Phylogeography and population history of the least weasel (Mustela nivalis) in the Palearctic based on multilocus analysis
Abstract
The least weasel (Mustela nivalis) is one of the most widely distributed carnivorans. While previous studies have identified distinct western and eastern mitochondrial DNA (mtDNA) lineages of the species in the western Palearctic, their broader distributions across the Palearctic have remained unknown. To address the broad-scale phylogeographical structure, we expanded the sampling to populations in Eastern Europe, the Urals, the Russian Far East, and Japan, and analyzed the mtDNA control region and cytochrome b, the final intron of the zinc finger protein on Y chromosome (ZFY), and the autosomal agouti signaling protein gene (ASIP). The mtDNA data analysis exposed the previous western lineage (Clade I) but poorly supported assemblage extending across Palearctic, whereas the previous eastern lineage (Clade II) was reconfirmed and limited in the south western part of the Palearctic. The ZFY phylogeny showed a distinctive split that corresponding to the mtDNA lineage split, although less phylogeographical structure was seen in the ASIP variation. Our data concur with the previous inference of the Black Sea–Caspian Sea area having an ancestral character. The Urals region harbored high mitochondrial diversity, with an estimated coalescent time of around 100,000 years, suggesting this could have been a cryptic refugium. Based on the coalescent-based demographic reconstructions, the expansion of Clade I across the Palearctic was remarkably rapid, while Clade II was relatively stable for a longer time. It seems that Clade II has maintained a constant population size in the temperate region, and the expansive Clade I represents adaptation to the cold regions.
1 INTRODUCTION
Some species have narrow distributions within limited geographic regions, while others are distributed broadly across the globe. A basic concern of biogeographical science is to understand how species have evolved and acquired their worldwide distributions. Phylogeography explores the evolutionary and dispersal histories of widespread species using the genealogical information embedded in their DNA (Garrick et al., 2015). Many studies of phylogeography have tried to reconstruct the migration histories of mammalian species in the Palearctic and to identify their glacial refugia, which have frequently been located in the Balkan and Iberian peninsulas, in the Caucasus and the Russian Far East (Frantz et al., 2014; Hewitt, 1999; Hope et al., 2010; Korsten et al., 2009). The location of these refugia evidently reflects the species' environmental tolerances (Schmitt & Varga, 2012). Moreover, Řičánková, Robovský, Riegert, and Zrzavý (2015) pointed out that Central Asia was important as a glacial refugium for the megafauna and that the “mammoth fauna” (these were the cold tolerant fauna, which developed and reached their peak during the late Pleistocene, see Vereshchagin & Baryshnikov, 1992) remained there with highest frequency in Palearctic.
The least weasel (Mustela nivalis Linnaeus, 1766) is one of the most widespread carnivore taxa in the Northern Hemisphere with a range covering most of the Palearctic: Europe, North Africa, Northern Asia including the Japanese islands, and North America. Considering this fossil records, the least weasel is included in the “mammoth fauna” (Sheffield & King, 1994; Youngman, 1993, and references therein). This species is considered a mesopredator, and their diet consists mainly of rodents, lagomorphs with some insects (King & Powell, 2007). The least weasel has significant variation in body and skull sizes and proportions throughout its huge distribution range (Abramov & Baryshnikov, 2000). Along with an overall high variation of cranial characters, there is a tendency toward an increase in body size and relative tail length from the north to south and to some extent from the east to west (Abramov & Baryshnikov, 2000). Abramov and Baryshnikov also suggested the least weasel has unique geographical variations such as the distribution of the two pelage colorations (nivalis and vulgaris): The nivalis type is distributed in northern Palearctic and the vulgaris type in the Mediterranean region, and the ancestral cranial type, in turn, is distributed in Northern Africa, Spain, Caucasus, Middle East, and Central Asia. It is also remarkable that the body sizes of the least weasels do not follow the Bergmann rule (Bergmann, 1847). King and Powell (2007) suggested that this phenomenon is affected by the unique evolutionary process of the least weasels, which have decreased their body sizes so that they can prevent the heat loss and forage the small rodents in the cold environment. In fact, Marciszak and Socha (2014) reported a correlation between the temperature and the cranial size using the fossil materials from Polish caves: The size decreases during colder periods and increases in warmer intervals. In addition, Zub, Szafrańska, Konarzewski, and Speakman (2011) suggested the winter survival rate is higher for smaller than for larger least weasels. It is plausible that several weasel characters including the ecological status, comprehensive distribution, and great geographical variation have resulted from adaptation to the various environments, and it is interesting to further understand the evolutionary history of the least weasel.
The chromosome number of the least weasel in Siberia and in Hokkaido, Japan, is 2n = 42, whereas on the Honshu Island it is 2n = 38 (Mandahl & Fredga, 1980; Obara, 1991). Distinct mitochondrial DNA (mtDNA) control region (CR) sequences were observed in least weasels from Hokkaido and Honshu islands, as reported by Kurose, Masuda, and Yoshida (1999). In a phylogeographic analysis of the mtDNA CR across Siberia, the Caucasus, Central Asia, and North America, the lineages around the Caucasus were the most variable probably due to the introgression or maintenance of polymorphic status of their ancestral population (Kurose, Abramov, & Masuda, 2005). Meanwhile, Lebarbenchon, Poitevin, Arnal, and Montgelard (2010) detected a subdivision of the Western Palearctic least weasel mitochondrial diversity into Western (Clade I) and Eastern (Clade II) lineages using the mtDNA CR and cytochrome b (Cytb) sequences. Using only Cytb data, Mcdevitt et al. (2012) identified a presence of a suture zone between the lineages in Poland and suggested that the Carpathians were one of the refugia for the least weasel and confirmed the existence of the two main lineages, similar to the western Palearctic region reported by Lebarbenchon et al. (2010). Rodrigues et al. (2016) revealed the taxonomic status of the Egyptian least weasel that shared a haplotype with weasels in Turkey and Mediterranean islands. Additionally, the least weasel could have been imported to some Mediterranean islands artificially, and the gene flow could have affected the genetic structure (Lebarbenchon et al., 2010; Rodrigues et al., 2017). However, the original dispersal and migration history of the least weasels still remains unclear in Palearctic.
To further understand the molecular phylogenetic and biogeographical relationships among least weasels, we have analyzed mtDNA CR and Cytb, as maternally inherited genes, from a range of new localities across the Palearctic region. In addition, we also sequenced and analyzed the final intron of the zinc finger protein locus on the Y chromosome (ZFY) as a paternally inherited gene and the agouti signaling protein locus (ASIP) as a biparentally inherited gene. Combining our data with the previous results, we discuss the phylogeography and migration history of the least weasel in Palearctic.
