Systematics and historical biogeography of the Aphanius dispar species group (Teleostei: Aphaniidae) and description of a new species from Southern Iran
Abstract
Among the species of Aphanius Nardo, 1827, Aphanius dispar (Rüppell, 1828) is the most common taxon and has long been viewed as representing a species group rather than a single species. This study provides comprehensive data on the phylogenetic relationships, morphology, and otoliths within the A. dispar species group, including the description of a new species. Our data demonstrate that the “true” A. dispar is restricted to the Red Sea drainages and that all other populations hitherto identified as A. dispar actually represent separate species. Four main clades are defined and named for the geographic areas in which the respective species of Aphanius occur. The oldest one is the “Red Sea clade,” it comprises A. dispar. The “Dead Sea clade” is represented by A. richardsoni (Boulenger, 1907). It is sister to both the “Hormuzgan clade” in S Iran (containing A. hormuzensis sp. nov. and A. ginaonis (Holly, 1929)) and the “Persian Gulf & Gulf of Oman clade” (comprising A. stoliczkanus (Day, 1872)). The species separation within the A. dispar group is confirmed by the distinctive otolith morphology of each species. Moreover, we present a time-calibrated phylogeny (chronogram) for the A. dispar species group using †A. princeps (16–17 Mya) as a minimum age and the first appearance of †Prolebias (33–34 Mya) as a maximum age for the genus Aphanius. The evolution and historical biogeography routes are discussed based on the outcome of the chronogram and in the context of the geological and climatic history of the Near East in Pliocene–Pleistocene times.
1 INTRODUCTION
The killifish genus Aphanius is the sole native representative of the family Aphaniidae in the Old World and represents a relic of the ancient ichthyofauna of the Tethys (Freyhof, Weissenbacher, & Geiger, 2017; Hrbek & Meyer, 2003; Kosswig, 1955). Its present-day species diversity and distribution have largely been shaped by vicariance events since the early Miocene, when the ancient Tethys Sea closed and uplift of the Anatolian and Iranian plateaus began (e.g., Hrbek & Meyer, 2003; Teimori, Esmaeili, Erpenbeck, & Reichenbacher, 2014).
Thirty-five nominal species of Aphanius have been described to date (Eschmeyer & Fong, 2017; Freyhof et al., 2017). The greatest species diversity appears in the Near East, especially Iran and Turkey (Coad, 2009; Coad & Abdoli, 2000; Hrbek & Meyer, 2003; Hrbek, Keivany, & Coad, 2006; Wildekamp, Kücük, Ünlüsayin, & Neer, 1999). Thirteen native and one widely distributed species (A. dispar) occur in drainage basins in Iran (e.g., Coad, 1988, 2009; Coad & Abdoli, 2000; Esmaeili, Teimori, Gholami, Zarei, & Reichenbacher, 2012; Gholami, Esmaeili, Erpenbeck, & Reichenbacher, 2014; Hrbek et al., 2006; Teimori et al., 2014; Teimori, Esmaeili, Gholami, Zarei, & Reichenbacher, 2012; Teimori, Schulz-Mirbach, Esmaeili, & Reichenbacher, 2012).
Species of Aphanius are well known for their remarkable capacity to adapt to adverse ecological conditions and evolve into new species when populations become isolated. This has made them particularly attractive as model species for biologists, and many researchers have studied their phenotypic variation, diversity, zoogeography, and phylogenetic relationships (e.g., Chiozzi et al., 2017; Ferrito, Mannino, Pappalardo, & Tigano, 2007; Ferrito et al., 2013; Hrbek & Meyer, 2003; Parenti, 1981; Tigano, Ferrito, & Nicosia, 1999; Tigano et al., 2006; Villwock, 1982; Wildekamp et al., 1999). Over the past decade, significant progress has also been made in elucidating the phylogeny of Aphanius in the Near East, with a strong focus on populations in Iran (Coad, 2009; Esmaeili, Teimori, Gholami, & Reichenbacher, 2014; Esmaeili et al., 2012; Esmaeili, Teimori, Sayyadzadeh, Masoudi, & Reichenbacher, 2014; Gholami et al., 2014; Gholami, Esmaeili, Erpenbeck, & Reichenbacher, 2015; Gholami, Esmaeili, & Reichenbacher, 2015; Gholami, Teimori, Esmaeili, Schulz-Mirbach, & Reichenbacher, 2013; Hrbek et al., 2006; Masoudi et al., 2016; Teimori, Esmaeili, et al., 2012; Teimori, Schulz-Mirbach, et al., 2012, 2012, 2014; Teimori, Jawad, Al-Kharusi, Al-Mamry, & Reichenbacher, 2012; Teimori et al., 2014). A recent study of A. dispar (Rüppell, 1828), the most common species of Aphanius in Iran and around the Persian Gulf, is performed by Freyhof et al. (2017). The objective of our study was to provide a comprehensive analysis for the populations from the drainages of southern Iran and to discuss the biogeographic history of the A. dispar species group in the Near East.
1.1 Aphanius dispar (Rüppell, 1828)
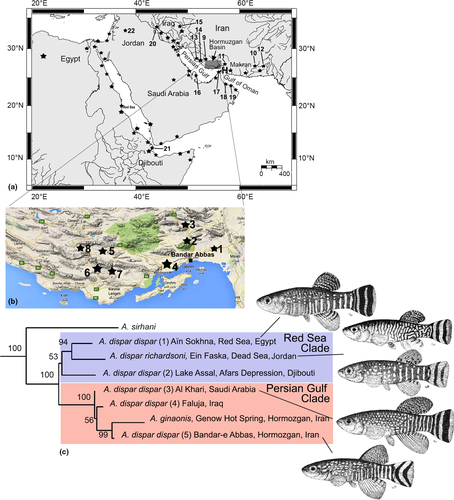
Aphanius dispar as currently understood (“A. dispar” in the following) is found over an area that extends from the easternmost Mediterranean Sea, the Red Sea, and the Persian Gulf to the Indian Ocean and the southern coastline of Pakistan (Wildekamp, 1993) (Figure 1a,b). Its principal habitats are coastal lagoons, but it is capable of thriving in inland waters to which it may have migrated from the coast in the past (Krupp, 1983; Reichenbacher, Feulner, & Schulz-Mirbach, 2009). A notable feature of “A. dispar” is its astonishing ecological tolerance. It can survive in all types of endorheic and exorheic river systems, including very small creeks and large streams, hot sulfur-rich springs (in Iran), and the springs in the Dead Sea Valley (Krupp, 1983; Teimori, Schulz-Mirbach, et al., 2012; Wildekamp, 1993).
Individuals of “A. dispar” may reach 50 mm (very rarely up to 70 mm) in total length and are characterized by a moderately slender body with a prominent pigmentation pattern consisting of blotches, flank bars, or a combination of the two. The name of the species refers to the clear sexual dimorphism with respect to pigmentation (only the males have two black vertical stripes on the caudal fin) and anal and dorsal fin lengths (which are larger in males) (Krupp, 1983; Rüppell, 1828; Villwock, Scholl, & Krupp, 1983; Wildekamp, 1993).
Formerly, two subspecies were accepted, that is, A. dispar dispar (Rüppell, 1828) and A. dispar richardsoni (Boulenger, 1907). One other subspecies has also been described as A. dispar stoliczkanus (Day, 1872), but this has been considered as a synonym for A. dispar dispar in more recent work (e.g., Wildekamp, 1993). Data on the phenotypic variation between isolated populations of “A. dispar” have been provided by several studies (Keivany & Ghorbani, 2012; Krupp, 1983; Masoudi et al., 2016; Teimori, Schulz-Mirbach, et al., 2012; Villwock et al., 1983; Wildekamp, 1993), and also variation in otolith morphology within “A. dispar” was described (Reichenbacher, Feulner, et al., 2009; Reichenbacher, Kamrani, Esmaeili, & Teimori, 2009; Reichenbacher, Sienknecht, Küchenhoff, & Fenske, 2007; Teimori, Schulz-Mirbach, et al., 2012; Teimori, et al., 2012). Furthermore, the impact of environmental parameters on the growth, feeding habits, and reproduction of “A. dispar” has been studied (Frenkel & Goren, 1997, 2000; Homski, Goren, & Gasith, 1994; Lotan, 1969).
