Soft sponges with tricky tree: On the phylogeny of dictyoceratid sponges
Abstract
Keratose (horny) sponges constitute a very difficult group of Porifera in terms of taxonomy due to their paucity of diagnostic morphological features. (Most) keratose sponges possess no mineral skeletal elements, but an arrangement of organic (spongin) fibers, with little taxonomic or phylogenetic information. Molecular phylogenetics have targeted this evolutionary and biochemically important lineage numerous times, but the conservative nature of popular markers combined with ambiguous identification of the sponge material has so far prevented any robust phylogeny. In the following study, we provide a phylogenetic hypothesis of the keratose order Dictyoceratida based on nuclear markers of higher resolution potential (ITS and 28S C-region), and particularly aim for the inclusion of type specimens as reference material. Our results are compared with previously published data of CO1, 18S, and 28S (D3-D5) data, and indicate the paraphyly of the largest dictyoceratid family, the Thorectidae, due to a sister group relationship of its subfamily Phyllospongiinae with Family Spongiidae. Irciniidae can be recovered as monophyletic. Results on genus level and implications on phylogenetic signals of the most frequently described morphological characters are discussed.
1 INTRODUCTION
In the last couple of decades, our knowledge on phylogenetic relationships of sponges, particularly demosponges, experienced major turmoil when molecular data demonstrated serious pitfalls in the classical, morphology-based classification (see, e.g., Boury-Esnault, 2006; Cárdenas, Pérez, & Boury-Esnault, 2012; Erpenbeck & Wörheide, 2007; Redmond et al., 2013; Wörheide et al., 2012). This resulted in a fundamentally revised classification at order level (Morrow & Cárdenas, 2015). However, revisions of most intra-ordinal relationships are still due for revision. A particularly difficult order of sponges is the Dictyoceratida (Subclass Keratosa), which possess a skeleton of organic material (spongin) only and lack mineral skeletal elements (with the exception of Vaceletia, which possesses a hypercalcified secondary limestone skeleton instead of spongin fibers, see Wörheide, 2008). Therefore, these sponges were historically assigned to the "horny" sponges. The spongin skeleton renders specimens of some genera useful as bathing sponges, but at the same time limits the suite of diagnostic features for morphological classification and phylogeny. Morphologically, all dictyoceratids share the presence of this anastomosing spongin fiber skeleton that often make up a significant proportion of the body volume. Fibers develop from multiple points and are organized into primary, secondary, and sometimes tertiary fibers (Cook & Bergquist, 2002e). Earlier molecular studies supported monophyly of Dictyoceratida, their sister group relationship to order Dendroceratida as subclass Keratosa, and their distinction from other horny sponge lineages (e.g., Verongiida, subclass Verongimorpha) (Borchiellini et al., 2004; Erpenbeck, Sutcliffe, et al., 2012; Hill et al., 2013; Redmond et al., 2013; Thacker et al., 2013). Internal relationships, however, are still insufficiently understood, although are mandatory for a variety of downstream research (Boufridi et al., 2017; Chianese et al., 2017; see e.g., Erpenbeck, Hooper, et al., 2012).
At the last major (morphology-based) revision of sponge classification, in the Systema Porifera (Hooper & Van Soest, 2002), Dictyoceratida were separated into the four taxa at the family level Dysideidae, Irciniidae, Spongiidae, and Thorectidae, with the latter being divided into the subfamilies Thorectinae and Phyllospongiinae (Cook & Bergquist, 2002d, 2002e). A fifth family, Verticillitidae, was added subsequently (Morrow & Cárdenas, 2015; Wörheide, 2008). So far, molecular studies targeting shallow-level relationships of Dictyoceratida provided insufficient resolution or conflicting data: The first comprehensive molecular approach based on the partial mitochondrial cytochrome c oxidase subunit 1 gene (CO1) and the D3-D5 partition of the nuclear large ribosomal subunit gene (28S) confirmed monophyly of the families Dysideidae and Irciniidae, and confirmed Dysideidae as sister to all other families as well, but failed to resolve Spongiidae and Thorectidae relationships (Erpenbeck, Sutcliffe, et al., 2012). Likewise, Redmond et al. (2013) and Thacker et al. (2013) confirmed the distinct position of Dysideidae, based on the nuclear small ribosomal subunit gene (18S) and full-length 28S, respectively, but could not robustly resolve the relationship of other dictyoceratid taxa either. Undoubtedly, the molecular markers used so far bear insufficient resolution potential to answer all dictyoceratid phylogenetic questions.
In the present study, we aim to unravel the phylogenetic relationships of dictyoceratid sponges by employing faster evolving molecular markers. We use the C-region of 28S, which has been successfully used in sponge molecular taxonomic studies (Erpenbeck, Voigt, et al., 2016; e.g., Voigt & Wörheide, 2016), and the internal transcribed spacers 1 and 2 (ITS, including the 5.8S rRNA gene). ITS is a classical marker on species level and below (see, e.g., Borchiellini, Chombard, Lafay, & Boury-Esnault, 2000), but in Dictyoceratida so far recruited for studying metabolite distribution only (Boufridi et al., 2017; Chianese et al., 2017; Erpenbeck, Hooper, et al., 2012).
Conclusive (molecular) phylogenies must be based on well-identified species. Most dictyoceratid phylogenies, however, suffer from incomplete and ambiguous specimen identification (Erpenbeck, Sutcliffe, et al., 2012; Redmond et al., 2013; Thacker et al., 2013) due to the difficult (morphology-based) taxonomy (see also Cook, 2007). Type specimens, particularly holotypes, are the only unambiguous reference points for taxonomic delineation, but not frequently used for sponge molecular phylogenetic studies due to difficult accessibility and bad DNA qualities (see review in Erpenbeck, Ekins, et al., 2016). The present study therefore attempts to use type material where possible, or other well-identified specimens such as Systema Porifera reference material. The results of the new dictyoceratid ITS and 28S (C-region) molecular analyses are compared with phylogenies obtained from 18S (Redmond et al., 2013), CO1, and 28S (D3-D5) (Erpenbeck, Sutcliffe, et al., 2012) markers in order to summarize our current knowledge and formulate a phylogenetic hypothesis for dictyoceratids.
