Inter- and intraspecific plasticity in distribution patterns of immunoreactive compounds in actinotroch larvae of Phoronida (Lophotrochozoa)
Abstract
Recent molecular data place Phoronida within the protostome superclade Lophotrochozoa, where they have been suggested to form a monophyletic assemblage with Brachiopoda and/or Nemertea. Herein, the anatomy of the nervous system and the structure of the apical organ are described for two larval stages of Phoronis muelleri in order to contribute to the discussion concerning the evolution of lophotrochozoan nervous systems. Specimens were investigated by confocal laser scanning microscopy using antibodies against the serotonin-like immunoreactive (serotonin-lir), the FMRF-amide-like immunoreactive (FMRFamide-lir) and the small cardioactive peptide B-like immunoreactive (small cardioactive peptide B-lir) compounds of the nervous system. Consistent with larvae of other phoronid species, we found a complex apical organ that consists of numerous serotonin-lir flask-shaped cells, additional bi- or multipolar serotonin-lir cells and several FMRFamide-lir perikarya. A detailed comparison between our results and those of a previous study on the same species shows significant differences in the innervation of the preoral lobe, the tentacles and the telotroch. Our work is the first to prove the presence of small cardioactive peptide B in phoronids. In larvae of P. muelleri, it is expressed in neurites along the margin of the preoral hood, in the mesosome, in the tentacles, in the trunk as well as in the apical organ. A positive signal for this peptide is also known from molluscs, annelids and arthropods, indicating that it was also part of the protostomian groundplan. In contrast to a recent study on another phoronid species, Phoronopsis harmeri, we did not find a ventral neurite bundle in the larval stages investigated herein, thus leaving the question open whether this structure was part of the phoronid groundplan or evolved de novo in P. harmeri.
Introduction
Although phoronids share some features with deuterostomes, such as radial cleavage (but see Pennerstorfer and Scholtz 2012 for a recent study that suggests spiral cleavage in at least one species), regulative development and maybe a (sometimes disputed) trimeric body organization, recent studies leave little doubt that they indeed represent protostomes that nest within the Lophotrochozoa (see, e.g., Halanych et al. 1995; Dunn et al. 2008; Hejnol et al. 2009). A previously suggested monophyletic Lophophorata comprising Phoronida, Ectoprocta and Brachiopoda (e.g. Nielsen 2002) is usually not recovered. Instead, a monophyletic assemblage of Phoronida and Brachiopoda, for which the term Brachiozoa was coined, is often proposed, and a close affinity of this clade with Nemertea has been suggested (Dunn et al. 2008; Helmkampf et al. 2008; Paps et al. 2009a,b). In accordance with the overall bodyplan disparity of the various lophotrochozoan representatives, the organization of their nervous systems varies widely among the different groups. Interestingly, however, several shared features were recently revealed by comparative developmental studies of species belonging to different lophotrochozoan phyla. For example, while adult annelids may exhibit anything between one and five ventral nerve cords, ontogenetic analyses have shown that these ventral cords (and the single ventral nerve cord of adult sipunculans and echiurans) always develop from a paired nerve (see Wanninger 2009). Two ventral longitudinal neurites are also present in juvenile brachiopods, while adults have a nerve plexus with little concentration, similar to the condition found in adult ectoprocts (Nielsen 2012; Schwaha and Wanninger 2012). The nervous system of adult molluscs includes a paired ventral (pedal) and a paired lateral (visceral) nerve cord (Wanninger et al. 2007; Wanninger 2009; Nielsen 2012). Such a tetraneurous condition is also present in the creeping-type larva of entoprocts, which, together with other apomorphic features, have led to the suggestion of a monophyletic molluscan–entoproct clade, the Tetraneuralia (Wanninger et al. 2007; Wanninger 2008, 2009).
The nervous system of adult phoronids consists of a ganglion that is located in the epidermis between the anus and the ring of tentacles. It gives rise to a nerve ring at the base of the lophophore and, in most species, one or two lateral giant nerve fibres (Bullock and Horridge 1965). In addition, they possess a basiepidermal nerve plexus along their entire body; a ventral nerve cord is absent in adult phoronids (Emig 1979). Interestingly, for the early actinotroch larva of Phoronopsis harmeri Pixell, 1912, a serotonin-like immunoreactive (serotonin-lir) and an FMRF-amide-lir ventral neurite bundle containing several repetitive commissures have recently been described (Temereva 2012; Temereva and Wanninger 2012). When the larva reaches the 6 primordial tentacle stage, the serotonin-lir ventral neurite bundle disappears, while the FMRFamide-lir ventral neurite bundle is present until at least the 6-tentacle stage (Temereva and Wanninger 2012).