It is obvious from the aforementioned studies that the wide geographical distribution of “A. dispar” and its presence in what are now isolated habitats have resulted in considerable intraspecific diversity with regard to fin sizes, pigmentation, morphometric and meristic characteristics, and otolith morphology. However, the only studies that have yet considered “A. dispar” based on molecular genetic analyses are those of Hrbek and Meyer (2003), Chiozzi et al. (2017), and Freyhof et al. (2017). Hrbek and Meyer (2003) showed that “A. dispar” splits into two major clades (a Persian Gulf clade and a Red Sea clade) and does not represent a monophyletic species because it also includes A. ginaonis (Holly, 1929) (see Figure 1c). In light of the work of Hrbek and Meyer (2003), it seemed likely that the broad range of intraspecific variation in “A. dispar” reflects diversification due to geographical isolation and that the taxon encompasses more than one species. A similar conclusion was presented by Chiozzi et al. (2017), who demonstrated that Aphanius cf. dispar in the Danakil Depression in northeastern Africa reveals high genetic diversity and is clearly separated from “A. dispar sensu stricto.” Freyhof et al. (2017) showed the validity of A. richardsoni from the Dead Sea drainages, and of A. stoliczkanus from the coastal areas of the Persian Gulf, the northern Arabian Sea east to Gujarat in India, the Gulf of Oman, and some endorheic basins in Iran and Pakistan. These authors also described A. kruppi Freyhof, Weissenbacher and Geiger, 2017 as new species within the A. stoliczkanus group from Oman.
In this study, we (i) clarify the phylogenetic relationships between the Persian Gulf, Red Sea, and Dead Sea populations of “A. dispar”, (ii) define the species subsumed within the taxon and describe a new species from southern Iran, (iii) present a time-calibrated phylogeny using fossils as calibration points, and (iv) discuss the biogeographical history.
2 MATERIALS AND METHODS
2.1 Ethics statement
This study was carried out in strict accordance with applicable national and international guidelines. The study was approved by the Ethics Committee of the Biology Department of the Shiraz University (SU-909830).
2.2 Sampling
The dataset comprises 286 individuals (+ four tissue samples) of “A. dispar” from 21 sites in the Persian Gulf and Gulf of Oman, the Red Sea, and the Dead Sea (Table 1; Figure 1). These individuals were used for molecular genetic and morphological analyses (see Table 2 for details). In addition, we collected 10 specimens of A. ginaonis (Holly, 1929) (site 2) and eight individuals of A. furcatus Teimori et al., 2014 (sites 1, 3, 4, 8) from the Hormuzgan basin.
| Locality | Basin - Country | Habitat type | Coordinates | Species collected |
|---|---|---|---|---|
| Shur (1) | Hormuzgan, Iran | Brackish water river | E 56°28′10.2″ N 27°19′37.6″ | A. furcatus, A. hormuzensis |
| Genow (2) | Hormuzgan, Iran | Hot sulfur spring | E 56°17′97.0″ N 27°26′77.2″ | A. ginaonis |
| Khurgu (3) | Hormuzgan, Iran | Hot sulfur spring | E 56°28′08.2″ N 27°31′34.1″ | A. furcatus, A. hormuzensis |
| Kol (4) | Hormuzgan, Iran | Brakish water river | E 55°45′31.2″ N 27°07′40.3″ | A. furcatus, A. hormuzensis |
| Rassul (5) | Hormuzgan, Iran | Brakish water river | E 54°36′30.5″ N 27°16′13.2″ | A. hormuzensis |
| Gotab (6) | Hormuzgan, Iran | Brakish water river | E 54°15′46.1″ N 27°08′39.8″ | A. hormuzensis |
| Kukherd (7) | Hormuzgan, Iran | Brakish water river | E 54°29′13.1″ N 27°04′28.7″ | A. hormuzensis |
| Faryab (8) | Hormuzgan, Iran | Hot sulfur spring | E 54°16′28.0″ N 27°25′16.2″ | A. furcatus, A. hormuzensis |
| Howba (9) | Mond, Iran | Hot sulfur spring | E 53°53′58.4″ N 27°57′30.5″ | A. stoliczkanus |
| Irandegan (10) | Jazmourian, Iran | Qanat | E 62°24′50.9″ N 27°13′18.4″ | A. stoliczkanus |
| Rudan (11) | Hormuzgan, Iran | Brakish water river | E 57°15′14.5″ N 27°28′24.4″ | A. stoliczkanus |
| Saravan (12) | Mashkid, Iran | Brakish water river | E 62°24′50.9″ N 27°34′39.9″ | A. stoliczkanus |
| Dalaki (13) | Helleh, Iran | Hot sulfur spring | E 51°16′35.4″ N 29°24′07.9″ | A. stoliczkanus |
| Mirahmad (14) | Helleh, Iran | Hot sulfur spring | E 51°16′50.9″ N 27°48′56.4″ | A. stoliczkanus |
| Sartang (15) | Tigris | Spring stream | E 45°51′48.5″ N 33°41′24.3″ | A. stoliczkanus |
| Al-Manameh (16) | Persian Gulf, Bahrain | Aquarium | E 50°29′57.7″ N 26°10′05.4″ | A. stoliczkanus |
| Khor Hulaylah (17) | Persian Gulf, UAE | Coastal habitat | E 56°03′23.9″ N 25°53′55.4″ | A. stoliczkanus |
| Sib city 1 (18) | Gulf of Oman, Oman | Coastal marsh | E 58°12′21.3″ N 23°40′19.6″ | A. stoliczkanus |
| Sib city 2 (19) | Gulf of Oman, Oman | Coastal marsh | E 58°11′33.3″ N 23°40′28.7″ | A. stoliczkanus |
| Fallujah (20) | Tigris, Iraq | Freshwater river | E 33°21′14.4″ N 33°45′28.8″ | A. stoliczkanus |
| Lake Assal (21) | Red Sea, Djibouti | River and stream | E 42°27′17.2″ N 11°40′36.6″ | A. dispar |
| Ain Abata (22) | Dead Sea, Jordan | Spring stream | E 35°30′32.3″ N 31°38′63.4″ | A. richardsoni |
- The population of site 17 has already been used in Reichenbacher, Feulner, et al. (2009). The numbers in parentheses behind the locality names refer to the sampling codes. UAE—United Arab Emirates.
| Species locality | n (♀/♂) | n morph. | n mol. | SL | Individuals, GenBank Accession numbers |
|---|---|---|---|---|---|
| A. furcatus | |||||
| Shur (1) | 5 (2/3) | 0 | 5 | 24.6 ± 3.40 | 1 (KF983848, MH194774), 2 (KF983847, MH194775), 3 (KF983846, MH194776), 4 (KF983845, MH194777), 5 (KF983844, MH194778) |
| Khurgu (3) | 1 (1/0) | 0 | 1 | 22.4 ± 3.15 | 1 (KF983843, MH194779) |
| Kol (4) | 1 (1/0) | 0 | 1 | 21.7 ± 2.30 | 1 (KF983842, MH194780) |
| Faryab (8) | 1 (1/0) | 0 | 1 | 26.5 ± 0.40 | 1 (MH194782, MH194781) |
| A. ginaonis | |||||
| Genow (2) | 10 (4/6) | 10 | 4 | 30.5 ± 2.65 | 1 (MH188347, MH194713), 2 (MH188348, MH194714), 3 (MH188349, MH194715), 4 (MH188350, MH194716) |
| A. hormuzensis sp. nov. | |||||
| Shur (1) | 10 (5/5) | 10 | 2 | 30.6 ± 3.40 | 1 (MH188384, MH194750), 2 (MH188394, MH194760) |
| Khurgu (3) | 10 (5/5) | 10 | 3 | 31.9 ± 3.80 | 1 (MH188380, MH194746), 2 (MH188381, MH194747), 3 (MH188382, MH194748) |
| Kol (4) | 10 (5/5) | 5 | 3 | 28.6 ± 3.70 | 1 (MH188378, MH188378), 2 (MH188379, MH188379), 3 (MH188383, MH194749) |
| Rassul (5) | 10 (5/5) | 10 | 5 | 27.7 ± 2.80 | 1 (MH188385, MH194751), 2 (MH188386, MH194752), 3 (MH188387, MH194753), 4 (MH188388, MH194754), 5 (MH188389, MH194755) |
| Gotab (6) | 10 (5/5) | 10 | 2 | 29.4 ± 3.35 | 1 (MH188392, MH194758), 2 (MH188393, MH194759) |
| Kukherd (7) | 10 (5/5) | 10 | 2 | 33.5 ± 3.50 | 1 (MH188390, MH194756), 2 (MH188391, MH194757) |
| Faryab (8) | 10 (5/5) | 10 | 2 | 29.6 ± 5.30 | 1 (MH188376, MH194742), 2 (MH188377, MH194743) |
| A. stoliczkanus | |||||
| Howba (9) | 10 (5/5) | 10 | 1 | 32.0 ± 4.00 | 1 (MH188363, MH194729) |
| Irandegan (10) | 6 (6/0) | 0 | 2 | 29.3 ± 2.95 | 1 (MH188370, MH194736), 2 (MH188371, MH194737) |
| Rudan (11) | 42 (20/22) | 20 | 2 | 28.5 ± 3.45 | 1 (MH188368, MH194734), 2 (MH188369, MH194735) |
| Saravan (12) | 4 | 0 | 4 | Tissue only | 1 (MH188372, MH194738), 2 (MH188373, MH194739), 3 (MH188374, MH194740), 4 (MH188375, MH194741) |
| Dalaki (13) | 10 (5/5) | 10 | 2 | 33.0 ± 2.45 | 1 (MH188355, MH194721), 2 (MH188361, MH194727) |
| Mirahmad (14) | 31 (16/15) | 10 | 2 | 28.9 ± 4.40 | 1 (MH188356, MH194722), 2 (MH188360, MH194726) |
| Sartang (15) | 12 (6/6) | 12 | 4 | 34.6 ± 3.65 | 1 (MH188351, MH194717), 2 (MH188352, MH194718), 3 (MH188353, MH194719), 4 (MH188358, MH194724) |
| Al-Manameh (16) | 4 (2/2) | 4 | 1 | 34.4 ± 2.95 | 1 (MH188354, MH194720) |
| Hulaylah (17) | 5 (3/2) | 4 | 2 | 33.6 ± 2.70 | 1 (MH188357, MH194723), 2 (MH188359, MH194725) |
| Sib city1 (18) | 26 (14/12) | 15 | 2 | 32.5 ± 2.80 | 1 (MH188364, MH194730), 2 (MH188365, MH194731) |
| Sib city2 (19) | 16 (9/7) | 16 | 2 | 32.8 ± 2.35 | 1 (MH188366, MH194732), 2 (MH188367, MH194733) |
| Fallujah (20) | 30 (10/20) | 10 | 1 | 29.8 ± 5.25 | 1 (MH188362, MH194728) |
| A. dispar | |||||
| Lake Assal (21) | 14 (8/6) | 14 | 6 | 32.03 ± 3.75 | 1 (MH188395, MH194761), 2 (MH188396, MH194762), 3 (MH188397, MH194763), 4 (MH188398, MH194764), 5 (MH188399, MH194765), 6 (MH188400, MH194766) |
| A. richardsoni | |||||
| Ain Abata (22) | 10 (5/5) | 10 | 7 | 30.03 ± 2.75 | 1 (MH188401, MH194767), 2 (MH188402, MH194768), 3 (MH188403, MH194769), 4 (MH188404, MH194770), 5 (MH188405, MH194771), 6 (MH188406, MH194772), 7 (MH188407, MH194773) |
- The numbers in parentheses behind the locality names refer to the sampling codes. n, number of individuals used for the morphometric and meristic (morph.) and for the molecular genetic analyses (mol.); SL, standard length (in mm) given as mean value ± standard deviation; UAE, United Arab Emirates. For each specimen two accession numbers are provided, which correspond to the 12S rRNA-tRNA-Val and cyt b genes, respectively.