2 MATERIALS AND METHODS
Sponge specimens or fractions thereof, including type material, were borrowed or obtained from the Queensland Museum (Brisbane, Australia), Australian Museum (Sydney, Australia), from the Universalmuseum Joanneum (Graz, Austria; formerly Landesmuseum Joanneum Graz), from the Naturhistorisches Museum Basel (Basel, Switzerland), from the Zoological Museum Amsterdam (now NCB Leiden, the Netherlands), from the Natural History Museum (London, Great Britain), and from the collections of Steve de C. Cook (Auckland, New Zealand) (see Appendix 1 for a complete list of specimens).
PCR amplifications were conducted in 12.5 μl reactions: 5X Green GoTaq® Flexi Reaction Buffer (Promega), 25 mM MgCl2 (Promega), 10 mM dNTP (Bioline), 5 mM of each primer (Metabion), and 1 unit of Taq polymerase (GoTaq, Promega). Usage of the additive bovine serum albumin (BSA, 10 mg/ml) significantly improved the amplification yields. Polymerase chain reactions (PCRs) for both ITS and 28S were conducted under the following conditions: 3 min at 95°C (denaturation), 35 cycles at 95°C for 30 s (heating), 51°C for 30 s (annealing, for primer combinations, see Table 1), and 72°C for 1 min (extension), followed by 72°C for 5 min (final extension). For some samples, touchdown PCRs prove to be more efficient than the standard protocol: 3 min at 95°C (denaturation), 20 cycles at 95°C for 30 s (heating), 55–45°C (annealing; −0.5°C per cycle), and 72°C for 1 min (extension), followed by 20 cycles at 95°C for 30 s (heating), 50°C (annealing), and 72°C for 1 min (extension), concluded by 72°C for 5 min (final extension). PCR products were isolated cleaned up with the freeze-squeeze method (Tautz & Renz, 1983) from 1.5% agarose gels. Cycle sequencing products were generated with BigDye Terminator v3.1 followed by Sanger sequencing on an ABI 3730 in the Genomic Sequencing Unit of the LMU Munich. Forward and reverse reads were assembled and corrected with CodonCode Aligner 3.7.1 (http://www.codoncode.com) after checking for contaminants by BLAST against NCBI GenBank. Intragenomic polymorphisms (IGP) were recoded following the IUPAC ambiguity codes for nucleotides. The assembled and checked sequences were aligned with MAFFT (Katoh & Standley, 2013) under default settings as implemented in Geneious Prime® 2019.0.4 (http://www.geneious.com; Kearse et al., 2012) and subsequently optimized by eye. The data set was complemented with homologous sequences of the ITS regions and 28S C-region as published in GenBank (see Figures S1–S4). Data for CO1 and 28S (D3-D5) consist predominantly of previously published sequences (see Figures S1–S4), plus 39 yet unpublished sequences (1 of 28S (D3-D5), 38 of CO1) generated in course of the study of Erpenbeck, Sutcliffe, et al. (2012). See boldfaced accession numbers in Appendix 1 and Erpenbeck, Sutcliffe, et al. (2012) for details of sequence generation.
| Name (reference) | Nucleotide sequence | Target region | Amplicon size |
|---|---|---|---|
| RA2_keratose (fwd)a | 5′ GRA TGG TTT AGT GAG ATC TT 3′ | ITS | |

|
~660 bp | ||
| ITS2.2_keratose (rev)a | 5′ AAA TTC AGC GGG TAG YCT GG 3′ | ITS | |

|
~365 bp | ||
| 5.8S_keratose (fwd)a | 5′ TGA CAA CTT CTG ACG GT 3′ | ITS-2 | |
| 28S-C2_keratose (fwd)a | 5′ GAA AAG AAC TTT GRA RAG AGA GTC 3′ | 28S | |

|
~340 bp | ||
| 28S-D2_keratose (rev)a | 5′ CCG TGT TTC AAG ACG GGT CGR ACG AG 3′ | 28S | |
| RA2-fwdb | 5′ GTC CCT GCC CTT TGT ACA CA 3′ | ITS | |

|
~660 bp | ||
| ITS2.2-revb | 5′ CCT GGT TAG TTT CTT TTC CTC CGC 3′ | ITS | |
| ~330 bp | |||
| 5.8S-1-fwdc | 5′ GTC GAT GAA GAA CGC AGC 3′ |
ITS-2

|
|
| 28S-C2-fwdc | 5′ GAA AAG AAC TTT GRA RAG AGA GT 3′ | 28S | |
| ~340 bp | |||
| 28S-D2-revc | 5′ TCC GTG TTT CAA GAC GGG 3′ |
28S

|
All sequences are submitted to the European Nucleotide Archive (see Appendix 1 for accession numbers [LR######]). For all four data sets (ITS, 28S C-region, (28S (D3-D5), and CO1) maximum-likelihood reconstructions were generated with RAxML 8 (Stamatakis, 2014) as implemented in Geneious Prime® 2019.0.4 under the GTR GAMMA I model and 1,000 rapid bootstrap replicates. The alignments used in this study are freely available at https://github.com/PalMuc/Soft-Sponges-Tricky-Tree.
3 RESULTS AND DISCUSSION
For a total of 236 dictyoceratid specimens, new sequences were generated (see Appendix 1). As not all fragments for every specimen were amplifiable and/or available from NCBI GenBank, the data sets for ITS (93 taxa (of which 91 newly sequenced for this study)/ 808 characters), 28S C-Region (148 (121)/ 347), 28S D3-D5 region (76 (1)/ 549), and CO1 (152 (38)/ 495) (see Appendix 1 and Figures S1–S4 for the individual gene trees) differ in their taxon content. The summarizing overview on the phylogenetic results is given in Figure 1.