Recently, numerous immunocytochemical studies on the development of the nervous system have been carried out on various lophotrochozoan taxa (e.g. Santagata 2002; Voronezhskaya et al. 2002, 2003; Wanninger 2005; Wanninger et al. 2005a,b, 2007; Fuchs and Wanninger 2008; Kristof et al. 2008; Altenburger and Wanninger 2010; Schwaha and Wanninger 2012; Temereva 2012; Temereva and Wanninger 2012). These studies have shown that the larvae of most lophotrochozoans possess a simple apical organ that contains only a few serotonin-lir flask-shaped cells, often about four (Voronezhskaya et al. 2003; Fuchs and Wanninger 2008; Wanninger 2008; Altenburger and Wanninger 2010; Nielsen and Worsaae 2010). However, in polyplacophoran molluscs and in the creeping-type larva of entoprocts, the apical organ is more complex and contains six to eight serotonin-lir flask-shaped cells and several additional peripheral cells (Voronezhskaya et al. 2002; Wanninger et al. 2007). The larva of phoronids is sometimes considered a modified trochophore (Salvini-Plawen 1980) and has numerous flask-shaped cells in its apical organ (Hay-Schmidt 1990a; Lacalli 1990; Santagata 2002; Temereva and Wanninger 2012), similar to most echinoderm larvae (Nakajima et al. 1993; Beer et al. 2001; Byrne et al. 2007; but see Bishop et al. 2013). However, a recent study of the larval nervous system of P. harmeri has shown that initially only four serotonin-lir flask-shaped cells differentiate simultaneously in the mid-gastrula stage, thus suggesting that this may reflect the ancestral condition not only for spiralian but also for phoronid larvae (Temereva 2012; Temereva and Wanninger 2012).
The gross morphology of the nervous system of the actino-troch larva of Phoronis muelleri has been described previously (Hay-Schmidt 1990a), but several details remain vague. Therefore, our study not only deals with the analysis of the nervous system of the actinotroch larva of P. muelleri in general, but also focuses on the detailed structure of the apical organ. Moreover, this work presents the first data on small cardioactive peptide B-like immunoreactivity in the nervous system of an actinotroch larva. This neuropeptide was originally characterized from molluscan nervous tissue, where it is involved in regulation of the heart beat rate. A positive signal was previously found for gastropods (Lloyd et al. 1984, 1987; Mahon et al. 1985; Kempf et al. 1987; Fox and Lloyd 1997; Perry et al. 1999; Ohsuga et al. 2000; Kempf and Page 2005), bivalves (Candelario-Martinez et al. 1993; Gainey et al. 1999; Willows et al. 2000; Ellis and Kempf 2011), cephalopods (Kanda and Minakata 2006), annelids (Evans and Calabrese 1989) and arthropods (Settembrini and Villar 2005). In larval and adult molluscs, it is expressed in the central and peripheral nervous system, and in adult arthropods, the distribution of this neuropeptide is concentrated in the brain and the subesophagal ganglion, while in adult annelids, it is mainly expressed in the segmental ganglia (Evans and Calabrese 1989; Perry et al. 1999; Settembrini and Villar 2005; Ellis and Kempf 2011). Taken together, our work increases the morphological database concerning the larval neuroanatomy of Phoronida and contributes to a better understanding of the evolution of the lophotrochozoan nervous system.
Materials and Methods
Animal collection and fixation
Actinotroch larvae of Phoronis muelleri (Selys-Longchamps, 1903) were collected in August 2011 at the Sven Lovén Centre for Marine Sciences, Sweden. Vertical plankton tows were collected from 55 m depth (58°15.66N, 11°27.20E; 58°15.7N, 11°26.30E; 58°15.65N, 11°27.22E) and 15 m depth (58°15.02N, 11°27.23E), respectively. In addition, a hori-zontal tow was collected from 3 to 5 m depth (58°15.66N, 11°27.20E). Specimens could be divided into two developmental stages (12–16 tentacles, 18 and more tentacles). For each stage, 4–11 individuals were investigated for each neurotransmitter. The specimens were narcotized by adding 3.5% magnesium chloride to the seawater and were subsequently fixed for 2–3.5 h at room temperature in 4% paraformaldehyde (PFA) made up in 0.1 M phosphate-buffered saline (PBS) with 10% sucrose added (pH 7.4). After fixation the samples were rinsed 3–5 times in 0.1 M PBS with 0.1% sodium azide (NaN3) for 10 min each (pH 7.4) and stored at 4°C.