The sites in the Persian Gulf area comprised (i) river drainages in SW Iran (Helleh and Karoun-Tigris drainage), S Iran (Hormuzgan basin), and SE Iran (Makran-Jazmourian-Mashkid drainages), and (ii) coastal sites in the Gulf of Oman, the United Arab Emirates, and Bahrain (see Figure 1a,b). The samples from the Red Sea and the Dead Sea, respectively, come from a stream near Djibouti (drainage of Lake Assal) and a spring close to Ain Abata in Jordan. Details of the sampling sites and numbers of studied specimens (including comparative materials) are given in Table 1. No material was available from A. kruppi and A. sirhani, and our differential diagnosis concerning these species is based on Freyhof et al. (2017).
2.3 Molecular genetic analyses
All samples of “A. dispar,” A. ginaonis and A. furcatus shown in Table 1 were used for the molecular genetic studies. Sequences from GenBank were added for the outgroup taxa, that is, further species of Aphanius from Iran, Turkey, and the Mediterranean Sea, and some other cyprinodontid taxa (see Table 2).
DNA was extracted from fragments (on the order of a few mm3) of muscle tissue from the right side of the caudal peduncle, using a commercial DNA extraction kit (DNeasy Tissue Kit, Qiagen) and following the manufacturer's protocol. Three mitochondrial genes (cytochrome b (cyt b), 12S rRNA, and tRNA-Val) were amplified by polymerase chain reaction (PCR) using the specific primers shown in Table 3. The amplification reaction was performed as follows: initial denaturation at 94°C (2 min), 35 cycles of denaturation at 94°C for 45 s, annealing at 58°C for 60 s and extension at 72°C for 90 s, and a final extension phase at 72°C for 10 min. The PCR mixtures were prepared with 2.5 μl of 10× Standard Taq Reaction Buffer (New England BioLabs), 0.5 μl of 10 mM dNTPs, 0.5 μl of each primer (0.2 RM final concentration), 0.2 μlTaq DNA polymerase and 1–2 μl DNA (<1,000 ng), and brought to a final volume of 25 μl with ddH2O. The products were visualized on a 1% agarose gel and then purified by precipitation with PEG solution (PolyEthylene Glycol, modified from A. Rosenthal, Coutelle, & Craxton (1993), which contains 10.0 g PEG, 7.3 g NaCl, and 45 ml ddH2O. Forward and reverse strands were sequenced with the PCR primers and BigDye 3.1 chemistry following the manufacturer's protocol (Applied Biosystems) on an ABI3730 automated sequencer in the Genomic Sequencing Unit at LMU Munich. Sequences were deposited in GenBank (Table 2).
| Gene | Primer | Primer sequence | Reference | |
|---|---|---|---|---|
| 12S rRNA | H1782 | TTTCATCTTTCCCTTGCGGTAC | Forward primer (5′→3′) | Hrbek and Larson (1999) |
| 12S rRNA | L1090 | AAACTGGGATTAGATACCCCACTA | Revers primer (5′→3′) | Hrbek and Larson (1999) |
| tRNA-VAL | H1782 | TTTCATCTTTCCCTTGCGGTAC | Forward primer (5′→3′) | Hrbek and Larson (1999) |
| tRNA-VAL | L1090 | AAACTGGGATTAGATACCCCACTA | Revers primer (5′→3′) | Hrbek and Larson (1999) |
| Cyt b | Glu-F | AACCACCGTTGTATTCAACTACAA | Forward primer (5′→3′) | Machordom and Doadrio (2001) |
| Cyt b | Thr-R | ACCTCCGATCTTCGGATTACAAGACCG | Revers primer (5′→3′) | Machordom and Doadrio (2001) |
After primer trimming in Geneious 10.1.3, the new data sets consisted of 1,134 alignable characters for cytb, and a total of 518 characters for 12S rRNA and tRNA-Val. To provide a data matrix for analyses, sequences from all studied genes were trimmed to the size of the smallest fragment to minimize the amount of missing data; the final data set (cyt b + 12S rRNA and tRNA-Val) consisted of 1,064 bp.
Sequences of the cytochrome b gene were aligned using MUSCLE 3.6 (Edgar, 2004), as implemented in Geneious 10.1.3 (Biomatters Ltd, Auckland, New Zealand). Alignments of 12S rRNA and tRNA-Val were constructed manually based on secondary structural models. All regions whose alignment was ambiguous were excluded from the phylogenetic analyses.
The models best fitting the concatenated data set were obtained with JModelTest (Darriba, Taboada, Doallo, & Posada, 2012), using the Akaike information criterion (AIC) (Akaike, 1973) and Bayesian information criterion (BIC). JModelTest output based on both criteria suggested the GTR + G + I model as the best fit for the current data (Table S1). Accordingly, Bayesian inference was accomplished by MrBayes v. 3.1.2 program (Ronquist & Huelsenbeck, 2003) using the GTR + G + I model.
MrBayes carries out Metropolis-coupled Markov chain Monte Carlo sampling. Bayesian analyses employed four Markov chains, with random starting trees, default priors, and chains with 1 × 106 generations length. Analyses were terminated once the average standard deviation of split frequencies for the simultaneous analyses fell below 0.01. Maximum-likelihood reconstruction searches of the concatenated dataset were conducted with RAxML v. 7.2.5 (Stamatakis, 2006) using a GTR + G + I model of nucleotide substitution, randomized MP starting trees, the fast hill-climbing algorithm, GTR rates, with CAT approximation of rate heterogeneity and fast bootstrap (2,000 bootstrap replicates).
2.4 Morphological analyses
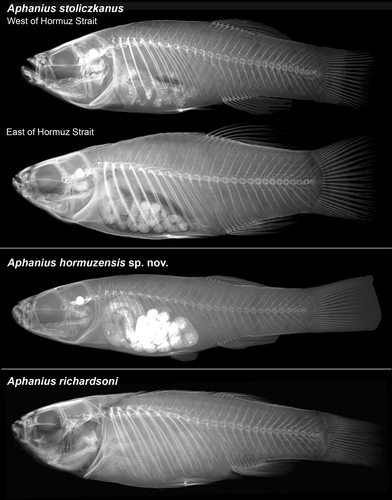
As mentioned in the introduction, “A. dispar” exhibits a strong sex dimorphism, which is visible in the pigmentation pattern and coloration of the males and females, respectively, but can also concern morphometric and meristic characters (see Teimori, Schulz-Mirbach, et al., 2012). As a result, morphometric analyses and meristic counts were carried out separately on all studied male and female specimens (see Supporting Information, Tables S2 and S3). Eighteen linear distances were measured to the nearest 0.5 mm using a Vernier calliper. Measurements were standardized based on standard length (SL), pre-anal distance, head width, head length (HL), eye diameter, and minimum body depth, and 17 morphometric variables were calculated. Meristic counts included the numbers of the dorsal, anal, pectoral, and pelvic fin rays and gill rakers under a stereomicroscope. Vertebra counts include the last vertebra (= uroterminal centrum) and were based on X-ray photographs of specimens of all studied species, with the exception of A. ginaonis. All data are presented in Tables S2 and S3 of the Supporting Information.