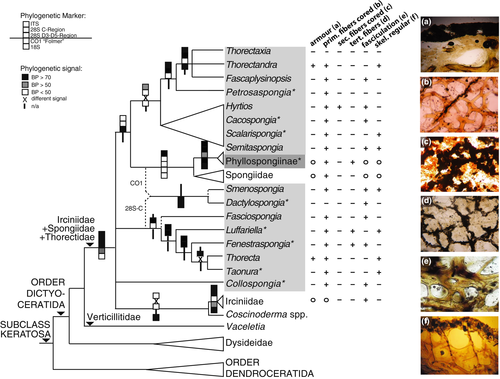
The dictyoceratid taxa fall into clades differently supported by the individual fragments (see Figure 1). These molecular analyses, as currently the most comprehensive to unravel the phylogenetic relationships of dictyoceratid sponges including type (and other reference) material, demonstrate that family Thorectidae sensu Cook and Bergquist (2002d) cannot be upheld. Thorectidae was erected by Bergquist (1978) who regarded concentric fiber lamination as a distinct and combining feature among dictyoceratid sponges as opposed to the homogeneous fibers in Spongiidae. However, Sanders and van Soest (1996) remarked that several members of Spongiidae possess laminated fibers, usually invisible with light microscopy rendering fiber lamination an unsuitable phylogenetic character. Despite these concerns, Bergquist at al. (1999) reclassified foliose Dictyoceratida from Spongiidae into Thorectidae, based on fiber structure, erecting a distinct subfamily Phyllospongiinae (foliose sponges) alongside all other thorectids (which formed Subfamily Thorectinae). Nevertheless, Cook and Bergquist (2002d) regarded Thorectinae as "heterogeneous group of sponges," "difficult to objectively define," and as a "catch-all" for all non-phyllospongiine thorectids. Our studies demonstrate that Thorectidae (particularly Subfamily Thorectinae) constitute a paraphyletic assemblage of dictyoceratid taxa, as indicated in earlier molecular studies (Erpenbeck, Sutcliffe, et al., 2012; see discussion in Morrow & Cárdenas, 2015; Redmond et al., 2013; Thacker et al., 2013). Fiber laminations (see, e.g., fig. 15 in Cook, 2007), as observed in Thorectidae (see, e.g., Cook & Bergquist, 2002d), are also reported for Dysideidae (Cook & Bergquist, 2002a) that branch first from all other dictyoceratid families. Therefore, such thorectid fiber lamination should be regarded as an ancient dictyoceratid trait, plesiomorph in thorectids, and therefore not suitable to morphologically define any phylogenetic clade within the Dictyoceratida.
Subfamily Phyllospongiinae, however, can be recovered, although with a taxon composition emended back to Keller's (1889) core taxa Carteriospongia and Phyllospongia, plus Strepsichordaia. This constellation is underlined by several in-depth studies that included types of Strepsichordaia lendenfeldi Bergquist, Ayling & Wilkinson (AM Z5026), and Carteriospongia foliascens (Pallas) BMNH 1925.11.1.41 (see Abdul Wahab, Fromont, Whalan, Webster, & Andreakis, 2014; Galitz et al., 2018) (sequencing of the holotype of Phyllospongia papyracea (Esper) BMNH 1931.4.1.1 was attempted but unsuccessful). Of the remaining phyllospongiine genera (Cook & Bergquist, 2002d), Candidaspongia Bergquist, Sorokin & Karuso, 1999 has been identified as Dysideidae (Galitz et al., 2018; Redmond et al., 2013) and Lendenfeldia Bergquist, 1980 requires revision—lectotype sequencing of its type species L. frondosa (Lendenfeld) (BMNH 1877.5.21.1697) has been attempted, but without success. Further details on the internal relationships of Phyllospongiinae and paraphyly of its genera are given in Abdul Wahab et al. (2014) and Galitz et al. (2018).
However, Phyllospongiinae form a clade with Spongiidae, thereby corroborating the former Spongiidae sensu Gray. Spongiidae were mostly recovered as monophyletic. This clade comprises all the specimens of Spongia (including a Systema Porifera reference of type species Spongia officinalis (Linnaeus) SDCC/RF001), Rhopaloeides (including a Systema Porifera reference of type species Rhopaloeides odorabile Thompson, Murphy, Bergquist & Evans, SDCC/RF067), and Hippospongia from several different studies as published in NCBI GenBank. Several specimens identified or published as Spongia do not form a clade and prompt for a revision of the spongiid taxa (see also Redmond et al., 2013). Unfortunately, success rate of type and reference material of Spongiidae was low, as PCR of the neotype of S. officinalis BMNH 1883.12.4.28 failed, likewise sequencing the holotypes of R. odorabile (AM Z4965) and Leiosella levis (Lendenfeld) (BMNH 1886.8.27.319) furthermore historic comparative material for Hippospongia communis (Lamarck) (as H. equina (Schmidt) BMNH 1899.5.2.2, see Cook & Bergquist, 2002c) did not result in sequences suitable for phylogenetic analyses. Consequently, we refrain from hypothesizing on the internal phylogenetic relationships of Spongiidae until more molecular data from reference material are obtained. A morphological feature combining Phyllospongiinae and spongiids might be found in the apparently more homogeneous fiber structure in contrast to Thorectinae. Phyllospongiinae were described with "successive fibrous layers," which remain tightly adherent, producing an overall homogeneous structure with visible contiguous laminae" (Cook & Bergquist, 2002e), and Spongiidae are defined by their homogenous fiber structure (Cook & Bergquist, 2002c; objected by Sanders & Van Soest, 1996).
The thorectid genera Thorectandra, Thorectaxia, Fascaplysinopsis, and Petrosaspongia form a clade with the latter splitting first. Genus Petrosaspongia Bergquist, 1995 currently comprises two species, and the holotype of the type species Petrosaspongia nigra Bergquist (QM G304685) was analyzed. Thorectandra, Thorectaxia, and Fascaplysinopsis form a monophyletic group. The holotype for Thorectandra corticatus Lendenfeld, type species of Thorectandra, is unknown (Hooper & Wiedenmayer, 1994), but its reference material analyzed for the Systema Porifera was sequenced (SDCC/RF016, see Cook & Bergquist, 2002d). Although histologically regarded as similar (Cook & Bergquist, 2002d), Thorectandra is phylogenetically distant to Thorecta (see below), prompting a re-evaluation of histological characters for keratose sponge systematics. Instead, Thorectandra is recovered close to the monotypic genus Fascaplysinopsis. Bergquist (1980) remarks Fascaplysinopsis recalling Thorectandra species in the "pronounced gelatinous appearance of the matrix, the yellow internal pigmentation and the coarse nature of the fibres" besides similarities in secondary metabolites. Unfortunately, DNA extraction from the holotype of Fascaplysinopsis reticulata Bergquist (Aplysinopsis reticulata Hentschel SMF904) was yet unsuccessful, but we managed to include the reference sample SDCC/RF017 from Systema Porifera (see Cook & Bergquist, 2002d). However, several additional cf. Fascaplysinopsis samples in our data set urge for a revision of this genus. We found a close relationship of Fascaplysinopsis and Thorectandra to the monotypic genus Thorectaxia, of which a sample of Thorectaxia papuensis Pulitzer-Finali & Pronzato from the type location (Papua New-Guinea) could be sequenced.