2 MATERIALS AND METHODS
2.1 Specimens
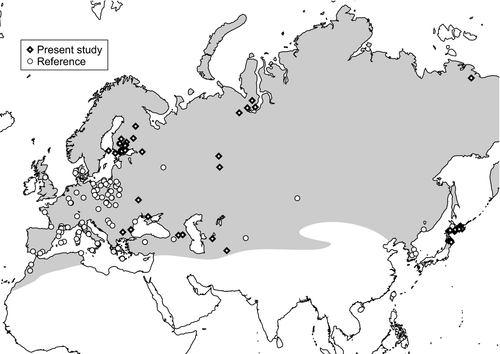
Tissue samples were obtained from collaborative laboratories and museums in Bulgaria, Russia, Finland, and Japan (76 samples consisting of 65 ethanol-preserved muscle tissues and 11 dried skins; Table 1 and Figure 1). Of these, 31 were also used in the previous mtDNA CR studies of Kurose et al. (1999), Kurose et al. (2005). In addition, we employed published data from public databases, as specified in Tables 1 and S1.
| Voucher No. | Geographic origins | Accession Nos. | |||
|---|---|---|---|---|---|
| CR | Cytb | ZFY | ASIP | ||
| Mn4 | Japan, Hokkaido, Shari-cho | LC314620 | LC324904 | LC325040 | LC324973 |
| Mn5 | Japan, Hokkaido, Shari-cho | LC314621 | LC324905 | – | LC324974 |
| Mn6 | Japan, Hokkaido, Shari-cho | LC314624 | LC324906 | – | LC324975 |
| Mn7 | Japan, Hokkaido, Shari-cho | LC314622 | LC324907 | – | LC324976 |
| NEM01 | Japan, Hokkaido, Nemuro | LC314625 | LC324908 | – | LC324977 |
| OBH01 | Japan, Hokkaido, Obihiro | LC314623 | LC324909 | – | LC324978 |
| S12 | Japan, Hokkaido, Shari-cho | AB006720a | LC324910 | LC325041 | LC324979 |
| S13 | Japan, Hokkaido, Shari-cho | AB006721a | – | – | LC324980 |
| S14 | Japan, Hokkaido, Shari-cho | Same as S13a | LC324911 | LC325042 | LC324981 |
| YOT1 | Japan, Hokkaido, Mt. Yōtei | AB006719a | LC324912 | – | LC324982 |
| OB1 | Japan, Hokkaido, Makubetsu-cho | AB006718a | LC324913 | LC325043 | LC324983 |
| OB2 | Japan, Hokkaido, Shihoro-cho | Same as S13a | LC324914 | – | LC324984 |
| HIT1 | Japan, Hokkaido, Sapporo | AB006722a | LC324915 | – | LC324985 |
| HIT2 | Japan, Hokkaido, Sapporo | AB006723a | LC324916 | LC325044 | LC324986 |
| N26 | Japan, Hokkaido, Unknown | AB006724a | LC324917 | LC325045 | LC324987 |
| N27 | Japan, Hokkaido, Sapporo | AB006725a | LC324918 | LC325046 | LC324988 |
| N28 | Japan, Hokkaido, Sapporo | Same as N27a | LC324919 | – | LC324989 |
| N29 | Japan, Hokkaido, Sapporo | Same as N27a | LC324920 | – | LC324990 |
| N30 | Japan, Hokkaido, Sapporo | AB006726a | LC324921 | – | LC324991 |
| N31 | Japan, Hokkaido, Sapporo | Same as YOT1a | LC324922 | LC325047 | LC324992 |
| N32 | Japan, Hokkaido, Shibecha-cho | AB006727a | LC324923 | LC325048 | LC324993 |
| N33 | Japan, Hokkaido, Sapporo | Same as N30a | LC324924 | LC325049 | LC324994 |
| N34 | Japan, Hokkaido, Sapporo | Same as HIT1a | LC324925 | – | LC324995 |
| N35 | Japan, Hokkaido, Shibecha-cho | Same as N32a | LC324926 | – | LC324996 |
| N37 | Japan, Hokkaido, Sapporo | Same as HIT1a | LC324927 | – | LC324997 |
| AKI1 | Japan, Akita, Kazuno-shi | AB006728a | LC324928 | LC325050 | LC324998 |
| IWA1 | Japan, Iwate, Kunohe-gun | Same as AKI1a | LC324929 | AB491594c | LC324999 |
| IWA2 | Japan, Iwate, Iwaizumi-cho | Same as AKI1a | LC324930 | LC325051 | LC325000 |
| 151646 | Russia, Staroutkinsk | LC314601 | LC324931 | – | LC325001 |
| 278252 | Russia, Karpinsk | LC314602 | LC324932 | – | LC325002 |
| 79252 | Russia, Priuralsky District | LC314603 | LC324933 | – | – |
| 79253 | Russia, Priuralsky District | LC314604 | – | – | – |
| 79254 | Russia, Priuralsky District | LC314605 | – | – | – |
| 79255 | Russia, Priuralsky District | LC314606 | LC324934 | – | LC325003 |
| 79271 | Russia, oz. Yarato 2-e | LC314607 | LC324935 | LC325052 | – |
| 79272 | Russia, oz. Yarato 2-e | LC314608 | – | – | – |
| 79273 | Russia, oz. Yarato 2-e | LC314609 | – | – | – |
| 79274 | Russia, Yamalsky District | LC314610 | – | – | – |
| 79304 | Russia, Yamalsky District | LC314611 | LC324936 | – | LC325004 |
| 79307 | Russia, Priuralsky District | LC314612 | LC324937 | – | LC325005 |
| RLEN3 | Russia, Leningrad Province | Same as RALTIb | LC324938 | LC325053 | LC325006 |
| RIND1 | Russia, Indigirka | AB049772b | LC324939 | – | LC325007 |
| ROMS1 | Russia, Omsk, West Siberia | LC314613 | – | – | – |
| BUSG1 | Bulgaria, Sredna Gora | LC314614 | LC324940 | LC325054 | LC325008 |
| BUV1 | Bulgaria, Varna | LC314615 | LC324941 | LC325055 | LC325009 |
| BUV2 | Bulgaria, Varna | LC314616 | LC324942 | LC325056 | LC325010 |
| BUV3 | Bulgaria, Varna | LC314617 | LC324943 | LC325057 | LC325011 |
| BUV4 | Bulgaria, Varna | LC314618 | LC324944 | LC325058 | LC325012 |
| BUV5 | Bulgaria, Varna | LC314619 | LC324945 | LC325059 | LC325013 |
| RTUR2 | Turkmenistan, Murgab | Same as RCAU1b | LC324946 | – | LC325014 |
| RKAR2 | Turkmenistan, South East Karakum | AB049774b | LC324947 | – | LC325015 |
| RASK1 | Ukraine, Askania, Nova | AB049765b | LC324948 | – | LC325016 |
| RASK2 | Ukraine, Askania, Nova | AB049768b | LC324949 | – | LC325017 |
| RUMA1 | Ukraine, Kiev Province | Same as RALTIb | LC324950 | – | LC325018 |
| RCAC1 | Georgia, Tbilisi | AB049770b | LC324951 | – | – |
| RCAU2 | Georgia, Lagodekhi | AB049771b | LC324952 | – | LC325019 |
| KS.KN 39356 | Finland, Porvoon mlk | LC314626 | LC324953 | LC325060 | LC325020 |
| KS.KN 39357 | Finland, Joutsa | LC314627 | LC324954 | LC325061 | LC325021 |
| KS.KN 34388 | Finland, Siuntio | LC314628 | LC324955 | – | LC325022 |
| KS.KN 34462 | Finland, Helsinki, Vuosaari | LC314629 | LC324956 | – | LC325023 |
| KS.KN 34463 | Finland, Inkoo | LC314630 | LC324957 | – | LC325024 |
| KS.KN 39310 | Finland, Heinolan mlk | LC314631 | LC324958 | – | LC325025 |
| KS.KN 34644 | Finland, Vantaa, Korso | LC314632 | LC324959 | – | LC325026 |
| KS.KN 38719 | Finland, Siuntio | LC314633 | LC324960 | – | LC325027 |
| KS.KN 34740 | Finland, Vihti, Selki | LC314634 | LC324961 | – | LC325028 |
| KS.KN 33901 | Finland, Vantaa, Riipilä | LC314635 | LC324962 | – | LC325029 |
| KS.KN 47736 | Finland, Finström | LC314636 | LC324963 | LC325062 | LC325030 |
| KS.KN 47078 | Finland, Dragsfjärd | LC314637 | LC324964 | LC325063 | LC325031 |
| KS.KN 47020 | Finland, Keuruu, Haapamäki | LC314638 | LC324965 | LC325064 | LC325032 |
| KS.KN 47872 | Finland, Espoo | LC314639 | LC324966 | LC325065 | LC325033 |
| KS.KN 47874 | Finland, Tampere | LC314640 | LC324967 | LC325066 | LC325034 |
| KS.KN 47882 | Finland, Asikkala | LC314641 | LC324968 | LC325067 | LC325035 |
| KS.KN 47906 | Finland, Pernaja | LC314642 | LC324969 | – | LC325036 |
| KS.KN 47907 | Finland, Porvoo | LC314643 | LC324970 | LC325068 | LC325037 |
| KS.KN 48312 | Finland, Heinävesi | LC314644 | LC324971 | – | LC325038 |
| KS.KN 48372 | Finland, Kuhmo | LC314645 | LC324972 | – | LC325039 |
2.2 DNA extraction, amplification, and sequencing
Total DNA was extracted from the samples using the DNeasy Tissue & Blood Kit (QIAGEN) or QIAamp DNA Investigator Kit (QIAGEN), following the manufacturer's protocols, including extraction blanks for the negative control. All experiments were done with filter tips and disposal tubes for preventing the contamination in the present study.