Morphometric variables and meristic data were analyzed using PASW 21.00 (SPSS Inc, 2015) and PAST (Paleontological Statistics, version 2.17c; Hammer, Harper, & Ryan, 2001). Most of the morphometric variables were normally distributed (Shapiro–Wilk, p > .05). The exceptions were “length of pelvic fin/HL,” “length of pelvic fin/SL,” and “length of dorsal fin/SL’.” Principal component analysis (PCA) and canonical discriminant analysis (CDA) were used to display the differences between the groups in multivariate space (see Section 2).
2.5 Divergence time estimates
The extinct species †A. princeps Gaudant & Rovira-Sendrós, 1998; from the late Early Miocene (16–17 Mya) of Spain is the oldest fossil that can be securely assigned to Aphanius because it displays the characteristic dentary and tricuspid jaw teeth of the genus (see Gaudant & Rovira-Sendrós, 1998). We used this fossil to define a minimum age for Aphanius. Other fossil species of Aphanius cannot be used in our analysis because their phylogenetic position within the tree is not known due to the lack of a character matrix for Aphanius that is based on morphological characters (and thus applicable to a fossil). For the same reason, the only fossil species from Iran, that is, A. persicus from the Late Miocene (7–9 Mya) of northwestern Iran (see Gaudant, 2011; Reichenbacher et al., 2011) cannot yet be used as additional calibration point. We have set the maximum age for Aphanius at 34 Ma using the oldest known fossil of the Old World killifishes, that is †Prolebias stenoura Sauvage, 1874 (see Gaudant, 2012).
Divergence time was estimated in BEAST v1.7 (Drummond, Suchard, Xie, & Rambaut, 2012) using the uncorrelated log-normal relaxed clock model (UCLN) (Drummond, Ho, Phillips, & Rambaut, 2006) and the birth–death model. One major advantage of BEAST is its ability to estimate the topology and divergence time simultaneously (Drummond & Rambaut, 2007). BEAUti (Bayesian Evolutionary Analysis Utility version) v1.6.1 (Drummond et al., 2012) was utilized to generate initial xml files for BEAST. A starting chronogram that satisfied all monophyly and initial divergence was generated under penalized likelihood in r8s v1.71 (Sanderson, 2002) using the RAxML tree. The substitution model was GTR + G + I with four rate classes. Five replicates of the Markov chain Monte Carlo (MCMC) analyses were run for 100 million generations. The maximum credibility tree, with means and 95% highest posterior density of divergence times, was identified with TreeAnnotator v1.6.1 (Rambaut & Drummond, 2010).
2.6 Nomenclature
The electronic edition of this article conforms to the requirements of the amended International Code of Zoological Nomenclature, and hence, the new names contained herein are available under that code from the electronic edition of this article. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information viewed through any standard web browser by appending the LSID to the prefix “http://zoobank.org/”. The LSID for this publication is urn:lsid:zoobank.org:pub: DA995390-A7F3-4455-A9B5-E1F2436FAB3C. The electronic edition of this work was published in a journal with an ISSN and has been archived and is available from the following digital repositories: PubMed Central, LOCKSS.
3 RESULTS
3.1 Molecular genetic analysis
Maximum likelihood and Bayesian estimation methods resulted in similar topologies of relationships among the studied groups and revealed a clear differentiation among the specimens previously identified as “Aphanius dispar” (Figures 2, S1 and S2). Four distinct clades can be recognized, which reflect the main areas of distribution of the taxon: (i) the Red Sea, (ii) the Dead Sea, (iii) Hormuzgan (S Iran), and (iv) the Persian Gulf & Gulf of Oman (Figure 2). The Hormuzgan clade is sister to the Persian Gulf & Gulf of Oman clade, and these two represent the youngest of the four. They, in turn, display a sister group relationship to the Dead Sea clade, and all three are sister to the Red Sea clade, which is the oldest one. Furthermore, two subclades can be distinguished within the Hormuzgan clade, one is represented by A. ginaonis, while the second includes all other specimens from the Hormuzgan Basin studied here (Figure 2).
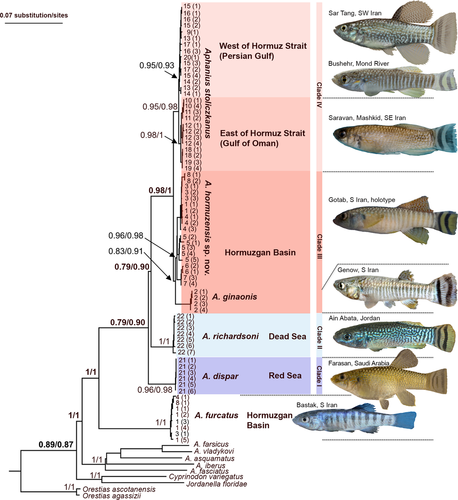
Thus, the phylogenetic relationships revealed by the analyses of the three mitochondrial genes imply that the designation “A. dispar” actually covers four distinct species. The species from the Hormuzgan basin is introduced here as A. hormuzensis sp. nov. The remaining three correspond to the former subspecies and are designated here as A. dispar (Rüppell, 1828), A. richardsoni (Boulenger, 1907), and A. stoliczkanus (Day, 1872).
3.2 Systematics
Subdivision Teleostei sensu Arratia (1999)
Order Cyprinodontiformes Berg, 1940
Suborder Cyprinodontoidei Parenti, 1981
Family Aphaniidae Hoedeman, 1949
Genus Aphanius Nardo, 1827 (type species A. fasciatus Valenciennes, in Humboldt and Valenciennes, 1821)
Aphanius hormuzensis sp. nov. urn:lsid:zoobank.org:act:DA995390-A7F3-4455-A9B5-E1F2436FAB3C (Figure 3c)
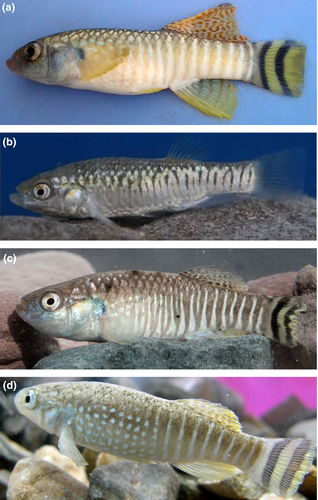
Holotype Male (ZM-FISBUK 157) (Figure 3a).
Paratypes 11 males (22.1–33.9 mm SL), five females (ZM-FISBUK 164–179).
Referred specimens See Table 2.
Type locality Iran, Hormuzgan Province (S-Iran), 330 m, Mehran River, Gotab village, 15 km south of Bastak (E 54°,15′,46.1″ N 27°,08′,39.8″), 29 August 2015, Azad Teimori.
Further localities See Table 2.
Material used in the molecular genetic analysis See Table 2.
Etymology The name refers to the basin name “Hormuzgan” in S Iran.
Diagnosis This species is well distinguished by combination of the fish coloration (in males) and a unique combination of otolith characters. The males have mostly 13–17 vertical light flank bars and two crescent-shaped dark brown stripes on the caudal fin. The vertical flank bars are usually straight (Figure 3a), but can also be somewhat scattered (Figure 3c) or be replaced by vertical series of white spots (Figure 3d). Otoliths are rounded trapezoid to rounded triangular with a rounded or relatively straight dorsal rim and no dorsal tip; the rostrum and antirostrum are relatively short and terminally rounded, the rostrum is slightly longer than the antirostrum (see Figure 5). In addition, the new species has a slightly higher total number of vertebrae (27–28) than A. dispar (24–25) and A. richardsoni (26) and also slightly more caudal vertebrae (16) than A. dispar (13) and A. richardsoni (15) (see Tables S2 and S3; Figure 4).