Molecular data reveal phylogenetic signal of a close relationship of (Thorectandra + Thorectaxia + Fascaplysinopsis + Petrosaspongia) to Hyrtios, Cacospongia, Scalarispongia, and Semitaspongia, whose inter- and intrageneric relationships require revision. Genus Scalarispongia, represented by a sequence of the type species' holotype Scalarispongia scalaris (Schmidt) LMJG 15406/0, and several Hyrtios species, H. erectus (Keller), H. altus (Poléjaeff), and H. reticulatus (Thiele), form a clade, to which Cacospongia (including the lectotype LMJG 15405/19 of its type species C. mollior Schmidt) is sister. Cacospongia mycofijiensis (Kakou, Crews & Bakus), however, is distant, therefore resulting in the paraphyly of Cacospongia. Specimens of Hyrtios proteus Duchassaing & Michelotti, the nominal type species of Hyrtios, fall outside this clade. This confirms earlier findings on non-monophyly of the genus Hyrtios, demonstrating the need for a revision of this genus (Erpenbeck et al., 2017; Erpenbeck, Sutcliffe, et al., 2012; Redmond et al., 2013). Cook and Bergquist (2002d), remark that Cacospongia species other than C. mollior and C. serta (Lendenfeld) require revision. A partial ITS sequence of the C. serta holotype BMNH 1886.8.27.166, so far the only specimen of this species known (Cook & Bergquist, 2000), falls outside this clade, but verification from a longer sequence is required. In the past, C. mycofijiensis classification underwent numerous changes in its relatively young taxonomic history, triggered by overlapping morphological characteristics to other genera (see review in Sanders & Van Soest, 1996). An assignment of C. mycofijiensis to Petrosaspongia (suggested in Bergquist et al., 1999) can be rejected following our data, but assignment to Cacospongia (Sanders & Van Soest, 1996) or Scalarispongia (objected in Manconi, Cadeddu, Ledda, & Pronzato, 2013) requires thorough revision of the three genera. Both Scalarispongia and Semitaspongia have been erected by Cook and Bergquist (2000) to accommodate members of the "'Cacospongia' group" which is supported by the present data.
A further major clade unites Luffariella, Thorecta, Fenestraspongia, Taonura, and Fasciospongia. Thorecta Lendenfeld is in our data set represented by T. reticulata Cook & Bergquist [reference specimen SDCC/NZ097 in Cook and Bergquist (1996)] and a specimen of Thorecta freija Lendenfeld. Sequencing results from the holotype of the type species T. exemplum var. tertia Lendenfeld (BMNH 1886.8.27.188) were ambiguous. Santos et al. (2010) noted on the shortcomings in the classification of Thorecta and regarded eleven species as valid including T. reticulata, while T. freija was reclassified as Taonura. Genus Taonura in this analysis is represented by two specimens of the type species Taonura flabelliformis Carter (lectotype BMNH 1844.9.13.3 and the Systema Porifera reference specimen SDCC/RF024). Although only a partial ITS 2 fragment of the lectotype could be recovered, preventing the resolution of intergeneric relationships, the phylogenetic placement with Luffariella + Thorecta + Fenestraspongia clade is indicated. Our 28S reconstruction recovers Thorecta as paraphyletic with a sister group relationship between T. freija and T. flabelliformis, supporting Santos et al. (2010). Cook and Bergquist (2002d) described Taonura as a "hybrid of skeletal morphologies seen in Cacospongia, Semitaspongia, and Scalarispongia," but our molecular results cannot second the phylogenetic signal of Taonura skeletal morphology to those genera. Closely related to Thorecta is Fenestraspongia, represented by the holotype of its type species F. intertexta (Carter) BMNH 1886.12.15.238. Luffariella Thiele comprises the type species L. variabilis (Polejaeff) (holotype BMNH 1885.8.8.52), L. caliculata Bergquist (holotype QM G304686), and L. cylindrica Bergquist (holotype QM G304687) and outside Thorecta + Fenestraspongia. Luffariella and Fenestraspongia were regarded as the only Thorectinae with tertiary fibers (Cook & Bergquist, 2002d). A phylogenetic signal of tertiary fibers is not given due to the phylogenetic position of Thorecta and the presence of tertiary fibers in Phyllospongiinae and Petrosaspongia species (see Uriz & Cebrian, 2006). Genus Fasciospongia Burton is in our analyses represented by a F. costifera (Lamarck, 1814) from its type locality (Western Australia) and a South African F. cf. cycni sequence from GenBank. Type region of F. cycni (Lendenfeld) is Western Australia; therefore, the taxonomy of this sample remains to be confirmed.
For Smenospongia and Dactylospongia, Bergquist relationships to the other dictyoceratid taxa are unresolved as sister to either Luffariella + Thorecta + Fenestraspongia + Taonura or Phyllospongiinae + Spongiidae. Dactylospongia is here represented by the lectotype (NMB-PORI 44), several samples of the type species D. elegans (Thiele), and a reference specimen for the Systema Porifera [SDCC/RF047 D. metachromia (Laubenfels)]. For Smenospongia the type species, S. aurea (Hyatt) and other Smenospongia samples (Redmond et al., 2013) were considered. Dactylospongia was erected to accommodate Luffariella elegans Thiele, which appeared morphologically distinct to Luffariella (Bergquist, 1965). Dactylospongia was subsequently assigned to Thorectidae based on its stratified fiber structure and due to morphological and pigment biochemical similarity to Smenospongia (Cook & Bergquist, 2002d). Both, distinction from Luffariella and similarity to Smenospongia, can be confirmed by our molecular data. A transfer of D. metachromia to the genus Petrosaspongia as suggested by Kwak, Schmitz, and Kelly (2000) based on terpenic compounds is in strong conflict with our molecular findings (see Uriz and Cebrian (2006) for a discussion).