Immunocytochemistry, data acquisition and analysis
Prior to immunocytochemical staining, the larvae were washed in PTA (0.1 M PBS with 0.1% NaN3 and 4% Triton-X-100) for 1 h at room temperature. Unspecific binding sites were blocked overnight in a solution of 6% normal goat serum (Invitrogen, Molecular Probes, Eugene, OR, USA) in 0.1 M PBS and 4% Triton-X-100 (block-PTA). The samples were incubated for 24 h at room temperature in block-PTA containing the primary antibodies: anti-rabbit serotonin (Immunostar, Hudson, WI, USA, dilution 1 : 800); anti-rabbit FMRFamide (BioTrend, Cologne, Germany, dilution 1 : 1000); anti-mouse acetylated α-tubulin (Sigma-Aldrich, St. Louis, MO, USA, dilution 1 : 500). For small cardioactive peptide B staining, the samples were incubated for 48 h at room temperature in block-PTA containing the primary antibody (developed by B. Masinovsky, A.O.D. Willows and S.C. Kempf, University of Washington; dilution 1 : 300). After 4 washes in block-PTA for 6–12 h or overnight, the secondary fluorochrome-conjugated antibody (goat anti-rabbit Alexa Fluor 568, Invitrogen; goat anti-mouse Alexa Fluor 568, Invitrogen; goat anti-mouse Alexa Fluor 633, Invitrogen), was added in a 1 : 100 or 1 : 300 dilution in block-PTA, depending on the optimal signal-to-noise ratio. Incubation was for 24 h in the dark at room temperature in all cases except for anti-small cardioactive peptide B, where it was 48 h. Multistainings were performed by a cocktail of the desired antibodies in the respective working concentrations. Specimens were rinsed 4 times in PBS without NaN3 for 6–12 h or overnight and subsequently mounted in Flouromount G (Southern Biotech, Birmingham, AL, USA) on glass slides. Negative controls included either omitting the primary antibody, the secondary antibody, or both of them, and rendered no signal.
Animals were analysed using a Leica TCS SP 5 II confocal laser scanning microscope (Leica Microsystems, Wetzlar, Germany). Specimens were scanned with 0.1 μm–1.5 μm step size, and maximum projection images were created with the Leica las af software (Leica Microsystems, Wetzlar, Germany). 3D reconstructions were generated with the software imaris 7.3.1 (Bitplane, Zurich, Switzerland). Differential interference contrast (DIC) images were taken with a Nikon Eclipse E800 microscope (Nikon, Tokyo, Japan) equipped with a Nikon DS-Fi2 U3 camera. The figure plates were created with Adobe Photoshop CS5 (Adobe, San Jose, CA, USA) and the line drawings with Adobe Illustrator CS5 software.