3.2.1 Description
Male (holotype)
Standard length 35.8 mm; body moderately robust and oval; greatest body depth just anterior of pelvic fins; head profile flat and dorsal profile convex; ventral profile convex; snout rounded; lower jaw directed upward. Dorsal and anal fin triangular, with distal margin straight to slightly concave; pectoral fins rounded; dorsal fin origin in front of vertical through pelvic fin origin; anal fin positioned slightly posterior to the dorsal fin origin; pectoral fin inserted below midline of body, not reaching the pelvic fins and shorter than head length; pelvic fins short, positioned anterior to the dorsal fin origin, not reaching the anal fin; anus situated slightly in front of anal fin origin. Dorsal fin with eight rays, anal fin with nine, pectoral fin with 15, pelvic fin with seven rays; no pelvic axillary scale. Caudal fin truncate. Scales along the flank 17. Gill rakers 15. Body coloration dark brown, dorsal surface of head and upper flank dark brown; belly and ventral part of head whitish cream; 13 light vertical flank bars from insertion of pectoral fin to base of caudal fin, no spots between bars; irregular network of anastomosing narrow brown stripes on anterior most flank bar and dorsal part of body; chin and snout with dense black pigmentation, darker than rest of ventral head; one row of dense dark gray pigments below eyes, especially in anterioventral region of eyes; dorsal and anal fins spotted on bright background, with irregular dark and light brown pigmentation on membranes and rays, rays more pigmented than membranes; pectoral and pelvic fins hyaline, few dark brown pigments at base and on rays of pectoral fin; caudal fin with two dark brown crescent-shaped bars (concave side posterior) on light background.
Male (paratypes)
Standard lengths 25.5–38.7 mm; description of body, head and fins as for holotype; dorsal and anal fin each with 9–10 rays; pectoral fin with 15–16, pelvic fin with 7–8 rays; no pelvic axillary scale. Caudal fin truncate. Scales along the flank 26–29. Gill rakers 15–16. Total vertebrae 27–28 (11–12 + 16) (n = 3). Flank with 15–17 narrow flank bars.
Female (paratypes)
Standard lengths 24.1–36.3 mm; description of body, head and fins as for holotype; dorsal and anal fin each with 9–10 rays, pectoral fin with 15–16, pelvic fin with 7–8 rays; no pelvic axillary scale. Caudal fin truncate. Scales along the flank 27–29. Gill rakers 14–15. Total vertebrae 27 (11 + 16) (n = 2). Coloration of dorsal surface of head and upper flank dark brown; belly and lower head whitish cream; flank with 15–17 narrow dark brown bars; dark brown pigments on snout and around eyes, small and denser than opercular pigments; all fins hyaline (see Figure 3b).
Other material
General description and coloration as for holotype and paratypes; dorsal and anal fin each with 8–10 rays; pectoral fin with 13–17, pelvic fin with 6–8 rays; scales along the flank 25–34; gill rakers 14–16.
Synonyms
This species has previously been described as A. dispar in Wildekamp (1993, pro parte), Hrbek and Meyer (2003, pro parte), Keivany and Ghorbani (2012), Reichenbacher, Kamrani, et al. (2009), Teimori et al. (2012b, pro parte); Teimori, Schulz-Mirbach, et al. (2012, pro parte) and Teimori et al. (2014).
Distribution and habitat
The new species is endemic to the Hormuzgan Basin in S Iran. It occurs mainly in two types of habitats, that is, brackish rivers of exorheic drainages and hot sulfur springs. In both habitat types, A. hormuzensis sp. nov. can be found sympatrically with A. furcatus. During sampling, the endemic cichlid, Iranocichla hormuzensis Coad, 1982, was also collected from the type locality.
3.3 Analysis of phenotypic variation in the Aphanius dispar species group
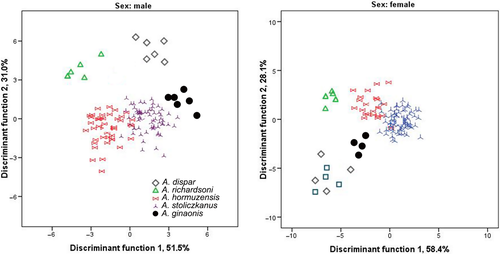
As is usually the case in Aphanius, the morphometric and meristic characters of the species of Aphanius studied here show considerable overlap (Tables S2 and S3). As a result, it is difficult to separate them on the basis of a single morphometric or meristic character. This is consistent with the outcome of earlier work, which in most cases has found broad overlap in morphometric and meristic characters between closely related species of Aphanius (e.g., Coad, 2009; Esmaeili, Teimori, Gholami, et al., 2014; Hrbek et al., 2006; Villwock et al., 1983). Nevertheless, compared to the other studied species, A. ginaonis tends to have a longer head (relative to the SL), while A. dispar usually has the highest number of dorsal fin rays (8–11) and lowest numbers of total (24–25) and caudal (13) vertebrae (see Tables S2 and S3). Furthermore, species separation is possible in multivariate space. The canonical discriminant analysis based on the morphometric variables revealed high overall classification success both for males (95.0%) and females (95.90%) (Table 4; Figure 6). In contrast, principal component analysis (PCA) of the morphometric and meristic characters failed to separate species, neither among the males nor between the females (plot not shown).
| Sex | Species | A. stoliczkanus | A. hormuzensis | A. ginaonis | A. richardsoni | A. dispar | n |
|---|---|---|---|---|---|---|---|
| Males | A. stoliczkanus | 97.0 | 3.0 | 96 | |||
| A. hormuzensis | 9.1 | 90.9 | 35 | ||||
| A. ginaonis | 100 | 6 | |||||
| A. richardsoni | 100 | 5 | |||||
| A. dispar | 100 | 6 | |||||
| Females | A. stoliczkanus | 96.6 | 3.4 | 96 | |||
| A. hormuzensis | 4.2 | 95.8 | 35 | ||||
| A. ginaonis | 100 | 4 | |||||
| A. richardsoni | 20.0 | 80.0 | 5 | ||||
| A. dispar | 100 | 8 |
- Percentages of classification into the species of each group are given in columns (correct classifications are indicated in bold type). Wilks_k = 0.05 in males and k = 0.04 in females.
3.4 Analysis of otolith variation in the Aphanius dispar species group
Otoliths of Aphanius and other genera among the Cyprinodontiformes are well known for their species-specific characteristics (e.g. Gholami et al., 2014; Reichenbacher & Reichard, 2014; Reichenbacher et al., 2007). Here, we refer to the otolith characters that were introduced in Reichenbacher et al. (2007) and have proven to be useful for species separation in subsequent work (Gholami, Esmaeili, & Reichenbacher, 2015; Reichenbacher, Kamrani, et al., 2009; Teimori, Esmaeili, et al., 2012; Teimori et al., 2014).
The general otolith morphology of the A. dispar species group is characterized by rounded, triangular, or trapezoid otolith shapes, a well-developed rostrum, a prominent antirostrum, and an almost straight sulcus that is bent toward the ventral rim terminally (see Figures 5b,c and 7a,b for otolith terminology). Aphanius dispar (in its new definition) is unusual insofar as its sulcus is very flat and the posterior part of the cauda is not clearly delimited, as in the other three species.
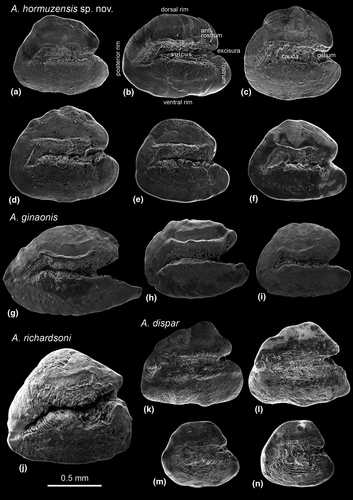
Apart from A. stoliczkanus, the otoliths of which are quite variable, each species can be identified by its characteristic otolith contour. In A. dispar, the shape is triangular to triangular trapezoid and a dorsal tip is present in some specimens (Figure 5k–n); in A. richardsoni, the contour is triangular (Figure 5j); in A. ginaonis, the shape is ovate to elongate and a dorsal tip is lacking (Figure 5g–i); and in A. hormuzensis sp. nov., the contour is rounded trapezoid or rounded triangular and no dorsal tip is present (Figure 5a–f).
Furthermore, the species are well separated from each other based on the dimensions and proportions of rostrum, antirostrum, and excisura. In both A. dispar and A. richardsoni, the relative height of the rostrum is greater than in the other two species, while the rostrum is relatively longer in A. dispar compared to A. richardsoni. In the two species from the Hormuzgan Basin, A. ginaonis, and A. hormuzensis sp. nov., otolith morphology appears to reflect their sister group relationship, because a relatively long and flat dorsal rim (without a dorsal tip) and a deeply incised sulcus are present in both species. However, the A. ginaonis otolith is clearly differentiated from that of A. hormuzensis sp. nov. by its greater relative rostrum length and excisura depth (see Figure 5g–i versus a–f)).
