Polymorphism of a genital organ under sexual selection in Monacha kuznetsovi from the Caucasus (Gastropoda: Hygromiidae)
Abstract
During mating the dart apparatus of the land snail group Helicoidea transfers an allohormone that increases paternity success. Thus, this organ is under sexual selection. Despite this selective advantage, the dart sac has independently been lost in many lineages in the Helicoidea, presumably because natural selection counteracts sexual selection. However, the selective pressures that result in the loss of the organ have not been examined experimentally because most species show no variation in the presence or absence of the dart sac. Using nuclear multilocus data and mitochondrial sequences, we show that a so far misidentified morph of the genus Monacha without appendicula, a homologue of the dart sac, is conspecific with M. kuznetsovi with appendicula. This is only the second case of polymorphy with regard to the presence or absence of the dart sac or its homologue in the Helicoidea. Monacha kuznetsovi might therefore be a suitable model organism to study how the interplay between sexual selection and natural selection may affect the evolution of the genital organs.
1 INTRODUCTION
The land snail group Helicoidea is characterized by the evolution of a dart apparatus that is usually equipped with one or more calcareous darts that are shot or pushed into the mating partner during courtship (Chase, 2007). The function of this behavior has long puzzled biologists. It has been hypothesized that the dart is a nuptial gift of calcium for the production of the eggs (Charnov, 1979; Leonard, 1992), that dart shooting is a sexual signal and dart recipients select on dart shooting effectiveness (Landolfa, 2002; Leonard, 1992) or that the dart is used to manipulate the mating partner (Adamo & Chase, 1996). The latter hypothesis could be corroborated by experimental studies (Chase, 2007). It has been shown that the dart transfers an allohormone produced by the glands of the dart apparatus, the glandulae mucosae, into the body of the recipient (Stewart, Wang, Koene, Storey, & Cummins, 2016). This allohormone inhibits the intake of the spermatophore into a digesting organ so that more sperm can reach the sperm-storing organ and fertilize eggs. The sexual conflict between dart shooter and dart receiver led to a co-evolutionary arms race resulting in a continuous improvement of the spermatophore receiving organs and the allohormone transfer, for example, by more complex surfaces of the darts (Koene & Schulenburg, 2005). Because of the strong selective advantage, more effective traits become quickly fixed. Thus, there is little variation of such genital characters within species. This is one reason, why differences in genital characters are often useful to distinguish species.
It is less clear how an organ that is under strong sexual selection like the dart sacs might be reduced or lost. Actually, such losses have been documented in various lineages of the Helicoidea (Hirano, Kameda, Kimura, & Chiba, 2014; Neiber & Hausdorf, 2017; Neiber, Razkin, & Hausdorf, 2017; Wade, Hudelot, Davison, Naggs, & Mordan, 2007). Neiber and Hausdorf (2015) hypothesized that natural selection might counteract sexual selection, for example, when it results in injuries or an increased infection risk of the dart recipient or a prolongation of the mating because of the delayed intake of the spermatophore, during which the individuals are under risk of desiccation or predation. However, the selective pressures and the circumstances that result in the loss of genital characters under strong sexual selection like the dart sac can hardly be studied experimentally because of the lack of intraspecific variation. The possession of one or several dart sacs is usually a species-specific character. The only species of the Helicoidea which is known to be polymorphic with regard to the presence or absence of a dart sac is Circassina frutis (Pfeiffer, 1859) from the Caucasus region (Hausdorf, 2001; Neiber & Hausdorf, 2015; Schileyko, 1978). Schileyko (1996) also reported a lack of the dart sac in one of six examined Cylindrus obtusus (Draparnaud, 1805) from the eastern Alps. Schileyko, Baminger, and Sattmann (1997) examined the genitalia of 52 specimens, Schileyko, Kleewein, Zopp, and Sattmann (2016) those of 38 specimens and Zopp, Haring, Kruckenhauser, Schileyko, and Sattmann (2017) those of 76 specimens of Cylindrus obtusus (samples partly overlapping) and found variation in the lengths of the dart sac and the glandulae mucosae. However, none of these studies has found additional specimens without dart sac. Thus, Cylindrus obtusus is not polymorphic with regard to the presence of the dart sac. Rather, the lack of the dart sac in one specimen was apparently an extreme variation or individual anomaly. The partial reduction in the glandulae mucosae in some populations of Cylindrus obtusus could be explained by selfing (Kruckenhauser et al., 2017).
In the north-western Caucasus, we found individuals of a Monacha taxon that differs from Monacha kuznetsovi only in the lack of the appendicula (Figure 1). Specimens with and without appendicula were collected in the same region in the north-western Caucasus, and even occur in sympatry at one locality (Figure 2d). The appendicula is a hollow tube, which replaces the dart and accessory sac in the Monachaini (Hygromiidae). Its exact function is unknown, but it is not unlikely that it stores the secretion of the glandulae mucosae containing the allohormone and squeezes it onto the copulation partner during courtship.
