly illustrates this scenario. We have access to topologies built from a huge molecular dataset (Borowiec et al., 2015), but understanding animal evolution continues to be interpreted directly through what we know about larval structure (see, e.g., Nielsen, 1994). Several groups that were widely supported in molecular analysis make a lot more sense when the synapomorphies of larvae are considered (see, e.g., Dunn et al., 2008). Having a reliable reconstruction of the ancestral eumetazoan larva (Nielsen, 2013), for instance, is crucial for understanding how the evolution of animals took place. Here, the Haeckelian shortfall meets Haeckel himself, as he was able to propose a theory, the gastraea theory, that all metazoans have evolved from a pelagic, planktotrophic ancestor (Haeckel, 1874; see Nielsen, 2013). Testing Haeckel's hypothesis and all those that continue to be proposed completely depend on good phylogenies and on the extensive knowledge of the morphology of the largest possible number of semaphoronts.
An interesting example was presented by Peters et al. (2014) using a transcriptome-based phylogenetic analysis to hypothesize the relations among holometabolous insects. From the inferred topology, they used a morphological database for mapping the evolutionary transformations of character states in order to infer the ground plan of the ancestral larva and the ancestral adult. In the specific case of larvae, they were able to propose that “an orthognathous larva belongs to the ground plan of Holometabola, with compound eyes and well-developed thoracic legs, externally feeding on plants or fungi” (Peters et al., 2014).
Overcoming the Darwinian and Linnean shortfalls relies on assessing fine phylogenetic hypotheses but also on ensuring that entities have meaningful names at the different levels of the taxonomic hierarchy. Thus, the Haeckelian shortfall becomes even more central in cases where acquiring molecular information of some species is difficult, and especially when dealing with name-bearing types.
6 CONCLUSION AND PERSPECTIVES
We proposed here the Haeckelian shortfall referring to the lack of knowledge about the distinct semaphoronts of species/taxa and, ultimately, to what we do not know about the ontogeny of the individuals that instantiate a species. This shortfall comes to join the other several shortfalls previously presented (see Hortal et al., 2015) as it captures, to our understanding, a dimension of biodiversity that had not been explicitly recognized.
Much of the relevance of the Haeckelian shortfall can be perceived by its own simplicity and, so to speak, obviousness. We can assume a general agreement that we have to unite forces against our ignorance on most of semaphoronts of the individuals of the species we already know, and that we have to decisively consider the ontogeny in the studies on biodiversity. As noted by Hortal and colleagues, today we have only a glimpse of our ignorance because a lot has already been done. But much remains to be uncovered, and we understand that the Haeckelian shortfall should really be considered among the main tasks. For achieving this task, we should strongly rely on integrative research (constructing morphological databases, rearing organisms, and using molecular techniques available to taxonomy and systematics), which can play a decisive role on removing the Haeckelian shortfall.
Biodiversity studies are increasingly expected to provide knowledge able to support decision making (e.g., Magnusson, 2019). And such task encompasses both the production of relevant idiographic and nomothetic knowledge (for a fine discussion on idiographic and nomothetic knowledge, see Currie, 2019; for a context more applied to biodiversity studies, see Gould, 1980; Cotterill & Foissner, 2010). Accordingly, the importance of studying semaphoronts increases: If in the idiographic sphere it represents at first sight only the purest and most descriptive access to the ontogeny of the individuals that constitute a species, this will support the production of relevant nomothetic knowledge, mainly in an applied context, where ontogeny could be used in building good mechanistic models able to support effective conservation actions (see, e.g., Turlure et al., 2009). Adults, surely the most studied semaphoront, comprise only the “surface” of the phenotypic morphospace, a small slither of the entire morphological diversity. If we consider the morphospace as a multidimensional volume, studying only one semaphoront implies accessing merely a single face of this construct. Exploring the developmental morphospace would certainly contribute to the development of a nomothetic eco–evo–devo (we are aware that the nomothetic dimension is not equally achievable by “eco,” “evo,” and “devo,” considering the very idiographic nature of some aspects of them). We should remember that, as stated by Assis (2018:2865), “we are entering into the developmental evolutionary dimension of biodiversity conservation, rather than focusing only on the particularities of both genetic sequences and adult forms.”
Although we wished the Haeckelian shortfall was completely overcome (i.e., all the semaphoronts of all species were known), this is an epistemologically impossible (and also a naive) endeavor. But we should start with all those species or groups that we already recognize as relevant to answer central questions in evolution, ecology, and conservation. We should try to “maximize nomothetic return for each idiographic investment” (to quote a brilliant sentence by Jenner & Wills, 2007:312) in order to remove the Haeckelian shortfall.
We dedicate the present study as a tribute to Ernst Haeckel for the recent 100th anniversary of his death.
ACKNOWLEDGEMENTS
We thank Conselho Nacional de Desenvolvimento Científico e Tecnológico, CNPq/Brazil (grant/award numbers 301636/2016-8 to MRP and 309666/2019-8 to FFS), for support. We also thank Dr. Thomas Stach and the anonymous reviewers for all their constructive and pertinent comments.