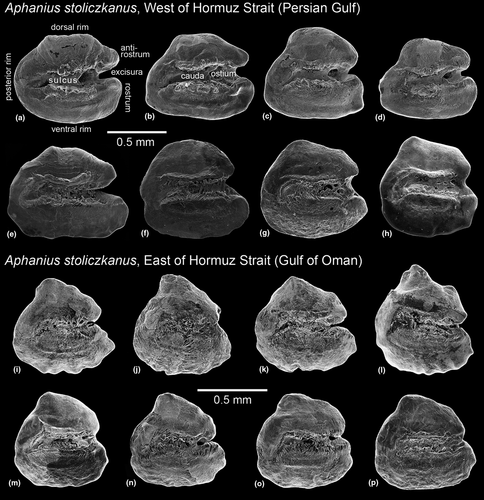
The otoliths of A. stoliczkanus from the population east of the Hormuz Strait possess a consistent overall morphology (Figure 7i–p): The otolith shape is rounded triangular with a prominent dorsal tip, the sulcus is not as flat as in A. dispar or as deep as in the two species from the Hormuzgan Clade, and the ventral and posterior rims are crenulated. As for the population west of the Hormuz Strait, only the specimens from the coastal sites of the Persian Gulf (Bahrain, Khor Hulaylah) show an overall otolith morphology similar to that seen in the population east of the Hormuz Strait, but their otolith rims are not crenulated (Figure 7h). In contrast, trapezoid to triangular shapes characterize the otoliths of A. stoliczkanus from the inland sites of Iran and Iraq, with the populations displaying distinctive variations in rostrum, antirostrum, and excisura sizes and proportions (see Figure 7a–g).
3.5 Revision of the Aphanius dispar species group
3.5.1 The Red Sea clade: new definition of A. dispar (Rüppell, 1828)
Rüppell (1828) originally described his new species “Lebias dispar” on the basis of material from the Red Sea region, but provided no information about the location of his sampling site(s). He mentions in his description a flattened head as seen in mugilids, a very small and weakly protrusible mouth, scales on the upper part of both the head and the body, relatively narrow dorsal and anal fins that are positioned slightly behind the middle of the body and are distinctively higher in males than in females, and ventral fins that are positioned very close to the anal fin. Furthermore, he reported the presence of nine rays in both dorsal and anal fins, 14 rays in the pectoral, six rays in the pelvic, and 22 rays in the caudal fin. As regards pigmentation, he noted the presence of three vertical stripes on the otherwise hyaline caudal fin of the males, while no such stripes occur on the caudal fin of the females.
Villwock et al. (1983) re-investigated the original material (11 specimens) of Rüppell's “Lebias dispar,” which is kept in the Senckenberg Museum in Frankfurt/Main, Germany, under collection numbers SMP 821 and SMO 1988. These authors designated SMF 821 (a male individual) as lectotype, and SMO 1988 (five males, five females) as paralectotypes, and counted 9–10 (mostly nine) dorsal fin rays, 9–11 (mostly 10) anal fin rays, 24–27 (mostly 26) lateral-line series scales, and 13–16 (mostly 14) gill rakers for them. The meristic data for our newly collected specimens (eight females, six males) from the drainages of Lake Assal in Djibouti (Red Sea) are consistent with the previous reports of Rüppell (1828) and Villwock et al. (1983) (Tables S2 and S3).
The descriptions given by Rüppell (1828), Krupp (1983), and Wildekamp (1993), as well as our investigation of the specimens studied here, reveal that A. dispar males are characterized by a distinctive color pattern (see Figure 2). Spots and blotches cover the anterior or entire body, while flank bars, if present, occur on the posterior part. The caudal fin has three black vertical stripes, of which the posterior two are most prominent. In addition, A. dispar tends to have fewer rays in the pectoral fin (13) and also smaller number of gill rakers (11–12) and total vertebrae (24–25) than its relatives, whereas the numbers of rays in the dorsal (8–11) and anal fins (10–12) tend to be higher (Tables S2 and S3). Furthermore, A. dispar can be recognized by its unique combination of otolith characters as described above. Otolith shape is usually triangular trapezoid; the sulcus is flat; excisura weakly or moderately incised; rostrum large and longer than antirostrum; antirostrum short and rounded; posterior rim sloping; ventral rim straight or slightly curved; dorsal rim completely curved, in some specimens with a weakly developed dorsal tip (Figure 5k–n).
The reefs and shore regions of the Red Sea represent the natural habitat of the “true” A. dispar. For long time, the taxonomic status of the populations from the easternmost Mediterranean Sea was not clear (see Kornfield & Nevo, 1976), but in the recent study by Freyhof et al. (2017), these populations have also been considered as A. dispar. Further studies based on additional material will be necessary to determine whether the populations in the freshwater systems near the coast of Ethiopia and northern Somalia (see Wildekamp, 1993) belong to A. dispar as newly defined here, because Chiozzi et al. (2017) demonstrated that additional divergence ensued in these regions.
3.5.2 The Dead Sea clade: validation of Aphanius richardsoni (Boulenger, 1907)
The populations from the Dead Sea Valley (western Jordan) have been considered as A. dispar richardsoni in previous work (Goren, 1974; Kornfield & Nevo, 1976; Krupp, 1983; Krupp & Schneider, 1989; Villwock et al., 1983; Wildekamp, 1993). Here, we support the conclusion of Freyhof et al. (2017) that A. richardsoni is a valid species because the specimens from the Dead Sea form a well-supported monophyletic clade and are characterized by their unique color pattern of the males (among others, see below).
The initial name of A. richardsoni was “Cyprinodon richardsoni” Boulenger, 1907. Boulenger (1907) reported his new species from the shore regions of the Dead Sea in Syria. Krupp and Schneider (1989: 389) designated a lectotype based on an individual from the “brine spring near Usdum, H. Poole” (35° 25′ E; 31° 51′ N, see Wildekamp, 1993: 46), deposited in the collections of the British Museum of Natural History (BMNH 1856.5.2: 4). Aphanius richardsoni is endemic to the Dead Sea area and nowadays restricted to springs or small streams of low salinity in Western Jordan (Villwock et al., 1983; Wildekamp, 1993).
Boulenger (1907) based his designation “Cyprinodon richardsoni” on the rounded caudal fin and the presence of only 12–14 teeth in each jaw. Krupp (1983) and Villwock et al. (1983), by investigating large sample sets, concluded that only the coloration is unique for the Dead Sea species, as its jaw tooth number varies between 12 and 20 and it, therefore, cannot be differentiated from A. dispar by this character. Goren (1974) provided a description for the Dead Sea species based on a very large number of specimens (>500). According to this analysis, A. richardsoni has 24–28 lateral-line scales, while fin rays number is 8–9 in the dorsal, 9–10 in the anal, 15–16 in the pectoral, 7 in the ventral fin. Villwock et al. (1983), who inspected a total of 60 specimens, found slightly higher ranges for ray numbers in the dorsal fin (8–10) and anal fin (9–12) and reported 25–27 lateral-line scales and 13–16 gill rakers. These numbers were confirmed in the study by Krupp and Schneider (1989). Our newly collected specimens yielded meristic data that are consistent with these records (Tables S2 and S3).
Judging from the aforementioned publications and the specimen available for our study (see Figure 2), A. richardsoni has a unique color pattern in males. Its complex pattern of pigmentation mainly consists of irregular and anastomosing silvery stripes and blotches on a brown background, with overlying irregular, dark vertical bars (for additional details see Wildekamp, 1993: p. 46). In addition, Villwock et al. (1983) noted shorter lengths for the dorsal and anal fin bases than are seen in A. dispar (19% of SL versus 23% of SL in A. dispar). This is in agreement with Wildekamp (1993: p. 46), who stated that the anal and dorsal fins are “much smaller” than in A. dispar. Moreover, A. richardsoni displays a characteristic combination of otolith characters (see above). Otolith shape is triangular; excisura weakly incised; rostrum blunt; antirostrum small and pointed; posterior rim slightly rounded; ventral rim straight, dorsal rim completely curved, and with a weak dorsal tip (Figure 5j).
3.5.3 The Hormuzgan clade: A. hormuzensis sp. nov. and A. ginaonis (Holly, 1929)
The Hormuzgan clade comprises two subclades (A. hormuzensis sp. nov., A. ginaonis), each of which is supported by high ML and Bayesian values (Figure 2). Our result is consistent with the work of Hrbek and Meyer (2003) who also demonstrated the presence of two distinctive lineages of Aphanius in the Hormuzgan Basin. While A. hormuzensis sp. nov. (=“A. dispar” in Hrbek and Meyer (2003)) has a wide distribution in the Hormuzgan Basin, A. ginaonis is restricted to the Genow hot sulfur spring (Coad, 1980). The taxonomic status of the latter has been in doubt for some times. Coad (1980) considered it a separate species, whereas Wildekamp (1993) suggested that it could be a subspecies of A. dispar. Reichenbacher, Kamrani, et al. (2009) confirmed the distinctness of A. ginaonis at the species level based on a comparative study of the otoliths. This view is now further supported by our new data, because the two species can be clearly distinguished by their general otolith contour (rounded trapezoid to rounded triangular in A. hormuzensis sp. nov. versus ovate elongate in A. ginaonis), relative rostrum length (clearly longer in A. ginaonis), and excisura shape (more deeply incised in A. ginaonis) (see above and Figure 5).