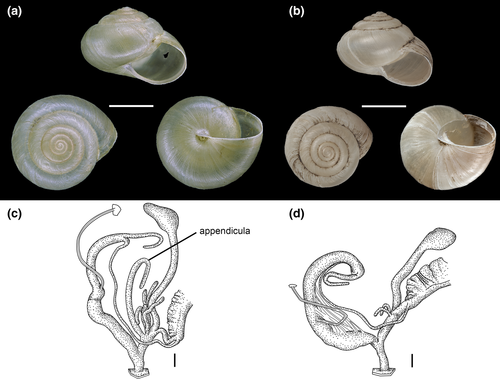
For a long time, the presence or absence of an appendicula was used as a diagnostic character to differentiate between subgenera of Monacha (Gittenberger & de Winter, 1985; Hausdorf, 2000a, 2000b; Hesse, 1914). However, a molecular phylogeny has shown that the appendicula was repeatedly lost in the evolution of Monacha (Neiber & Hausdorf, 2017). Nevertheless, no Monacha species was known so far that is polymorphic with regard to the presence/absence of the appendicula, that is, the appendicula is either present or completely lacking in all specimens of a species.
Hausdorf (2000b) reported on a specimen from the north-western Caucasus that he classified preliminary as M. (Metatheba) subcarthusiana (Lindholm, 1913) because of the lack of the appendicula, although it differed from that species in the low epiphallus:penis ratio and the narrow umbilicus. We found additional specimens of this morph. The form agrees with regard to the narrow umbilicus and the low epiphallus:penis ratio with M. (Paratheba) kuznetsovi Hausdorf, 2000, from which it differs, however, in the lack of the appendicula. Thus, it has originally been classified in a different subgenus.
However, Neiber and Hausdorf (2017) showed that most Monacha species from the Caucasus do not belong to different subgenera, but form together with M. fruticola (Krynicki, 1833) from Crimea and adjacent parts of Ukraine a well-supported monophyletic radiation. Within this clade, specimens of M. kuznetsovi with appendicula formed a maximally supported monophyletic group with the questionable morph lacking an appendicula. However, the relationships within this group could not be resolved. Here, we use additional mitochondrial sequences and nuclear multilocus AFLP data to elucidate the relationships of individuals of the questionable morph without appendicula and typical M. kuznetsovi with appendicula.
2 MATERIALS AND METHODS
For molecular genetic analyses, specimens housed at the Zoological Museum Hamburg (ZMH) were used: the holotype (ZMH 2767) and a paratype (ZMH 2768) of M. kuznetsovi (see Hausdorf, 2000b) and four samples either identified as M. kuznetsovi (with appendicula, ZMH 100112) or as M. cf. kuznetsovi (without appendicula, ZMH 2776 and ZMH 86697) that formed a well-supported monophyletic group in the analyses presented by Neiber and Hausdorf (2017) as well as a specimen (ZMH 100112) not previously analyzed. For information on sampling sites and specimens, see Tables 1 and 2. For the reconstruction of phylogenetic relationships previously published sequences of M. subcarthusiana, M. roseni (Hesse, 1914), M. claussi Hausdorf, 2000 and M. ciscaucasica Hausdorf, 2000 from the Caucasus region and M. fruticola from the Crimean Peninsula and adjacent mainland regions of Ukraine were used (for GenBank accession numbers and locality data, see Neiber & Hausdorf, 2017).
| Locality Number | Longitude | Latitude | Altitude | Locality |
|---|---|---|---|---|
| P1 | 44°40′27″ N | 37°35′10″ E | 160 m | Russia, Krasnodar, Abrau-Dyurso, near Camp Limanchik |
| P2 | 44°49′12″ N | 37°38′31″ E | 290 m | Russia, Krasnodar, 2 km along the road SW of Verkhnebakanskiy, pass between Anapa and Novorossisk, dry meadow |
| P3 | 44°43′20″ N | 37°52′00″ E | 500 m | Russia, Krasnodar, Gelendzhik, SW slope of Gora Markotkh |
| Taxon | Collection Data | GenBank Accession Number | AFLP | |||
|---|---|---|---|---|---|---|
| Collection number | DNA voucher number | Locality number | cox1 | 16S rRNA gene | ||
| M. kuznetsovi | ZMH 100112 | ZMH-DNA 1666 | P1 | KX507194 | KX495383 | + |
| M. kuznetsovi | ZMH 100112 | ZMH-DNA 2244 | P1 | KX507194a | KX495383a | + |
| M. cf. kuznetsovi | ZMH 86697 | ZMH-DNA 1770 | P2 | KX507195 | KX495384 | − |
| M. cf. kuznetsovi | ZMH 86697 | ZMH-DNA 2252 | P2 | KX507205 | KX495394 | + |
| M. cf. kuznetsovi | ZMH 2776 | ZMH-DNA 2254 | P3 | KX507207 | KX495396 | + |
| M. kuznetsovi (paratype) | ZMH 2768 | ZMH-DNA 2256 | P3 | − | − | + |
| M. kuznetsovi (holotype) | ZMH 2767 | ZMH-DNA 3145 | P3 | KX507216 | KX49540 | + |
- a Sequence identical to that listed under DNA voucher number ZMH-DNA 1666
Total genomic DNA was extracted from tissue samples following a slightly modified version of the protocol described by Sokolov (2000) as detailed in Scheel and Hausdorf (2012). Amplification of parts of the mitochondrial 16S rRNA and cytochrome c oxidase subunit 1 (cox1) genes, sequencing and sequence assembly was conducted as detailed in Neiber and Hausdorf (2017). The sequences were aligned with MAFFT 7 (Katoh & Standley, 2013) using the Q-INS-i algorithm and otherwise default settings.