Two fragments of the mtDNA, CR (around 550 base-pairs, bp) and Cytb (1,140 bp), and one from the Y-chromosomal ZFY (687 bp) and one from autosomal ASIP gene (480 bp; intron and exon), were amplified by PCR with the primers shown in Table S2. For DNA degraded samples, we amplified small fragments (165–705 bp) overlapping each other. In the samples of dried skins, relatively shorter DNA fragments were amplified. Furthermore, we duplicated the experiments for the data confirmation on the degraded samples.
PCR was carried out in a volume of 10 μl including 2.0 μl of 5× Prime STAR GXL DNA Buffer (Takara), 0.8 μl of dNTP mixture (2.5 mM each dNTP; Takara), 0.2 μl of Prime STAR GXL DNA Polymerase (1.25 U/μl, Takara), 0.4 μl for each of forward and reverse primers (10 pmol/μl), 0.4 of bovine serum albumin (0.4 μg/μl, Roche), and 1.0–2.0 μl of the DNA extract, and the volume was adjusted to a total of 10 μl with distilled water. The amplification was performed with 30–45 cycles of 98°C for 10 s, 54–61.4°C for 15 s, and 68°C for 1min using the Thermal Cycler TP400 (Takara). To check the amplicon, 3μl of the PCR product was electrophoresed on a 2% agarose gel, stained with ethidium bromide, and observed under an ultraviolet illumination. Then, the PCR products were purified with the QIAquick PCR Purification Kit (QIAGEN). In addition, we confirmed no PCR amplification in the negative controls.
The DNA cycle sequencing was performed with the BigDye v3.1 or 1.1 Cycle Sequencing Kit (Applied Biosystems, ABI), using the PCR primers shown in Table S2. Sequencing reaction was performed in a volume of 10 μl containing 1.75 μl of 5× BigDye Sequencing Buffer (ABI), 0.5 μl of Ready Reaction Premix (ABI), 0.5 μl for each of the primers, and 2.0 μl of DNA template, and the volume was adjusted to 10 μl with distilled water. Cycle sequencing was performed with a preheating at 96°C for 1 min and 25 cycles of 96°C for 10 s, 50°C or 55°C for 5 s, and 60°C for 4 min. The cycle PCR products were precipitated with ethanol, and dissolved in 10 μl of formamide, and applied to an ABI 3,730 DNA Analyzer for sequencing. The sequence alignment for all loci was performed using MUSCLE in MEGA6 (Tamura, Stecher, Peterson, Filipski, & Kumar, 2013).
2.3 Phylogenetic trees and networks
The nucleotide substitution models for analyses of all loci were selected using the Bayesian information criterion (BIC) with PartitionFinder v1.1.1 (Lanfear, Calcott, Ho, & Guindon, 2012). The selected models were used to reconstruct phylogenetic trees (gene genealogies) with two approaches and programs: MrBayes v3.2.6 (Ronquist et al., 2012) for analyses under Bayesian inference (BI) and Garli v2.01 (Bazinet, Zwickl, & Cummings, 2014) for Heuristic maximum-likelihood analyses (ML). For analysis of the concatenated CR and Cytb sequence segments, separate substitution models were estimated for the CR segment and for each of three codon positions in the Cytb (1,2,3): These were K81uf + I + G, K80 + G, HKY, and TrN + G, but in the phylogenetic analysis HKY and GTR models were used instead of K81uf and TrN models, respectively, because these models were not implemented in MrBayes (Hasegawa, Kishino, & Yano, 1985; Kimura, 1980, 1981; Lanave, Preparata, Saccone, & Serio, 1984; Tamura & Nei, 1993). The list of the other nucleotide substitution models is shown in Table S3.
For the BI trees, Markov chain Monte Carlo (MCMC) analyses were run for 5 × 106 to 1 × 107 generations with trees sampled every 1,000 generations, and the first 25% of the trees were discarded as burn-in. The convergence of MCMC analyses was confirmed by indicating the average standard deviation of split frequencies was below <0.01, and the parameter values sampled from MCMC runs were checked in Tracer ver. 1.6 (http://tree.bio.ed.ac.uk/software/tracer/). The Bayesian posterior probabilities (PP) were also obtained from MrBayes. In the reconstruction of the ML trees, the effort to search for the best tree was repeated 20 times independently and terminated at 20,000 generations with Garli. The maximum-likelihood bootstrap percentages (BP) were obtained from 1,000 pseudoreplicates with Garli to assess the confidence values of tree nodes.
Two confidence values (PP and BP) for the reconstructed nodes were mapped on the trees using SumTrees 3.3.1 of the DendroPy package (Sukumaran & Holder, 2010). Nodes of the trees were regarded as well supported when their PP was ≥0.95 and BP was ≥70%. Haplotypes from other three closely related species (Mustela nudipes Desmarest, 1822: AB601587 for CR, AB285332 for Cytb; M. kathiah Hodgson, 1835: AB601575, AB285331; M. erminea L., 1758: AB006730, AB026101) were used as outgroups in the mtDNA tree. The M. erminea sequences of ZFY and ASIP (AB491595 and JX130732, respectively) were used as the references for these genes. The SINEs (short interspersed nuclear elements) region of the M. erminea's ZFY sequence was excluded in the analysis. These phylogenetic trees were visualized and edited with FigTree v1.4.2 (http://tree.bio.ed.ac.uk/software/figtree/).
Median-joining networks of the concatenated mtDNA CR and Cytb, of ZFY, and of ASIP separately were reconstructed with POPART 1.7 (Leigh & Bryant, 2015). The program PHASE implemented in DnaSP v5 (Librado & Rozas, 2009) was used to estimate the haplotypes of ASIP. In all network analysis, the gap sites were treated as missing.