Family Irciniidae, currently consisting of the genera Ircinia, Psammocinia, Bergquistia, and Sarcotragus, is monophyletic. Irciniidae share the apomorphic fine collagenous filaments in the mesohyl (Cook & Bergquist, 2002b). While molecular studies unequivocally supported irciniid monophyly of its largest genus Ircinia, this remains uncertain in respect to Sarcotragus (Erpenbeck, Sutcliffe, et al., 2012; see also Pöppe, Sutcliffe, Hooper, Wörheide, & Erpenbeck, 2010). Cook and Bergquist (2002b) regard the status of Sarcotragus, which differs from Ircinia only by the extent of fiber fasciculation and coring, as uncertain, likewise the distinction of Bergquistia, from which so far no molecular marker has been published, to Sarcotragus is uncertain (Cook, 2007). Distinction between Psammocinia and Ircinia, however, has molecularly been shown (Pöppe et al., 2010). Irciniidae frequently resemble species of Coscinoderma in shape, texture, and surface (Sim & Kim, 2014). Genus Coscinoderma is a disjunct and species-poor genus with rare occurrence (but see Sim & Kim, 2014; Voultsiadou Koukoura, Van Soest, & Koukouras, 1991), currently classified as Spongiidae. Its species possess very fine, meandering ("woolly"), uncored secondary fibers. For example C. mathewsi (Lendenfeld), here represented by the reference specimen of the Systema Porifera (SDCC/RF077), is repeatedly recovered as sister to (this study) or within (Redmond et al., 2013) Irciniidae. A similar phylogenetic placement is observed from a GenBank specimen published as C. sporadense Voultsiadou-Koukoura, van Soest & Koukouras as published (KX866774, see Idan et al., 2018). In contrast, a C. lanuga Laubenfels specimen, a species described as poorly known, but valid (Bergquist, 1980;Voultsiadou Koukoura et al., 1991), falls into the Spongiidae resulting in a paraphyletic genus Coscinoderma. Clearly, examination of the type species C. pesleonis (Lamarck, 1813) is required to resolve the classification of this genus.
For the monospecific genus Collospongia, the holotype C. auris Bergquist, Cambie & Kernan (AM Z5035) has been analyzed (Galitz et al., 2018). Cook and Bergquist (2002c) remarked on morphological similarities with the Phyllospongiinae, but with different secondary metabolite composition and a unique skeletal structure, which allegedly makes classification into any of the thorectid subclasses difficult. We recover Collospongia among the first branching thorectid genera and clearly distant from Phyllospongiinae (see also Galitz et al., 2018).
Genus Vaceletia is the only lineage among the dictyoceratids with a mineral (although secondary hypercalcified aragonitic) skeleton. It is regarded as the only extant representative of the fossil family Verticillitidae on the basis of its sphinctozoan bauplan (see Vacelet, 2002). The lack of clear synapomorphies shared with any other extant sponge lineage hampered the (morphological) classification of Vaceletia (Vacelet, 2002) until molecular data unequivocally revealed the dictyoceratid origin (Wörheide, 2008), followed by the placement of Verticillitidae as fifth family of Dictyoceratida (Morrow & Cárdenas, 2015). Molecular data recover an early branching of Vaceletia from the remaining thorectid + spongiid + irciniid taxa, probably as sister group.
3.1 Implications for dictyoceratid morphological character evolution
Our reconstructed phylogenetic hypothesis has consequences for our current understanding of character evolution in dictyoceratid sponges. The sister group relationship of Dendroceratida to Dictyoceratida with Dysideidae splitting first from all other dictyoceratid families implies an ancestral nature of eurypylous choanocyte chambers for Keratosa in general and Dictyoceratida in particular (Erpenbeck, Sutcliffe, et al., 2012). Verticillitidae (Vaceletia) are the only Keratosa with aphodal choanocyte chambers, while the thorectid + spongiid + irciniid sister group can be distinguished by their diplodal choanocyte chambers, which are apomorphic within the Keratosa (Figure 1).
Possession of an armor, that is, a substantial ectosomal layer of foreign material, is frequently used for the discrimination of taxa, but our phylogenetic reconstruction does not indicate any phylogenetic signal in this character. Skeletal features constitute the most important source for phylogenetic and systematic characters in spiculose as well as non-spiculose sponges. Some of these characters have likewise been plotted on the phylogeny in Figure 1. The coring of primary or secondary fibers, that is, the inclusion of foreign mineral material into the fibers, did not harbor any phylogenetic signal. In Dysideidae, coring of both primary and secondary fibers potentially combines Dysidea, Lamellodysidea, and Acanthodendrilla, although the extent of this character as apomorphy in dysideids has yet to be shown (Erpenbeck, Sutcliffe, et al., 2012), particularly as secondaries in Candidaspongia are uncored (Cook & Bergquist, 2002a).
The possession of tertiary fibers is a combining character for the Phyllospongiinae, and the tertiary fiber-lacking alleged phyllospongiine Candidaspongia was revealed as dysideid (Galitz et al., 2018; Redmond et al., 2013). Tertiary fibers are further present in Luffariella and Fenestraspongia, two closely related genera. Some Spongia possess structures referred to as "pseudo-tertiary fibers" due to structural differences to those found in, for example, Luffariella (Cook & Bergquist, 2001), which leaves the possibility of tertiary fiber convergent evolution.
The arrangement of fibers into fascicles or into a regular (e.g., rectangular) skeleton does not constitute a reliable combining character either. While the closely related Thorecta and Taonura share this feature, histologically similar Thorectandra (cf. Cook & Bergquist, 2002d) are clearly distant.