3.5.4 The Persian Gulf & Gulf of Oman clade: validation of A. stoliczkanus (Day, 1872)
Berg (1949) found a population of Aphanius in the Bampur River (SE Iran), which he identified as A. dispar stoliczkanus (Day, 1872) because the specimens had only 6–9 (and very exceptionally 10) dorsal fin rays (versus 9–11, mostly 10 dorsal fin rays in “true” A. dispar, see above). The specimens reported here from SE Iran (sites 10 and 11; n = 48) and Oman (sites 18 and 19; n = 42) support the view of Berg (1949), because their dorsal fin ray number ranges from 7 to 9 (Tables S2 and S3). As a result, the species name A. stoliczkanus can now be revalidated, which has been recently also proposed by Freyhof et al. (2017).
The initial name of A. stoliczkanus was “Cyprinodon stoliczkanus.” Day (1872) reported his new species from small streams by the villages Joorun and Lodai, near the Rann River in western India (69°20′E; 22°30′N, see Wildekamp, 1993: 42). He counted nine dorsal fin rays, 17 pectoral fin rays, seven pelvic fin rays, nine anal fin rays, and 15 caudal fin rays, while the count of the lateral scale series was 27. In addition to his description of some characters that are generally present in species of Aphanius, he stated (Day, 1872: 259): “In the males the dorsal and anal fins when laid flat reach the base of the caudal; the anal commences below the last dorsal ray [comment: note that this seems to be a mistake, should be ‘first’ dorsal ray]. Caudal lunate, its outer rays being slightly elongated. Colors: male, yellowish green, reticulated with brownish green, a small black spot on the shoulder behind the opercle; dorsal fin spotted, anal more sparingly so; caudal yellowish with a crescentic black band in its outer third, and a second less wide (but still broader than the ground color) between the outer one and the root of the caudal fin. Female, silvery, with about nine vertical black bands extending from the back to the abdomen.” Our new specimens from SE Iran (sites 10–12) and Oman (sites 18, 19) display very similar crescentic black bands on their caudal fin (see Figure 2) as described for the type specimens by Day (1872), and also their meristic counts (Tables S2 and S3) are consistent with this original description of A. stoliczkanus. Accordingly, A. stoliczkanus is characterized by the distinctive caudal fin coloration of the males and by the slightly lunate shape of the caudal fin. Moreover, our phylogenetic analysis reveals that two populations can be recognized within A. stoliczkanus, one east of the Hormuz Strait, and one west of the Hormuz Strait (see Figure 2).
Aphanius stoliczkanus east of the Hormuz Strait
The natural range of A. stoliczkanus east of the Hormuz Strait extends from India and Pakistan to SE Iran and Oman (Teimori, Schulz-Mirbach, et al., 2012; Teimori et al., 2012; Wildekamp, 1993; in all studies as A. dispar). In SE Iran, the species usually inhabits brackish rivers that flow into the Gulf of Oman, while in Oman, it occurs in coastal lagoons. The populations east of the Hormuz Strait differ from those to the west in having slightly lower numbers of total and caudal vertebrae (26 versus 27–28 and 14 versus 16); however, only a few specimens could be X-rayed for this study (see Tables S2 and S3). Furthermore, they can be distinguished based on their otolith morphology (Figure 7i-p). Their otoliths present conspicuously crenulated rims (versus smooth rims in the population west of the Hormuz Strait) and a short antirostrum (versus a long antirostrum in the population west of the Hormuz Strait). It should be noted that another new species (A. kruppi) from the A. stoliczkanus group has recently been described from Oman (Al Hoota stream below Al Hoota Cave, 23°4′34″N 57°21′30″E, see Freyhof et al., 2017); it is not included in this study.
Aphanius stoliczkanus west of the Hormuz Strait
Aphanius stoliczkanus west of the Hormuz Strait is distributed in brackish rivers, freshwater springs, hot sulfur springs, and coastal habitats around the Persian Gulf and in inland sites of Iran and Mesopotamia (see Teimori, Schulz-Mirbach, et al., 2012, as A. dispar), but is not found in the Hormuzgan Basin. In the Karoun Basin in SW Iran, A. mesopotamicus Coad, 2009 is found sympatrically with A. stoliczkanus (Masoudi et al., 2016; as A. dispar group). Based on the genetic, morphometric, meristic, and otolith data presented here, it appears that the population of A. stoliczkanus west of the Hormuz Strait is highly variable (Figure 7a-h, Tables S2 and S3). This is additionally confirmed by a distinctive variation in the contour of the caudal fin and also in the pigmentation. Generally, the caudal fin is slightly widened distally and its end is truncated or slightly indented. However, in the specimens from coastal sites of the Persian Gulf (i.e., Khor Hulaylah (in UAE) and Bahrain), the caudal fin is narrow and tapers toward the tip, while in the Tigris Basin specimens (SW Iran), it has a broad, fan-like shape (Figure 2). The dark brown bars on the caudal fin can be slightly or strongly bent. In their study, Freyhof et al. (2017) have also considered the populations from the United Arab Emirates (UAE) and Bahrain as A. stoliczkanus (Figure 7).
4 DISCUSSION
4.1 Historical biogeography
Our new phylogenetic data reveal a clear pattern of distribution of the species previously identified as “A. dispar” (Figure 8). Aphanius dispar (in its new definition) occurs along the coasts of the Red Sea. The Dead Sea Basin is the home of A. richardsoni. Mesopotamia and the region fringing the Persian Gulf west and east of the Strait of Hormuz—with the exception of S Iran (Hormuzgan Basin)—are populated by A. stoliczkanus. The Hormuzgan Basin in S Iran is so far unique in that two closely related species of Aphanius, that is A. hormuzensis sp. nov. and A. ginaonis, live in this small basin.
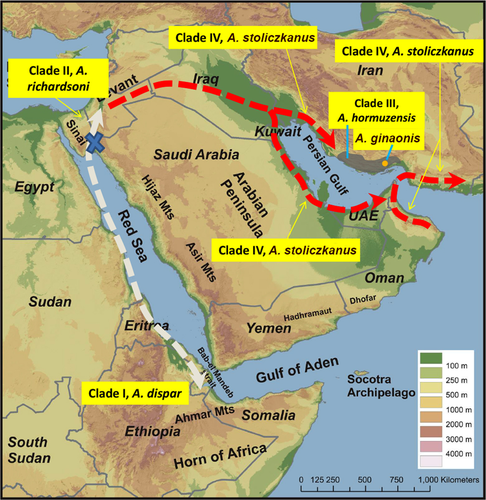
The biogeographical history of A. dispar and its descendants has been controversially discussed in previous works based on morphological comparisons of the populations from the different regions. Steinitz (1954) argued that a Pleistocene transgression of the Mediterranean Sea into the Jordan River valley had led to the immigration of the ancestor of A. richardsoni and that the today's Dead Sea Valley represents the refuge of its ancient Pleistocene distribution. This idea was taken up again by Por (1975) and Kornfield & Nevo (1976). Akşiray (1955) also argued that A. richardsoni originated during the Pleistocene, but, unlike the other authors cited, he proposed that A. richardsoni could have reached the Dead Sea Valley via dispersal from the Red Sea, by crossing the watershed between the two basins. Thus, all authors agree in proposing a Pleistocene age for the split of A. richardsoni, but disagree from where the ancestor of A. richardsoni has reached the Dead Sea Valley, that is, from the Mediterranean Sea or from the Red Sea. However, the time-calibrated tree of the A. dispar species group (Figure 9) suggests that the split between A. dispar and its sister group (including A. richardsoni) occurred at some time between the late Miocene and the Pliocene (9–4 Mya) and is thus older than that assumed in the above-mentioned studies.
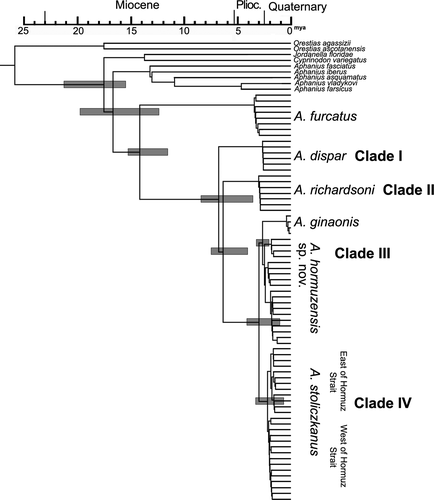
Krupp (1983) was the first to propose a model of past migration routes for A. dispar (in the old definition) that took account of its distribution in Mesopotamia and the Persian Gulf. According to him, A. dispar started to migrate during the early Pliocene (c. 5 Mya) from the Mesopotamian Basin to the Persian Gulf, the Arabian Sea, and further to the SE (Indian Ocean) from where it reached the coastal habitats of the Gulf of Aden and the reefs of the Red Sea. Like Akşiray (1955), Krupp (1983) thought that Pleistocene changes in the drainage systems might have enabled the Red Sea populations of A. dispar to invade the Dead Sea Valley. There, its populations became isolated by the middle or late Pleistocene (75,000–12,000 years B.P.), which eventually led to the evolution of A. richardsoni. However, in the case of this scenario, A. richardsoni should represent the youngest split in the phylogenetic tree, which is evidently not the case (Figure 2). Moreover, the chronogram (Figure 9) suggests that A. richardsoni originated prior to the Pleistocene, as indicated above.