2.4 Divergence time and demographic history
Population divergence times were estimated from the concatenated mtDNA two-locus data set using BEAST v2.4.8 (Bouckaert et al., 2014) package, with the outgroups mentioned above. Three calibration points were set on the basis of the divergence times among outgroup species and least weasels. Following Sato et al. (2012) and Kinoshita et al. (2015), a normal distribution with a mean of following time and SD was adopted to this analysis: 5.985 ± 0.315 million years ago (mya) between M. nudipes and the others, 5.365 ± 0.195 mya between M. kathiah and the others except M. nudipes, and 3.685 ± 0.285 mya between M. erminea and M. nivalis. In addition, the published estimate of least weasel mtDNA Cytb substitution rate of 2.1% Myr−1 from Dawson, Hope, Talbot, and Cook (2014) and the strict clock model were also used. The tree prior was set as the coalescent constant population model. The HKY + I + G substitution model was used for all loci, which was the best model for our data without partitions using BIC produced by PartitionFinder. The evolutionary rates of other loci were estimated based on these configurations. The MCMC analyses were run 1 × 108 generations with trees sampled every 1,000 generations, and the first 10% of the trees were discarded as burn-in. The effective sample sizes were confirmed by Tracer, with the requirements of convergence of MCMC chains and values for all parameters exceeding 200. The maximum clade credibility tree was selected using TreeAnnotator and visualized with FigTree.
Demographic changes (effective population size history) were simulated by the Extended Bayesian Skyline Plot (EBSP) approached in BEAST. The HKY + I + G model and the substitution rate of 2.1% Myr−1 for the mtDNA Cytb of the least weasel were applied to each of the two identified main mitochondrial clades separately (Clades I and II; 106 and 56 individuals, respectively). The MCMC analyzes were run 1 × 108 generations with trees sampled every 1,000 generations, and the first 10% of the trees were discarded as burn-in. The effective sample sizes and the convergence of MCMC chains were confirmed by Tracer.
2.5 Genetic structure
Genetic diversity and demographic history were estimated from each data set separately (i.e., concatenated mtDNA CR and Cytb, ZFY, and ASIP) with Arlequin ver. 3.5.1.3 (Excoffier & Lischer, 2010), calculating the haplotype diversity, the nucleotide diversity, the neutrality tests of Tajima's D (Tajima, 1989), and Fu's Fs (Fu, 1997).
Population structure was also assessed with SAMOVA (spatial analyses of molecular variance; Dupanloup, Schneider, & Excoffier, 2002) implemented in SPADS 1.0 (Dellicour & Mardulyn, 2014), using all 168 concatenated mtDNA CR and Cytb sequences. Specimens were grouped as populations by country, except for Japan where Hokkaido and Honshu were treated as different populations because there is a chromosomal difference between them. The number of a priori groups (K) was varied between 2 and 10, with 10,000 iterations and 10 repetitions. Following Magri et al. (2006), the preferred K was selected by choosing the highest FCT, and then, configurations with single-population groups were excluded. In addition, the pairwise differences among populations were estimated using the fixation index Φst (Excoffier, Smouse, & Quattro, 1992).
3 RESULTS
3.1 DNA sequencing
A total of 210 novel sequences (45 for CR, 69 for Cytb, 29 for ZFY and 67 for ASIP) were obtained in the present study and deposited to the DDBJ database with accession numbers: CR, LC314601-LC314645; Cytb, LC324904-LC324972; ZFY, LC325040-LC325068; and ASIP, LC324973-LC325039 (Table 1). In the alignments, the novel sequences were compared with the previously reported data (Tables 1 and S1), and then, overlapped regions were used to classify the haplotypes. All haplotype numbers were shown in Tables 2 and S1.
| Voucher No. | Haplotype Nos. | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CR | Cytb | CR and Cytb | ZFY | ASIP-1 | ASIP-2 | mtDNA and ZFY | mtDNA and ASIP | ZFY and ASIP | ALL | |
| Mn4 | A16 | B53 | C63 | D1 | E9 | E9 | F17 | G40 | H2 | I19 |
| Mn5 | A16 | B23 | C22 | – | E9 | E9 | – | G2 | – | – |
| Mn6 | A33 | B23 | C53 | – | E9 | E9 | – | G31 | – | – |
| Mn7 | A16 | B23 | C22 | – | E9 | E9 | – | G2 | – | – |
| NEM01 | A33 | B23 | C53 | – | E9 | E9 | – | G31 | – | – |
| OBH01 | A30 | B23 | C38 | – | E9 | E9 | – | G12 | – | – |
| S12 | A17 | B24 | C23 | D1 | E9 | E9 | F2 | G3 | H2 | I2 |
| S13 | A16 | – | – | – | E9 | E9 | – | – | – | – |
| S14 | A16 | B23 | C22 | D1 | E9 | E12 | F1 | G2 | H1 | I1 |
| YOT1 | A2 | B2 | C2 | – | E9 | E9 | – | G1 | – | – |
| OB1 | A31 | B23 | C40 | D1 | E9 | E9 | F4 | G14 | H2 | I4 |
| OB2 | A16 | B38 | C39 | – | E9 | E9 | – | G13 | H11 | – |
| HIT1 | A34 | B23 | C54 | – | E1 | E9 | – | G33 | H11 | – |
| HIT2 | A30 | B23 | C38 | D1 | E1 | E9 | F19 | G12 | H4 | I20 |
| N26 | A37 | B23 | C58 | D1 | E6 | E6 | F14 | G39 | H8 | I18 |
| N27 | A36 | B23 | C57 | D1 | E6 | E6 | F16 | G38 | H8 | I17 |
| N28 | A36 | B23 | C57 | – | E6 | E6 | – | G38 | H11 | – |
| N29 | A36 | B23 | C57 | – | E6 | E6 | – | G38 | H11 | – |
| N30 | A35 | B23 | C55 | – | E6 | E6 | – | G37 | H11 | – |
| N31 | A2 | B48 | C56 | D1 | E9 | E6 | F15 | G36 | H3 | I16 |
| N32 | A33 | B23 | C53 | D1 | E9 | E9 | F13 | G31 | H2 | I15 |
| N33 | A35 | B23 | C55 | D1 | E9 | E9 | F12 | G35 | H2 | I14 |
| N34 | A34 | B23 | C54 | – | E6 | E6 | – | G34 | H12 | – |
| N35 | A33 | B23 | C53 | – | E9 | E9 | – | G31 | H12 | – |
| N37 | A34 | B23 | C54 | – | E9 | E9 | – | G32 | H12 | – |
| AKI1 | A45 | B59 | C66 | D1 | E5 | E5 | F18 | G41 | H12 | I27 |
| IWA1 | A45 | B59 | C66 | D1 | E5 | E5 | F18 | G41 | H12 | I27 |