In conclusion, clear-cut and unambiguous morphological apomorphies for the discrimination and classification of dictyoceratid sponges are scarce and too prone to homoplasies. The current morphology-based classification of the inter- and intrafamiliar relationships of thorectids, spongiids, Irciniidae, and Verticillitidae is incongruent to phylogenetic hypotheses of independent molecular markers and prompt for a re-classification and re-evaluation of synapomorphies based on integrative taxonomy.
ACKNOWLEDGEMENTS
We like to thank Dorte Janussen (SMF, Frankfurt), Ulrike Hausl-Hofstätter (Universalmuseum Johaneum, Graz), Carsten Lüter (MfN, Berlin), Emma Sherlock (NHM, London), Urs Wüest (Naturhistorisches Museum Basel), Andreas Dietzel (now JCU), Ratih Aryasari (Universitas Gadjah Mada, Yogyakarta), Gabriele Büttner, Nora Dotzler, and Simone Schätzle (LMU) for various support for this study. DE acknowledges financial support of the European Union under a Marie-Curie outgoing fellowship (MOIF-CT-2004 Contract No 2882) and Deutsche Forschungsgemeinschaft (DFG: Er611/5-1). GW acknowledges funding by LMU Munich's Institutional Strategy LMUexcellent within the framework of the German Excellence Initiative. Renata Manconi, Roberto Pronzato, Editors, and anonymous reviewers are thanked for their constructive comments that improved the manuscript considerably.
APPENDIX 1
Specimens newly sequenced for this study. "HT", "NT," and "LT" following the voucher number indicate holotype, neotype, and lectotype, respectively. Accession numbers in bold indicate sequences newly obtained in the course of this study. Accession numbers of previously published sequences of the same specimen used in this study are given in regular font.
| Species | Voucher number | Accession numbers | |||
|---|---|---|---|---|---|
| Type status | CO1 | ITS | 28S-C | 28S-D3D5 | |
| Dysideidae | |||||
| Candidaspongia flabellata | QM G305536 | LR699438 | |||
| Candidaspongia flabellata | QM G305606 | LR699439 | |||
| Candidaspongia flabellata | QM G306588 | LR699440 | |||
| Candidaspongia flabellata | QM G307326 | LR699441 | |||
| Candidaspongia flabellata | QM G314439 | LR699322 | JQ082714 | ||
| Candidaspongia flabellata | QM G320157 | LR699323 | JQ082716 | ||
| Candidaspongia flabellata | QM G322756 | LR699442 | |||
| Dysidea cf. arenaria | QM G301096 | LR699478 | |||
| Dysidea cf. arenaria | QM G301107 | LR699479 | |||
| Dysidea cf. arenaria | QM G304690 | LR699480 | |||
| Dysidea cf. arenaria | QM G305915 | LR699481 | |||
| Dysidea cf. arenaria | QM G306542 | LR699482 | |||
| Dysidea cf. arenaria | QM G306942 | LR699483 | |||
| Dysidea cf. arenaria | QM G306943 | LR699484 | |||
| Dysidea cf. arenaria | QM G324696 | LR699485 | |||
| Dysidea fragilis | QM G301252 | LR699486 | |||
| Dysidea sp. | QM G333259 | LR699487 | |||
| Lamellodysidea herbacea | QM G301070 | LR699509 | |||
| Lamellodysidea herbacea | QM G301191 | LR699510 | |||
| Irciniidae | |||||
| Ircinia sp. | AM Z3989 | LR699350 | |||
| Ircinia sp. | QM G306067 | LR699351 | |||
| Ircinia sp. | QM G321282 | LR699352 | |||
| Ircinia sp. | QM G322564 | LR699353 | |||
| Psammocinia sp. | QM G303277 | LR699528 | |||
| Psammocinia sp. | QM G303290 | LR699529 | |||
| Psammocinia sp. | QM G303916 | LR699530 | |||
| Psammocinia sp. | QM G304115 | LR700203 | |||
| Sarcotragus muscarum | ZMA POR19029 | LR699420 | |||
| Sarcotragus sp. | QM G318919 | LR699372 | |||
| Spongiidae | |||||
| Cf. Coscinoderma nardorus | QM G303003 | LR699466 | |||
| Cf. Coscinoderma nardorus | QM G304469 | LR699467 | |||
| Coscinoderma lanuga | ZMA POR17975 | LR699329 | LR699454 | ||
| Coscinoderma mathewsi | QM G301075 | LR699455 | |||
| Coscinoderma mathewsi | QM G303125 | LR699456 | |||
| Coscinoderma mathewsi | QM G304249 | LR699457 | |||
| Coscinoderma mathewsi | QM G304282 | LR699458 | |||
| Coscinoderma mathewsi | QM G304283 | LR699459 | |||
| Coscinoderma mathewsi | QM G304295 | LR699460 | |||
| Coscinoderma mathewsi | QM G305068 | LR699461 | |||
| Coscinoderma mathewsi | QM G313086 | LR699330 | |||
| Coscinoderma mathewsi | QM G322760 | LR699331 | LR699462 | JQ082718 | |
| Coscinoderma mathewsi | QM G322762 | LR699463 | |||
| Coscinoderma mathewsi | QM G322765 | LR699332 | JQ082719 | ||
| Coscinoderma mathewsi | QM G324713 | LR699464 | |||
| Coscinoderma mathewsi | SDCC RF048 | LR699465 | |||
| Hippospongia ammata | QM G306900 | LR699344 | LR699493 | ||
| Hippospongia communis | ZMA POR14572 | LR699345 | |||
| Hyattella intestinalis | QM G300839 | LR699494 | |||
| Hyattella intestinalis | QM G304652 | LR699495 | |||