For phylogenetic analyses, the data set was initially divided into four partitions, that is the first, second and third codon positions of the partial cox1 gene and the partial 16S rRNA gene, respectively. An exhaustive search with PartitionFinder 2 (Lanfear, Calcott, Ho, & Guindon, 2012) was conducted to select an appropriate partitioning scheme and evolutionary models based on the Akaike information criterion allowing for separate estimation of branch lengths for each partition.
Heuristic maximum likelihood (ML) analyses were conducted with Garli 2.0 (Zwickl, 2006) allowing for independent estimation of parameters for individual partitions and best-fit models as suggested by PartitionFinder. Confidence values were computed by bootstrapping with 1000 replications. Bootstrap support values were mapped on the ML tree with Sumtrees 3.3.1, which is part of the DendroPy 3.8.0 package (Sukumaran & Holder, 2010). Bootstrap (BS) support values ≥70 were interpreted as positive support for a clade.
A median-joining network (Bandelt, Forster, & Röhl, 1999) using the program PopART (Leigh & Bryant, 2014) with ε = 0 was constructed on the basis of a reduced data set (alignments were prepared as detailed above) including only concatenated sequences of the partial cox1 and 16S rRNA genes of specimens either identified as M. kuznetsovi or M. cf. kuznetsovi. Pairwise p-distances were computed with MEGA7 (Kumar, Stecher, & Tamura, 2016).
AFLP data were generated using the protocol by Vos et al. (1995) with slight modifications as specified by Scheel and Hausdorf (2012). For adapters and primers used, see Neiber and Hausdorf (2015). In total, six selective primer combinations were used.
Detections and size calculations of AFLP bands were performed with Peak Scanner 1.0 (Applied Biosystems) using default parameters except for allowing a light peak smoothing. The add-on package RawGeno (Arrigo, Tuszynski, Ehrich, Gerdes, & Alvarez, 2009) for the statistical software suite R (R Core Team, 2013) was used for automated binning and scoring of AFLP bands. Scoring range was set to 50–200 base pairs (bp), minimum intensity to 100 relative fluorescence units (rfu), minimum bin width to 1 bp and maximum bin width to 1.5 bp. The remaining parameters were left at their default values.
From the resulting AFLP matrix, a neighbor-net (Bryant & Moulton, 2004) was constructed with SplitsTree4 4.13.1 (Huson & Bryant, 2006) using Jaccard distances.
3 RESULTS
The alignment of the partial sequences of the 16S rRNA gene for the ML analysis based on the larger data set had a length of 865 base pairs (bp) and that of the reduced data set for the construction of the median-joining network a length of 847 bp (one gap position in the sequence of a specimen from population P2, ZMH-DNA 1770, see Tables 1 and 2). The alignments of the partial cox1 sequences had a length of 655 (bp) for both analyses. Observed pairwise p-distances between specimens assigned to either M. kuznetsovi or M. cf. kuznetsovi were low (up to 1.3% for the partial sequences of the cox1 gene and up to 2% for the partial sequences of the 16S rRNA gene) and very low within populations (up to 0.4% for the partial sequences of the cox1 gene and up to 0.8% for the partial sequences of the 16S rRNA gene) (Table 3).