| IWA2 | A45 | B59 | C66 | D1 | E5 | E5 | F18 | G41 | H12 | I27 |
| 151646 | A27 | B88 | C118 | – | E1 | E2 | – | G52 | – | – |
| 278252 | A43 | B87 | C115 | – | E2 | E2 | – | G51 | – | – |
| 79252 | A77 | B86 | C109 | – | – | – | – | – | – | – |
| 79253 | A79 | – | – | – | – | – | – | – | – | – |
| 79254 | A80 | – | – | – | – | – | – | – | – | – |
| 79255 | A76 | B85 | C108 | – | E3 | E3 | – | G50 | – | – |
| 79271 | A75 | B84 | C107 | D1 | – | – | F26 | – | – | – |
| 79272 | A81 | – | – | – | – | – | – | – | – | – |
| 79273 | A82 | – | – | – | – | – | – | – | – | – |
| 79274 | A83 | – | – | – | – | – | – | – | – | – |
| 79304 | A74 | B83 | C106 | – | E2 | E2 | – | G49 | – | – |
| 79307 | A73 | B82 | C105 | – | E4 | E4 | – | G48 | – | – |
| RLEN3 | A18 | B27 | C27 | D1 | E2 | E2 | F3 | G6 | H5 | I3 |
| RIND1 | A21 | B30 | C29 | – | E1 | E1 | – | G8 | – | – |
| ROMS1 | A74 | – | – | – | – | – | – | – | – | – |
| BUSG1 | A15 | B79 | C102 | D2 | E2 | E2 | F25 | G47 | H11 | I26 |
| BUV1 | A11 | B78 | C101 | D2 | E2 | E2 | F24 | G46 | H11 | I25 |
| BUV2 | A70 | B77 | C100 | D2 | E2 | E2 | F23 | G45 | H11 | I24 |
| BUV3 | A64 | B76 | C99 | D2 | E2 | E2 | F22 | G44 | H11 | I23 |
| BUV4 | A69 | B75 | C98 | D2 | E2 | E2 | F21 | G43 | H11 | I22 |
| BUV5 | A64 | B74 | C97 | D2 | E1 | E2 | F20 | G42 | H10 | I21 |
| RTUR2 | A19 | B26 | C25 | – | E10 | E2 | – | G5 | – | – |
| RKAR2 | A20 | B29 | C28 | – | E2 | E2 | – | G7 | – | – |
| RASK1 | A25 | B34 | C33 | – | E1 | E2 | – | G11 | – | – |
| RASK2 | A24 | B33 | C32 | – | E1 | E6 | – | G10 | – | – |
| RUMA1 | A18 | B25 | C24 | – | E2 | E11 | – | G4 | – | – |
| RCAC1 | A23 | B32 | C31 | – | – | – | – | – | – | – |
| RCAU2 | A22 | B31 | C30 | – | E1 | E1 | – | G9 | – | – |
| KS.KN 39356 | A73 | B47 | C52 | D1 | E1 | E1 | F11 | G30 | H9 | I13 |
| KS.KN 39357 | A27 | B28 | C44 | D1 | E2 | E7 | F5 | G21 | H6 | I5 |
| KS.KN 34388 | A73 | B41 | C45 | – | E2 | E2 | – | G22 | – | – |
| KS.KN 34462 | A27 | B28 | C44 | – | E2 | E2 | – | G20 | – | – |
| KS.KN 34463 | A27 | B28 | C44 | – | E1 | E1 | – | G18 | – | – |
| KS.KN 39310 | A27 | B28 | C44 | – | E1 | E8 | – | G17 | – | – |
| KS.KN 34644 | A59 | B28 | C43 | – | E2 | E2 | – | G16 | – | – |
| KS.KN 38719 | A59 | B40 | C42 | – | E2 | E2 | – | G15 | – | – |
| KS.KN 34740 | A59 | B40 | C42 | – | E2 | E2 | – | G15 | – | – |
| KS.KN 33901 | A59 | B46 | C51 | – | E2 | E2 | – | G29 | – | – |
| KS.KN 47736 | A27 | B28 | C44 | D1 | E2 | E2 | F6 | G20 | H5 | I7 |
| KS.KN 47078 | A59 | B40 | C42 | D1 | E2 | E2 | F10 | G15 | H5 | I12 |
| KS.KN 47020 | A78 | B45 | C50 | D1 | E6 | E6 | F9 | G28 | H8 | I11 |
| KS.KN 47872 | A59 | B44 | C48 | D1 | E6 | E6 | F7 | G27 | H8 | I10 |
| KS.KN 47874 | A27 | B28 | C44 | D1 | E6 | E2 | F6 | G19 | H7 | I6 |
| KS.KN 47882 | A27 | B41 | C49 | D1 | E2 | E2 | F8 | G26 | H5 | I9 |
| KS.KN 47906 | A59 | B44 | C48 | – | E1 | E1 | – | G25 | – | – |
| KS.KN 47907 | A27 | B43 | C47 | D1 | E6 | E6 | F6 | G24 | H8 | I8 |
| KS.KN 48312 | A73 | B41 | C45 | – | E2 | E2 | – | G22 | – | – |
| KS.KN 48372 | A1 | B42 | C46 | – | E6 | E6 | – | G23 | – | – |
3.2 Diversity and genealogy of mtdna
In all, 92 distinct haplotypes were identified from the 215 sequences of the mtDNA CR. The length of CR was 514 bp including insert–deletion sites. The BI tree of CR was multifurcate and unstructured with no clear clusters except for the Hokkaido population (Cluster Ie; Figure S1 and Appendix S1). Likewise, 116 haplotypes of Cytb were detected from 221 sequences of 1,117 bp. The BI tree of Cytb was also poorly resolved, but some populations including the Hokkaido population and some European populations made up regional clusters (Figure S2 and Appendix S2).
In the concatenated CR and Cytb data (1,631 bp), 119 distinct haplotypes were recognized among 168 individuals (69 newly sequenced). This BI tree exposed two main clades (Figure 2 and Appendix S3), corresponding to the Clades I and II of Lebarbenchon et al. (2010); however, the support values were lower than the previous report. Clade I was in low monophyly with low supported values (0.77 PP and 9% BP), while Clade II had relatively high values (1.0 PP and 50% BP; cf, 1.0/ 93% for Clade I and 0.94 / 76% for Clade II in Lebarbenchon et al., 2010; Figure 2). All samples were divided to Clades I, II, and the others, based on the topology of this BI tree (Figure 2). The others consisted of the individuals of Turkmenistan (C25 and C28), Georgia (C30 and C31), and Ukraine (C32 and C33). These individuals were located at the most basal position. The ML tree in turn was multifurcated and did not have the main clades (Figure S3).
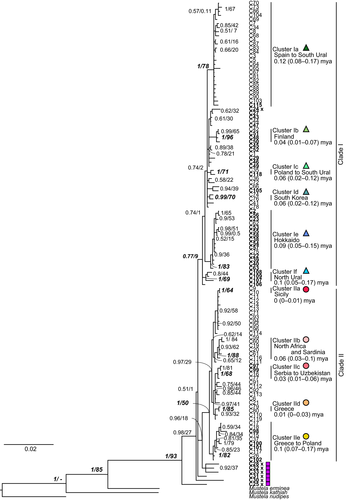
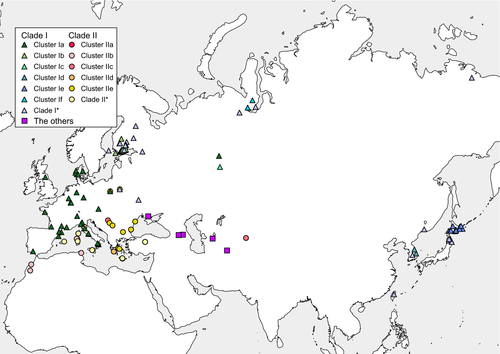
The CR and Cytb concatenated BI tree also exhibited eight clusters with high support values (Figure 2). Cluster Ia consisted of individuals from Spain to South Ural. Cluster Ib was shared by individuals from Finland. Cluster Ic distributed in Poland and South Ural. Cluster Ie consisted by only the Hokkaido population. Cluster If included only the North Ural individuals. Cluster IIb was shared by North African individuals and Sardinia. Cluster IId consisted of individuals from Greece, only. Cluster IIe was found from Greece to Poland. In addition, there were three clusters with relatively high support values: Cluster Id was consisted of South Korea, Cluster IIa was shared by Italian and its insular individuals, and Cluster IIc was distributed in Serbia, Bulgaria, and Uzbekistan. Individuals of Honshu Island of Japan shared haplotype C66, which is more closely related to that C105 of a North Ural individual than the Hokkaido population. The locations of clades and clusters were plotted on a Palearctic map (Figure 3).