| Rhopaloeides odorabile | QM G303923 | LR699531 | |||
| Rhopaloeides odorabile | QM G304220 | LR699532 | |||
| Rhopaloeides odorabile | QM G322761 | LR699417 | LR699369 | LR699533 | JQ082768 |
| Rhopaloeides odorabile | QM G322813 | LR699418 | LR699370 | JQ082769 | |
| Rhopaloeides odorabile | SDCC RF067 | LR699419 | LR699371 | LR699534 | |
| Spongia (Spongia) cf. irregularis | SDCC NZ002 | LR699375 | JQ082674 | ||
| Spongia (Spongia) cf. irregularis | SDCC NZ007 | LR699376 | LR699537 | JQ082675 | |
| Spongia (Spongia) hispida | QM G303209 | LR699538 | |||
| Spongia (Spongia) cf. hispida | ZMA POR19756 | LR699377 | |||
| Spongia (Spongia) officinalis | ZMA POR14396 | JQ082842 | LR699378 | LR699075 | |
| Spongia sp. | QM G324326 | LR699539 | |||
| Spongiidae sp. | QM G304328 | LR699379 | |||
| Spongiidae sp. | QM G305535 | LR699380 | |||
| Spongiidae sp. | QM G322786 | LR699423 | LR699381 | ||
| Spongiidae sp. | QM G322830 | LR699424 | LR699382 | ||
| Spongiidae sp. | RMNH 2283 | LR699425 | |||
| Thorectidae | |||||
| Cacospongia cf. mollior | SDCC RF139 | LR699316 | LR699437 | JQ082658 | |
| Cacospongia mollior | LMJG 15405, LT | LR699317 | |||
| Cacospongia mycofijiensis | QM G301467 | LR699396 | LR699318 | LR699435 | |
| Cacospongia mycofijiensis | QM G312707 | LR699398 | |||
| Cacospongia mycofijiensis | QM G313245 | LR699319 | |||
| Cacospongia mycofijiensis | ZMA POR18574 | LR699399 | LR699320 | ||
| Cacospongia mycofijiensis | ZMA POR18575 | LR699400 | LR699321 | LR699436 | |
| Cacospongia sp. | QM G306016 | LR699397 | LR700205 | ||
| Cacospongia sp. | QM G314076 | LR700206 | |||
| Cacospongia sp. | QM G315096 | LR700207 | |||
| Carteriospongia contorta | QM G303874 | LR699443 | |||
| Carteriospongia contorta | SDCC RF018 | LR699324 | LR699444 | JQ082663 | |
| Carteriospongia flabellifera | QM G303017 | LR699445 | |||
| Carteriospongia flabellifera | QM G304084 | LR699446 | |||
| Carteriospongia flabellifera | QM G304114 | LR699447 | |||
| Carteriospongia flabellifera | QM G304192 | LR699448 | |||
| Carteriospongia flabellifera | QM G306728 | LR699449 | |||
| Carteriospongia flabellifera | QM G313227 | LR699401 | JQ082664 | ||
| Carteriospongia flabellifera | QM G315231 | LR699325 | JQ082665 | ||
| Carteriospongia flabellifera | QM G322820 | JQ082662 | |||
| Carteriospongia flabellifera | QM G315298 | LR699450 | JQ082666 | ||
| Carteriospongia foliascens | BMNH 1925.11.1.411, NT | LR699326 | LR699451 | ||
| Carteriospongia foliascens | QM G304326 | LR699452 | |||
| Carteriospongia foliascens | QM G317494 | LR699402 | |||
| Carteriospongia foliascens | QM G322818 | LR699327 | JQ082667 | ||
| Collospongia auris | AM Z5035 HT | LR699453 | |||
| Dactylospongia elegans | NMB-PORI 44, LT | LR699333 | |||
| Dactylospongia elegans | QM G304125 | LR699468 | |||
| Dactylospongia elegans | QM G304225 | LR699469 | |||
| Dactylospongia elegans | QM G304296 | LR699470 | |||
| Dactylospongia elegans | QM G305092 | LR699471 | |||
| Dactylospongia elegans | QM G305998 | LR699472 | |||
| Dactylospongia elegans | QM G306931 | LR699473 | |||
| Dactylospongia elegans | QM G307754 | LR699474 | |||
| Dactylospongia elegans | QM G313054 | JQ082802 | LR699334 | ||
| Dactylospongia elegans | QM G313637 | LR699335 | JQ082683 | ||
| Dactylospongia elegans | QM G325555 | LR699475 | |||
| Dactylospongia metachromia | SDCC RF047 | LR699336 | LR699476 | JQ082684 | |
| Dactylospongia sp. | QM G311348 | LR699408 | LR699337 | JQ082682 | |
| Cf. Fascaplysinopsis reticulata | QM G322803 | JQ082812 | LR699338 | JQ082812 | |
| Cf. Fascaplysinopsis reticulata | SDCC RF017 | LR699339 | LR699489 | JQ082706 | |
| Cf. Fascaplysinopsis sp. | CASIZ300177 | LR699488 | |||
| Cf. Fascaplysinopsis sp. | QM G307325 | LR699405 | |||
| Cf. Fascaplysinopsis sp. | QM G313004 | LR699406 | LR699340 | ||
| Cf. Fascaplysinopsis sp. | QM G314831 | LR700208 | |||
| Cf. Fascaplysinopsis sp. | QM G320018 | LR699407 | LR699341 | LR699490 | |
| Cf. Fascaplysinopsis sp. | QM G331054 | LR699342 | LR699491 | ||
| Cf. Fascaplysinopsis sp. | QM G333241 | LR700209 | |||
| Cf. Fascaplysinopsis sp. | QM G333299 | LR700210 | LR700202 | ||
| Fenestraspongia intertexta | BMNH 1886.12.15.238, HT | LR699343 | LR699492 | ||
| Hyrtios altus | QM G311014 | LR699410 | |||
| Hyrtios erectus | QM G301134 | LR699496 | |||
| Hyrtios erectus | QM G301248 | LR699497 | |||
| Hyrtios erectus | QM G303305 | LR699498 | |||
| Hyrtios erectus | QM G303883 | LR699500 | |||
| Hyrtios erectus | QM G303906 | LR699501 | |||
| Hyrtios erectus | QM G303445 | LR699499 | |||