| Taxon | DNA voucher number | Locality number | |||||
|---|---|---|---|---|---|---|---|
| 1666 & 2244 | 1770 | 2252 | 2254 | 3145 | |||
| M. kuznetsovi | ZMH-DNA 1666 & 2244 | P1 | 0.020 | 0.018 | 0.003 | 0.003 | |
| M. cf. kuznetsovi | ZMH-DNA 1770 | P2 | 0.013 | 0.008 | 0.020 | 0.020 | |
| M. cf. kuznetsovi | ZMH-DNA 2252 | P2 | 0.013 | 0.001 | 0.018 | 0.018 | |
| M. cf. kuznetsovi | ZMH-DNA 2254 | P3 | 0.012 | 0.013 | 0.013 | 0.000 | |
| M. kuznetsovi (holotype) | ZMH-DNA 3145 | P3 | 0.008 | 0.012 | 0.012 | 0.004 |
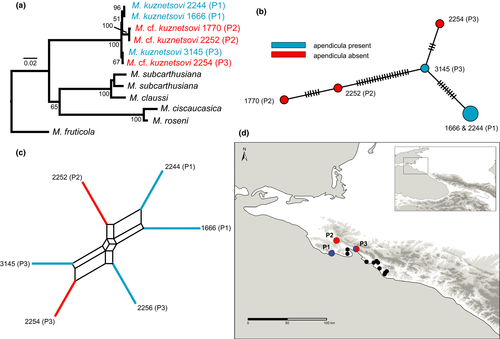
The analysis with PartitionFinder suggested to keep the predefined partitions and to apply the following evolutionary models: GTR + I for the first codon positions, HKY for the second codon positions and GTR + G for the third codon positions of the data set including the partial sequences of the cox1 gene and GTR + G for the data set including the partial sequences of the 16S rRNA gene. Like in the analyses of Neiber and Hausdorf (2017), specimens assigned to either M. kuznetsovi (including the holotype and a paratype) and specimens identified as M. cf. kuznetsovi were grouped together with maximal support in the ML analysis and were separated by a relatively long branch from the remaining Caucasian taxa included in the analysis. However, neither specimens identified as M. kuznetsovi nor specimens identified as M. cf. kuznetsovi were recovered as monophyletic groups within this clade (Figure 2a).
In the median-joining network based on the concatenated partial sequences of the cox1 and 16S rRNA genes (Figure 2b), a specimen from population P3 without appendicula was closer to a specimen from the same population with appendicula than to other specimens without appendicula from P2, that is, the specimens without appendicula did not form a monophyletic group.
The neighbor-net based on Jaccard distances of AFLP data (Figure 2c) obtained from six selective primer combinations (157 polymorphic loci) neither supported the monophyly of specimens with appendicula nor the monophyly of specimens without appendicula but rather grouped specimens according to populations, with specimens from populations P1 and P2 (Table 1 and Figure 2c, d) being closer to each other than to specimens from population P3 (Table 1 and Figure 2c, d).
4 DISCUSSION
The lack of monophyly of the Monacha specimens from the north-western Caucasus without appendicula including the specimen originally classified as M. subcarthusiana by Hausdorf (2000b) in the median-joining network based on the mitochondrial sequences (Figure 2b) implies that they do not form a species distinct from M. kuznetsovi. However, this result might be ascribed to incomplete lineage sorting or introgression between recently separated species. These possibilities could be excluded by the nuclear multilocus data. The network based on the AFLP data (Figure 2c) demonstrated that the specimens without appendicula are conspecific with specimens of M. kuznetsovi Hausdorf, 2000 with appendicula. This result warns against separating helicoid species based on presence versus absence of the dart sac or the appendicula only. However, it is a rare case that two forms differ only in the presence versus absence of the dart sac or the appendicula. Helicoid species that have lost the dart sacs or the appendicula, but also differ in additional characters from related taxa with dart sacs or appendicula are not affected by this conclusion.
Sexual selection should result in a rapid fixation of morphs with dart sac or appendicula in helicoid species, because a higher proportion of the sperm of mutants without dart sac (or appendicula) will be digested by the copulation partner (Chase, 2007). Actually, Monacha kuznetsovi is the only species of the Helicoidea besides Circassina frutis (Hausdorf, 2001; Neiber & Hausdorf, 2015; Schileyko, 1978) that is known to be polymorphic with regard to the possession of a dart sac or its homologue, the appendicula.
This polymorphism provides the opportunity to study the advantages of a loss of the appendicula by experimental crossings between individuals with and without appendicula. Such a study might help to understand how the polymorphism can persist, respectively why the morph without appendicula became independently fixed in several lineages within Monacha (Neiber & Hausdorf, 2017) despite the transfer of the secretion of the glandulae mucosae with the allohormone increases paternity success and, thus, fitness probably also in Monacha. Therefore, M. kuznetsovi is a suitable model organism to study how the interplay between sexual selection and natural selection may affect the evolution of the genital organs.
ACKNOWLEDGEMENTS
We are grateful to Pavel Kijashko (St. Petersburg) for assistance during fieldwork. We also thank the Volkswagen Foundation for funding this study within the project “Biogeography of the land molluscs of the Caucasus region.”