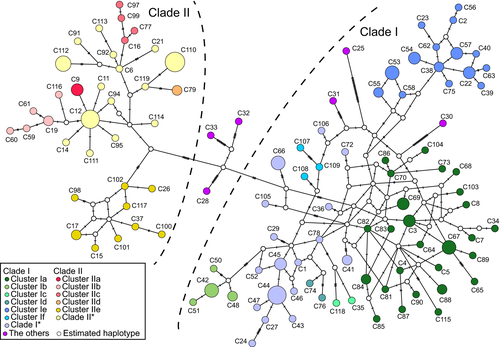
The mtDNA haplotype network indicated two main clades clearly, compared to the BI tree (Figure 4 and Appendix S4). Clade I was too complicated to read the phylogeographical relationships between haplotypes with many loops indicating the homoplasies. In contrast, Clade II had relatively clear internal structure and the mentioned clusters were supported. Haplotype C28 from Turkmenistan and the Ukrainian C32 and C33 were not placed in either of the two main clades but occupied an intermediate position of them.
3.3 Diversity in the paternal and biparental genes
Only two ZFY haplotypes, D1 and D2, were found from among 30 individuals, of which 29 were newly sequenced in this study. These two haplotypes were strongly diverged, with eight nucleotide differences and an 8-bp indel between them (Figure 5 and Appendix S5). D1 was shared by 12 individuals of Japan (Hokkaido and Honshu), two of Russia and nine of Finland, whereas D2 was common to all six individuals of Bulgaria. The Bulgarian D2 featured the 8-bp insertion, which was also present in the ZFY sequence of the closely related Mustela erminea (the stoat).
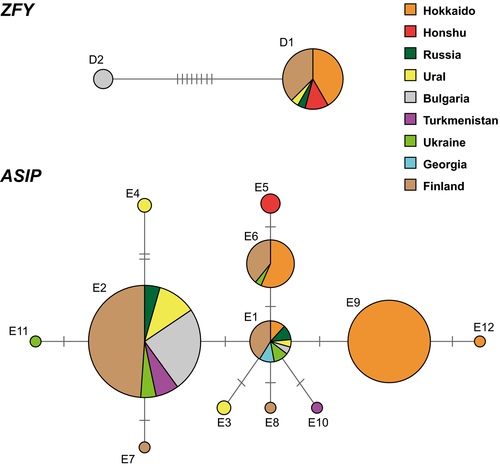
For ASIP, 12 haplotypes were detected from 67 individuals obtained in the present study (Figure 5 and Appendix S6), which had 12 polymorphic sites. A central haplotype E1 was found in all regions except Honshu, where all individuals shared another haplotype E5. Hokkaido in turn had five haplotypes. However, no further phylogeographical structure was seen, and the differences between haplotypes were just one or two substitutions.
3.4 Phylogenetic trees from multilocus data
All sequence data on the four loci (mtDNA CR, mtDNA Cytb, ZFY, and ASIP) obtained in the present study were concatenated to reconstruct a BI tree (Figure S4a and Appendix S7). This tree consisted of 29 individuals of 2,823 bp with the stoat (Mustela erminea) as an outgroup, and the two main clades were supported with higher bootstrap values compared to the mtDNA tree: 92% for Clade I and 98% for Clade II. On the other hands, if ZFY was excluded from the data set, no strongly supported major clustering was seen, except for some regional clusters such as Finland, Hokkaido, and Bulgaria (Figure S4 and Appendices S7–S10).
3.5 Divergence time and population expansion history
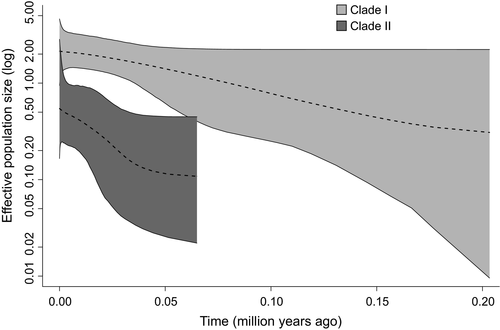
The Bayesian phylogram with BEAST analyses, based on the two concatenated mtDNA loci, had a topology similar to that from MrBayes (Figure S5). The time of most recent common ancestor (tMRCA) of the least weasels would have been on 740 (560–940, 95%HPD) thousand years before present (kyBP), at the split of the Turkmenistan lineage (C25) from the others. The coalescence of the remaining lineages would have been 480 (370–600) kyBP. The divergence time of the two main clades could not be traced from this tree, caused by the low PP support (<0.95). The tMRCA of Clade II was 200 (140–270) kyBP. The divergence times of the clusters were also obtained (Figure 2). Based on the demographic fluctuation analysis, both Clades I and II had experienced the population expansion from small populations. Clade II was relatively stable, whereas the expansion of Clade I was more rapid. (Figure 6, and Appendices S11 and S12).
3.6 Genetic structure
A hierarchical partition of genetic diversity in the concatenated mtDNA CR and Cytb data by region, and by clade and intra-clade clusters, is shown in Table 3. Overall, the nucleotide diversities were low (shallow genealogy) but haplotype diversities were high. Populations of the Black Sea–Caspian Sea region had the highest nucleotide diversities (0.028 for Turkmenistan; 0.025 for Ukraine; and 0.026 for Georgia), and that of the Ural population (0.009) was also relatively high. Cluster If had the highest nucleotide diversity among clusters. The Honshu population in turn was monomorphic at all loci. Significant values of Tajima's D and Fu's Fs statistics are suggestive of the rapid expansion in the Palearctic population. Likewise, treating the mitochondrial lineages separately, Clade I and Cluster Ia show signatures of rapid expansion. The Hokkaido population, Clade II and Cluster IIe, also had significant values of Fu's Fs. The autosomal ASIP showed an overall low nucleotide diversity (0.003) and a high haplotype diversity (0.78). The Y-chromosomal ZFY showed relatively low nucleotide and haplotype diversities (0.008 and 0.331). The data of ZFY and ASIP did not indicate an expansion.