| Hyrtios erectus | QM G303917 | LR699502 | |||
| Hyrtios erectus | QM G304193 | LR699503 | |||
| Hyrtios erectus | QM G304223 | LR699504 | |||
| Hyrtios erectus | QM G304346 | LR699505 | |||
| Hyrtios erectus | QM G304354 | LR699506 | |||
| Hyrtios erectus | QM G304362 | LR699507 | |||
| Hyrtios erectus | QM G305776 | LR699508 | |||
| Hyrtios erectus | SDCC RF049 | LR699346 | |||
| Hyrtios erectus | SNSB-BSPG.GW6170 | LR699347 | |||
| Hyrtios proteus | ZMA POR14381 | JQ082820 | LR699348 | ||
| Hyrtios reticulatus | SDCC RF031 | LR699349 | |||
| Lendenfeldia chondrodes | SNSB-BSPG.GW27611 | LR699513 | |||
| Lendenfeldia chondrodes | SNSB-BSPG.GW27619 | LR699514 | |||
| Lendenfeldia chondrodes | SNSB-BSPG.GW27699 | LR699515 | |||
| Lendenfeldia chondrodes | SNSB-BSPG.GW8481 | LR699354 | LR699516 | ||
| Lendenfeldia plicata | QM G303343 | LR699517 | |||
| Lendenfeldia plicata | QM G304093 | LR699518 | |||
| Lendenfeldia plicata | QM G319507 | LR699356 | |||
| Lendenfeldia plicata | QM G322766 | LR699412 | LR699394 | ||
| Lendenfeldia plicata | QM G312964 | LR699411 | LR699392 | ||
| Lendenfeldia cf. plicata | QM G304324 | LR699512 | |||
| Luffariella caliculata | QM G304686, HT | LR699357 | LR699519 | ||
| Luffariella cylindrica | QM G304687, HT | LR699358 | LR699520 | ||
| Luffariella variabilis | BMNH 1885.8.8.52, HT | LR699359 | |||
| Petrosaspongia nigra | QM G304685, HT | LR699360 | LR699521 | ||
| Petrosaspongia nigra | QM G313020 | LR699413 | LR699361 | JQ082747 | |
| Petrosaspongia nigra | QM G315543 | LR699414 | LR699362 | JQ082748 | |
| Phyllospongia lamellosa | QM G304169 | LR699522 | |||
| Phyllospongia lamellosa | QM G304677 | LR699523 | |||
| Phyllospongia lamellosa | QM G322790 | LR699363 | JQ082749 | ||
| Phyllospongia lamellosa | QM G322848 | LR699415 | LR699364 | ||
| Phyllospongia papyracea | QM G300316 | LR699524 | |||
| Phyllospongia papyracea | QM G304332 | LR699525 | |||
| Phyllospongia papyracea | QM G307267 | LR699416 | LR699365 | LR699526 | |
| Phyllospongia papyracea | QM G307268 | LR699527 | |||
| Phyllospongia papyracea | QM G318009 | LR699366 | JQ082750 | ||
| Phyllospongia papyracea | QM G322855 | LR699367 | JQ082751 | ||
| Phyllospongia papyracea | QM G322863 | LR699368 | JQ082752 | ||
| Phyllospongiinae sp. | SNSB-BSPG.GW26545 | LR735997 | |||
| Scalarispongia scalaris | LMJG 15406 | LR699373 | |||
| Semitaspongia sp. | SDCC NZ066 | LR699535 | |||
| Semitaspongia sp. | SDCC NZ121 | LR699421 | LR699374 | LR699536 | |
| Smenospongia aurea | ZMA POR13807 | LR699422 | |||
| Strepsichordaia aliena | RMNH 2284 | LR699426 | LR699383 | ||
| Strepsichordaia caliciformis | QM G311299 | JQ082843 | LR699384 | ||
| Cf. Strepsichordaia lendenfeldi | QM G322810 | LR700211 | JQ082775 | ||
| Strepsichordaia lendenfeldi | AM Z5026 HT | LR699427 | LR699385 | LR699540 | JQ082776 |
| Strepsichordaia lendenfeldi | QM G303854 | LR699541 | |||
| Strepsichordaia sp. | QM G306046 | LR699403 | LR699328 | JQ082669 | |
| Strepsichordaia sp. | QM G306072 | LR699404 | |||
| Taonura flabelliformis | BMNH 1844.9.13.3, HT | LR699386 | |||
| Taonura flabelliformis | SDCC RF024 | LR699542 | JQ082777 | ||
| Thorecta freija | QM G303743 | LR699387 | LR699543 | JQ082778 | |
| Thorecta reticulata | SDCC NZ097 | LR699544 | JQ082779 | ||
| Thorecta sp. | QM G303206 | JQ082780 | |||
| Thorectandra excavatus | QM G303331 | LR699428 | LR699389 | LR699545 | JQ082781 |
| Thorectandra excavatus | QM G303563 | LR699546 | |||
| Thorectandra excavatus | QM G303575 | LR699547 | |||
| Thorectandra excavatus | ZMA POR14042 | JQ082845 | LR699390 | JQ082782 | |
| Thorectandra sp. | SDCC RF016 | LR700212 | LR700204 | ||
| Thorectaxia papuensis | ZMA POR19767 | LR699548 | |||
| Thorectidae sp. | SNSB-BSPG.GW26569 | LR700215 | |||
| Thorectidae sp. | CASIZ302695 | LR699549 | |||
| Thorectidae sp. | QM G306003 | LR700213 | JQ082707 | ||
| Thorectinae sp. | CASIZ302698 | LR699550 | |||
| Thorectinae sp. | QM G301060 | LR699551 | |||
| Thorectinae sp. | QM G307378 | LR699431 | LR699391 | JQ082710 | |
| Thorectinae sp. | QM G313051 | LR699432 | LR699393 | ||
| Thorectinae sp. | SDCC RF053 | LR699552 | JQ082743 | ||
| Thorectinae sp. | SNSB-BSPG.GW26644 | LR699430 | |||
| Thorectinae sp. | ZMA POR11466 | LR699433 | |||
| Thorectinae sp. | ZMA POR15722 | JQ082831 | JQ082744 | ||
| Thorectinae sp. | ZMA POR16798 | JQ082813 | LR700214 | ||
| Thorectinae sp. | ZMA POR17995 | LR699434 | LR699395 | ||
| Uncategorized | |||||
| Dictyoceratida sp. | SDCC NZ147 | LR700201 | |||
| Dictyoceratida sp. | SNSB-BSPG.GW27609 | LR699477 | |||