The question remains whether A. richardsoni colonized the Dead Sea Valley from the north, via a transgression of the Mediterranean Sea into the Jordan River System, or by a transgression from the South, that is from the Red Sea. Kornfield and Nevo (1976) provided comparative electrophoretic evidence indicating that A. dispar from the easternmost Mediterranean Sea, A. dispar from the Red Sea and A. richardsoni from the Dead Sea are equally different from each other. This may indicate that a single event isolated the three populations from each other—and that the populations in the easternmost Mediterranean Sea might actually represent a further, as yet unrecognized new species. Such an isolating event could have happened in the aftermath of the early Pliocene transgression (c. 5 Mya) of the Mediterranean Sea up to the depression of the Dead Sea Valley (Lake Kinneret, Jordan Valley, Dead Sea) (Garfunkel, 1997; Horowitz, 2001; Rosenthal, Flexer, & Moller, 2006; Stein et al., 1997), which was connected to the Red Sea at that time (Ben-Avraham, Garfunkel, & Lazar, 2008). In the course of the Pliocene, the sea retreated and the shallow marine connection between the Red Sea, the Dead Sea Valley, and the Mediterranean Sea was closed (Ben-Avraham et al., 2008; Coleman, 1993; Garfunkel, 1997; Rosenthal et al., 2006). We assume that it was this Pliocene closure event that isolated the ancestral populations of A. dispar in the Red Sea, Dead Sea, and Mediterranean Sea from each other (Figure 8). This idea is compatible with the time-calibrated tree (Figure 9), which suggests a 95% credibility interval extending from the late Miocene (9 Mya) to the middle Pliocene (4 Mya) for the split between A. dispar and its relatives.
The subsequent split that separated A. richardsoni from the common ancestor of the species of Aphanius that make up Clades III and IV (Figures 2 and 9) probably happened within a relatively short time (see Figure 9). This implies that the common ancestor reached the shores of the Persian Gulf and the Gulf of Oman via the brackish water bodies of the Dead Sea Valley in the course of the middle Pliocene (Figure 8). This migration model is in principle consistent with Krupp's (Krupp, 1983) hypothesis that the A. dispar populations in the Persian Gulf originated in Mesopotamia, but it differs with respect to the time at which this happened (middle Pliocene versus Pleistocene).
Furthermore, the chronogram (Figure 9) reveals that some time must have elapsed between the arrival of the ancestor of Clades III and IV in the Persian Gulf and Gulf of Oman regions and the split between these clades, resulting in A. hormuzensis sp. nov. and A. stoliczkanus. It appears that this split can be linked to vicariance events during the Pleistocene, when tectonic uplift and compression led to the emergence of isolated drainage systems in SE Iran (Makran and Mashkid), S Iran (Hormuzgan), and SW Iran (Helleh) (e.g., Hatzfeld et al., 2010; Kompani-Zare & Moore, 2001; Regard et al., 2004). Clade III (Hormuzgan clade, A. ginaonis, and A. hormuzensis sp. nov.) diverged perhaps as a result of the development of unusual habitats such as hot sulfur springs in this part of Iran (Kosari Torbehbar & Haghnazar Liseroudi, 2015). A second example of a recently diverged endemic species from the Hormuzgan Basin (unrelated to the descendants of A. dispar) is A. darabensis (see Esmaeili, Teimori, Gholami, et al., 2014).
The two populations that can be recognized within A. stoliczkanus are probably the result of a vicariance event during the global cooling and sea-level fluctuations that occurred in the Pleistocene. The population west of the Hormuz Strait may have found a refuge in the Mesopotamian rivers (Tigris, Euphrates) and the rivers that rise in the Zagros Mountains. The population east of the Hormuz Strait would not have been affected by the fall in global sea level because this region was deeper and remained inundated (Lambeck, 1996; Teller, Glennie, Lancaster, & Singhvi, 2000). It appears that the Holocene sea-level rise at 11,000 BP and re-flooding of the Persian Gulf did not lead to mixing between the populations of A. stoliczkanus west and east of the Hormuz Strait. Most probably, the Strait of Hormuz acted as an ecological barrier to gene flow, since the waters of the Persian Gulf have higher salinities and exhibit more extreme shifts in water temperature than the Gulf of Oman (Hoolihan, Premanandh, D'Aloia-Palmieri, & Benzie, 2004).
The young (Pleistocene) age of the splits between and within Clades III and IV also explains why morphometric and meristic characters are of little use in separating the individual species. The same observation has previously been made with respect to the inland species of Aphanius from the Iranian plateau and Anatolia, which are all very similar in phenetic characters, but clearly different based on molecular genetic analyses (Esmaeili, Teimori, Gholami, et al., 2014; Esmaeili, Teimori, Sayyadzadeh et al., 2014; Gholami et al., 2014; Teimori, Esmaeili, et al., 2012).
5 CONCLUSIONS
Reconstruction of phylogenetic relationships among the Aphanius dispar species group based on sequence data for three mitochondrial genes in this study revealed four distinctive species within the species previously identified as A. dispar, one of which is the new species A. hormuzensis. Furthermore, the molecular genetic analyses confirm previous assumptions concerning intraspecific divergence within “A. dispar,” which, however, were based solely on conventional phenotypic characters (Teimori, Schulz-Mirbach, et al., 2012) or otoliths (Reichenbacher, Feulner, et al., 2009; Reichenbacher, Kamrani, et al., 2009; Teimori, Schulz-Mirbach, et al., 2012; Teimori et al.,2012).
The time-calibrated tree suggests that the factors that promoted interspecific differentiation within the A. dispar species group include (i) vicariance due to the middle Pliocene disconnection between the Red Sea, Dead Sea, and Mediterranean Sea, (ii) middle to late Pliocene dispersal from the Dead Sea via Mesopotamia to the Persian Gulf, and (iii) Pleistocene vicariance events due to global sea-level fluctuations. Moreover, A. dispar and its descendants obviously have a great capacity to adapt to new conditions, and this must have contributed to their survival and divergence since the late Miocene.
The phenotypic characters that most clearly differentiate A. dispar and its descendants from each other include body pigmentation, coloration of the caudal fin in males, and the shape of the caudal fin. In addition, their otoliths differ (Figures 5 and 7 of this study and Reichenbacher, Feulner, et al., 2009; Reichenbacher, Kamrani, et al., 2009; Teimori, Schulz-Mirbach, et al., 2012; Teimori et al., 2012). Interspecific differences in otoliths between closely related species may be linked to selection for species-specific hearing capabilities (Reichenbacher & Reichard, 2014), while specific pigmentation of male individuals could additionally serve for effective intraspecific communication (Teimori, Esmaeili, et al., 2012). This implies that, apart from vicariance and dispersal, evolution of hearing and visualization (sensorial evolution) might have played a role in the evolutionary history of A. dispar and its descendants.
ACKNOWLEDGEMENTS
The research work in Iran was funded by the Shahid Bahonar University of Kerman (Grant No. 1394 to the first author) and also by the Shiraz University (Grant No. 909830 to the second author), and in Germany by the German Academic Exchange Service (DAAD) (Grant No. 445-irn/irq-pi to the second author). We thank S.H. Hashemi from the Environment Department of Hormuzgan for providing the facilities for fish collection. R. Khaefi and A. Gholamifard assisted in the collection of fish specimens, and M. Kamran and M. Dehdar helped with the logistic support in the field (all Shiraz University). S. Bogotodeski and M.A. Abu-El-Regal (Port Said University, Egypt) kindly delivered the photograph of A. dispar from the Red Sea. G. Wörheide (LMU Munich) provided access to the Molecular Geo- and Palaeobiology Lab at LMU Munich, and G. Büttner and S. Schätzle assisted in the preparation of the data for the genetic analyses. To all, we express our sincere thanks. Finally, we are grateful to M. Dohrmann (LMU Munich) for constructive discussions.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: AZ, BR. Performed the experiments: AZ. Analyzed the data: AZ, BR, HRE. Contributed reagents/materials/analysis tools: AZ, HRE, NH, BR. Wrote the paper: BR, AZ, HRE.