| Gene | Group | n | h | Hd | π | D | F s |
|---|---|---|---|---|---|---|---|
| mtDNA | All | 168 | 119 | 0.992 | 0.0123 | −1.598* | −23.780* |
| Hokkaido (Cluster Ie) | 26 | 15 | 0.948 | 0.0023 | −0.898 | −6.035* | |
| Honshu | 4 | 1 | 0.000 | 0.0000 | 0.000 | NA | |
| Russia† | 3 | 3 | 1.000 | 0.0053 | 0.000 | 0.987 | |
| Ural | 7 | 7 | 1.000 | 0.0090 | −1.057 | −0.923 | |
| Bulgaria | 6 | 6 | 1.000 | 0.0062 | 0.592 | −0.947 | |
| Turkmenistan | 2 | 2 | 1.000 | 0.0283 | 0.000 | 3.829 | |
| Ukraine | 3 | 3 | 1.000 | 0.0251 | 0.000 | 2.591 | |
| Georgia | 2 | 2 | 1.000 | 0.0258 | 0.000 | 3.738 | |
| Finland | 22 | 11 | 0.875 | 0.0035 | 0.591 | −0.831 | |
| Clade I | 106 | 71 | 0.988 | 0.0084 | −1.590* | −24.152* | |
| Cluster Ia | 35 | 26 | 0.977 | 0.0030 | −1.614* | −18.823* | |
| Cluster Ib | 8 | 4 | 0.750 | 0.0012 | −0.705 | 0.119 | |
| Cluster Ic | 2 | 2 | 1.000 | 0.0037 | 0.000 | 1.792 | |
| Cluster Id | 2 | 2 | 1.000 | 0.0037 | 0.000 | 1.792 | |
| Cluster If | 3 | 3 | 1.000 | 0.0053 | 0.000 | 0.987 | |
| Clade II | 56 | 41 | 0.973 | 0.0059 | −1.185 | −19.949* | |
| Cluster IIa | 3 | 2 | 0.667 | 0.0000 | 0.000 | NA | |
| Cluster IIb | 6 | 6 | 1.000 | 0.0023 | −0.978 | −0.905 | |
| Cluster IIc | 4 | 4 | 1.000 | 0.0017 | 0.650 | −1.322 | |
| Cluster IId | 2 | 2 | 1.000 | 0.0006 | 0.000 | 0.000 | |
| Cluster IIe | 10 | 9 | 0.978 | 0.0031 | −0.301 | −3.619* | |
| ASIP | All | 67 | 12 | 0.780 | 0.003 | −0.702 | −3.367 |
| Hokkaido | 25 | 4 | 0.478 | 0.002 | 0.591 | 0.479 | |
| Honshu | 3 | 1 | 0.000 | 0.0000 | 0.000 | NA | |
| Russia† | 2 | 2 | 0.667 | 0.0014 | 1.633 | 0.540 | |
| Ural | 5 | 4 | 0.733 | 0.0032 | 0.324 | 0.017 | |
| Bulgaria | 6 | 2 | 0.167 | 0.0003 | −1.141 | −0.476 | |
| Turkmenistan | 2 | 2 | 0.500 | 0.0021 | −0.710 | 1.099 | |
| Ukraine | 3 | 4 | 0.867 | 0.0026 | −0.185 | −1.350 | |
| Georgia | 1 | 1 | 1.000 | 0.0000 | 0.000 | NA | |
| Finland | 20 | 5 | 0.631 | 0.0020 | 0.047 | −0.443 | |
| ZFY | All | 30 | 2 | 0.331 | 0.0077 | 0.946 | 13.074 |
Note
- Asterisks show that values of D or Fs are statistically significant (p < .05). A dagger means excluded the Ural individuals.
- Abbreviations: D, Tajima's D; Fs, Fu's Fs; h, number of haplotypes; Hd, haplotype diversity; n, number of individuals; NA, not analysis; π, nucleotide diversity.
Least weasels had genetic differences to each other (Φst = 0.556) in the Palearctic, but an attempt to divide the regional populations to subgroups by SAMOVA was not very successful with Appendix S4. A search for the number of independent groups (K) that show the maximum inter-group diversity FCT showed that this statistic increased with K and did not reach a plateau over K = 10 (Figure S6). A configuration of K = 3, for which FCT (0.368) was the highest among the others without single-population groups, was very similar to the mtDNA haplotype network (Figure 3). Group I represented populations distributed across the continent from Spain to Japan, which carry the mtDNA Clade I except for Georgia. Group II in turn included populations of Romania, Serbia, Bulgaria, Greece, Turkey, Tunisia, and Morocco, which harbor mtDNA Clade II. Group III was composed of the populations from Turkmenistan and Ukraine (Table 4).
| K | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Hokkaido | I | I | I | I | I | I | I | I | I |
| Honshu | I | I | II | II | II | II | II | II | II |
| Russia | I | I | I | II | II | III | III | III | III |
| Bulgaria | II | II | III | III | III | IV | IV | IV | IV |
| Turkmenistan | I | III | IV | IV | IV | V | V | V | V |
| Ukraine | I | III | I | IV | IV | V | V | V | V |
| Georgia | I | I | I | II | II | III | V | V | VI |
| Finland | I | I | I | II | II | III | III | III | III |
| France | I | I | I | V | V | VI | VI | VI | VII |
| Italy | I | I | I | V | V | VI | VI | VI | VII |
| Austria | I | I | I | V | VI | VII | VII | VII | VIII |
| Morocco | II | II | III | III | III | IV | VIII | VIII | IX |
| Switzerland | I | I | I | V | VI | VII | VII | VII | VIII |
| Romania | II | II | III | III | III | IV | IV | IV | IV |
| Greece | II | II | III | III | III | IV | VIII | IX | X |
| United Kingdom | I | I | I | V | VI | VII | VII | VII | VIII |
| Poland | I | I | I | V | V | VI | VI | VI | VII |
| Spain | I | I | I | V | V | VI | VI | VI | VII |
| Belgium | I | I | I | V | VI | VII | VII | VII | VIII |
| Germany | I | I | I | V | VI | VII | VII | VII | VIII |
| Serbia | II | II | III | III | III | IV | IV | IV | IV |
| Denmark | I | I | I | V | VI | VII | VII | VII | VIII |
| Luxembourg | I | I | I | V | VI | VII | VII | VII | VIII |
| South Korea | I | I | I | II | II | III | III | III | III |
| Uzbekistan | I | I | I | III | II | IV | V | V | III |
| Taiwan | I | I | I | II | II | III | III | III | III |
| Tunisia | II | II | III | III | III | IV | VIII | VIII | IX |
| Turkey | II | II | III | III | III | IV | VIII | IX | X |
| F CT | 0.3410 | 0.3685 | 0.3563 | 0.4010 | 0.4282 | 0.4722 | 0.4702 | 0.4849 | 0.5092 |
| F ST | 0.6510 | 0.6507 | 0.6461 | 0.5890 | 0.5820 | 0.5821 | 0.5762 | 0.5751 | 0.5764 |
| F SC | 0.4704 | 0.4470 | 0.4502 | 0.3138 | 0.2690 | 0.2083 | 0.2002 | 0.1750 | 0.1368 |
Note
- K is the number of groups.
4 DISCUSSION
4.1 Phylogeography of the least weasel in Palearctic
Our data from the concatenated mtDNA CR and Cytb sequences from a geographically expanded data set demonstrate a confused phylogeographic structure of the least weasels in Palearctic. The tree topologies agree with the two main lineages (Clades I and II) of Lebarbenchon et al. (2010), whereas the support values could not provide full confidence on Clade I's monophyly (Figure 2). Clade I has a broad distribution across northern Palearctic, from Spain in the west to Japan in the east, while Clade II is limited to the South western part of the Palearctic, between North Africa and East Europe. At a lower level, the least weasels were tentatively classified into eleven mtDNA clusters. The Turkmenistan lineage of Central Asia plausibly represents the most ancestral split among all Palearctic populations, as in the data of Kurose et al. (2005).
Our data expanded the coverage of European sampling, for example, Bulgaria and the Urals, supplementing the data of Lebarbenchon et al. (2010) and Rodrigues et al. (2016, 2017). The Bulgarian population was separated into two Clusters (IIc and IIe), and the Northern Urals population consisted of one distinctive Cluster (If) by itself. Individuals from around the Black and Caspian Seas had the new Cytb haplotypes that seemed to represent the most ancestral lineages branches of the genealogy (Figure 2). Furthermore, the Turkmenistan and Ukraine haplotypes appeared at an intermediate position between Clades I and II in the mtDNA haplotype network (Figure 4). This configuration probably brings about the lower confidence values of the main clades, relative to those reported by Lebarbenchon et al. (2010) and McDevitt et al. (2012).
The SAMOVA analysis also suggests the ambiguity for the phylogeographic structure, although the interpopulation component of variation is large (Φst = 0.556). The data point to a history of demographic and geographic expansions that have taken place across large areas while more ancestral diversity has been retained in certain segments of the range. The nucleotide diversity through the Palearctic population is quite low (0.012), while the haplotype diversity is very high (0.992; Table 3). This suggests that the least weasels experienced a rapid demographic expansion from a small population, as also indicated by the Bayesian simulation, Tajima's D and Fu's Fs statistics (Figure 6 and Table 3). Remarkably, similar results were also reported in the closely related species stoat M. erminea (Dawson et al., 2014). The data showed low genetic differences and suggested a rapid expansion of M. erminea from Spain across the continent and through Beringia to North America. Such continent-wide expansions have also been inferred from other carnivorans, such as Red fox (Vulpes vulpes Linnaeus, 1758) and Brown bear (Ursus arctos Linnaeus, 1758; Korsten et al., 2009; Statham et al., 2014).
The phylogeographic structure also shows some phenotypic correlates. It seems that Clade II has survived and maintained a relatively constant population size and high genetic diversity in the temperate region in the south western part of the Palearctic, whereas the expansive Clade I represents adaptation to the cold regions. On the other hand, the SAMOVA grouping (K = 2) coincided with the distribution of the two coloration types recognized by Abramov and Baryshnikov (2000). Group I correlates with the nivalis-type coat color and Group II with the vulgaris type. Atmeh, Andruszkiewicz, and Zub (2018) demonstrated a clear relationship between the predation pressure and the camouflage of pelage coloration in a field experiment. One could speculate that this also affected the demographic and evolutionary histories. Clade I (Group I) expanded rapidly across Palearctic including the cold regions.
Our study also for the first time addressed the signals of population history in variation of a biparentally inherited gene ASIP and the paternally inherited ZFY. The ASIP haplotype network, however, did not have any phylogeographic variation (Figure 5). Even the Turkmenistan individual, which had a distinct mtDNA lineage, shared haplotype E2 widespread across the range, in Bulgaria, Urals, Russia, Finland, and Hokkaido. The ZFY haplotype network in turn had a distinct subdivision of two main lineages that corresponded with the mtDNA Clade I-II split (Figure 5). Haplotype D1 was distributed from Finland to Japan. This distribution pattern is indeed similar to that in the gray red-backed vole (Craseomys rufocanus Sundevall, 1846) which exhibits a monomorphism from western to far eastern Russia, within the partial sequence of the Y-chromosomal DNA (Abramson, Petrova, Dokuchaev, Obolenskaya, & Lissovsky, 2012), and the spatial distribution of mtDNA and Y-chromosomal DNA haplotypes was slightly different. Our spatial distribution of the haplotypes also differed by each gene. As one hypothesis, this discordance could be caused by behavioral differences between sexes of least weasels. The least weasel has the significant sexual dimorphism in body size (greater male vs. female) and sex-biased differences in habitat use (King & Powell, 2007). Furthermore, McDevitt et al. (2013) suggested the dispersal of least weasels was sex-biased toward males. The male wide dispersal could be attributed to the phylogeographical status. In fact, the two main clades are supported with high values if the BI trees included ZFY sequences in data sets (Figure S4). Similar conclusions about discordant diversity in multiple loci from the same species have been drawn from other studies, such as Abramson et al. (2012) and Jones and Searle (2015). They also suggested that the discordant may have been caused by the sex biased dispersal in male and female voles and mice. If the male-biased dispersal of least weasels is true, it is easy to suppose that the large males had been strongly affected by cold temperature and could not distribute in high latitude, considering that Zub et al. (2011) suggested larger least weasel had low survival rate in cold environments.
4.2 Characteristics of clusters and local populations
We recognized some lower-level structure in the mtDNA genealogy of the least weasels, represented by eleven mtDNA clusters with relatively high support values (Figure 2). Three of these Clusters (Ib, Ic, and If) were newly recognized in the present study. Cluster If, only found in the Northern Urals, has the highest intra-cluster diversity. The tMRCA of this cluster was dated approximately 100 kyBP, close to MIS (marine isotope stage) 5c (105–93 kyBP, Räsänen, Huitti, Bhattarai, Harvey, & Huttunen, 2015). The population might then represent a relict that survived the glacial period, and the Ural region would have been one of the cryptic refugia for the species. Actually, based on the paleontological study, Kosintsev, Gasilin, Gimranov, and Bachura (2016) reported the least weasel appeared consistently from MIS 5e in the Ural caves. Likewise, previous studies of other mammalian species also suggested possible refugia in the Ural Mountains. For example, the sables (Martes zibellina Linnaeus, 1758; Kinoshita et al., 2015) and the bank voles (Myodes glareolus Schreber, 1780; Deffontaine et al., 2005) from the Ural Mountains had relatively higher genetic diversities, suggesting the polymorphic status of relict populations of them. The Ural Mountains were reported as refugia for the common shrews (Sorex araneus Linnaeus, 1758) by Polyakov et al. (2001). Cluster Ib consisted of Finnish individuals, and Cluster Ic consisted of just two individuals from Poland and South Ural.
The remaining eight clusters were already reported previously (Kurose et al., 2005, 1999; Lebarbenchon et al., 2010; Rodrigues et al., 2016, 2017). Cluster Ia (=subclade Ia) is now shown to be distributed from Spain to South Urals, and its estimated tMRCA is projected twofold older than by Lebarbenchon et al. (2010) (120 kyBP vs. 62kyBP). Kurose et al. (1999), Kurose et al. (2005) first reported the Cluster Ie (Hokkaido). This tMRCA was estimated as less than 200 kyBP, but the present study showed around 90 kyBP. The result suggests that ancestors of the Hokkaido population could be prevented to pass the Tsugaru Strait, which separates Honshu and Hokkaido, because that strait was formed around 100–150 kyBP (Ohshima, 1991).
Remarkably, the Honshu population in Japan has no variation on any loci, and this lineage (C66) was more closely related to the North Ural lineage (C106) than the Hokkaido population in the mtDNA analysis. The ZFY haplotype D1, however, was shared among Honshu, Hokkaido, and North Ural. The number of chromosomes was also different between the Honshu population (2n = 38) and the Hokkaido population (2n = 42; Obara, 1991). In addition, all studied populations from Eurasia and North America bear the karyotypes similar to that of the Hokkaido population (Mandahl & Fredga, 1980; Zima & Grafodatskij, 1985). According to our result and the previous studies, the migration history could be different between the Honshu and Hokkaido populations.
The Bulgarian individuals were separated into two Clusters (IIc and IIe). Cluster IIc is distributed from Serbia in the Balkan Peninsula to Uzbekistan in Central Asia. Cluster IIe includes the individuals from Greece to Poland and could have experienced the demographic expansion shown by Fu's Fs statistics. Populations around the Black and Caspian Seas (Turkmenistan, Ukraine, and Georgia) had higher nucleotide diversities and were differentiated from the other populations. It has been reported that the populations of the Black and Caspian Seas area could be the ancestor type of least weasel, using morphological characters (Abramov & Baryshnikov, 2000) and mtDNA CR (Kurose et al., 2005). Our results emphasize this possibility.
ACKNOWLEDGEMENTS
We would like to thank T. Saitoh, Y. Masuda, H. Yanagawa, F. Sekiyama, M. Takahashi, M. Hisasue, the Finnish Museum of Natural History, and the Museum at the Institute of Plant and Animal Ecology (Ural Branch of the Russian Academy of Sciences) for providing samples, and Y. Nishita for suggestions. This study was supported in part by Joint Research Project Grants from the Japan Society of the Promotion of Science (JSPS) and the Russian Foundation for Basic Research, Russian State program AAAA-A17-117022810195-3, and a grant from the Joint Research Program of the Japan Arctic Research Network Center.