Results
12–16 tentacle stage
Serotonin-lir nervous system
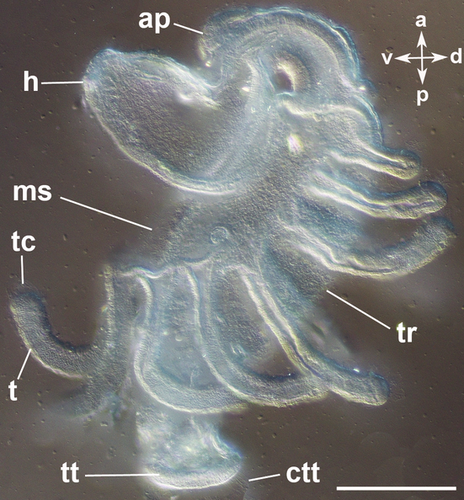
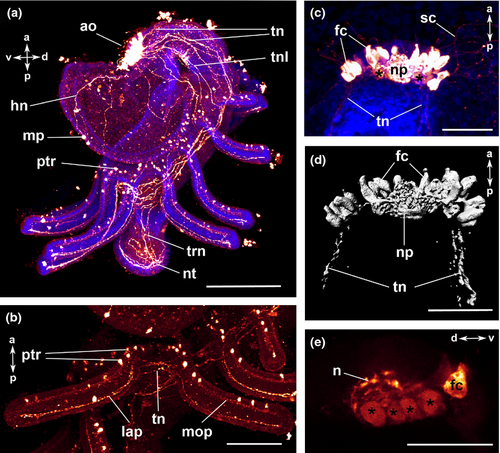
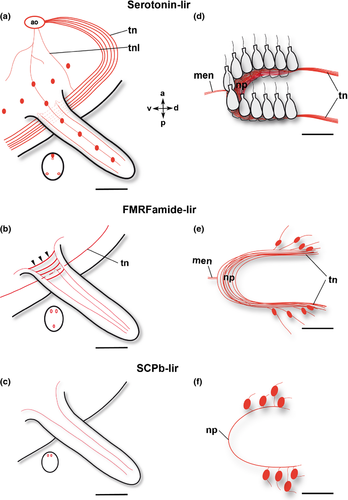
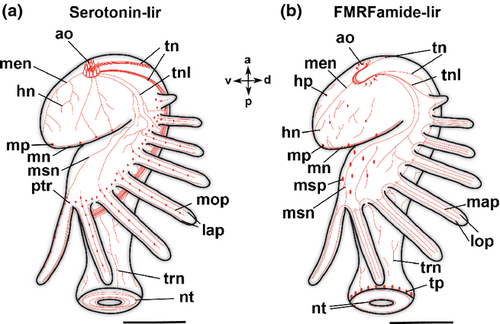
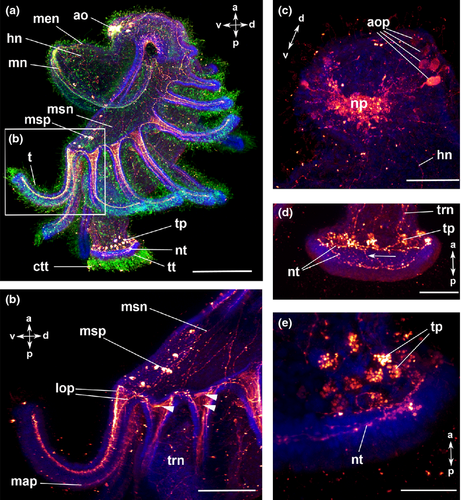
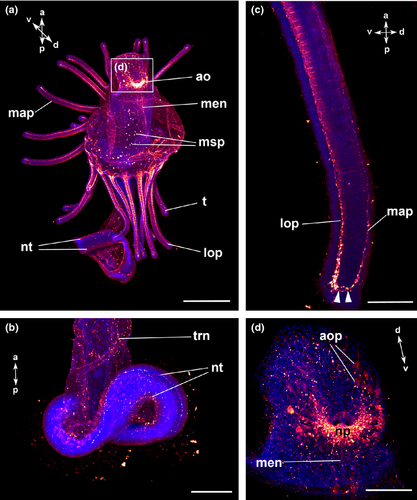
The body of the actinotroch larva is divided into three regions: the prosome, the mesosome and the metasome. The prosome, also called preoral lobe or hood, overhangs the mouth and includes the apical organ. The mesosome or collar region bears the tentacles. The metasome (trunk) is elongated, bears the telotroch and terminates in the anus (Fig. 1). In the preoral hood, a U-shaped apical organ is situated (Fig. 2a). It contains about 22 flask-shaped cells, each projecting into the neuropil (Fig. 2c–e). Every flask-shaped cell bears a single cilium. Bi- or multipolar serotonin-lir cells are located under the neuropil and are likewise arranged in a horseshoe-like pattern (Fig. 2a, e; Fig. 3d). Neurites emerge from the apical organ, project posteriorly and give rise to the tentacular neurite bundle, which runs under the tentacles and sends two lateral aboral processes into each tentacle (Fig. 2a, b; Fig. 3a; Fig. 4a). The lateral part of the tentacular neurite bundle projects towards the mesosome and sends a median oral process into each tentacle (Fig. 2a, b; Fig. 3a; Fig. 4a). Along the tentacular ridge and the oral side of each tentacle, a band of serotonin-lir perikarya is present (Fig. 2a, b; Fig. 3a). From the apical organ, the median hood neurite bundle extends and runs towards the margin of the preoral hood, where the hood margin neurite bundle with several perikarya is situated. Serotonin-lir neurites are present along the entire preoral hood (Fig. 2a; Fig. 4a). The neurites of the trunk region connect the tentacular neurite bundle to the ring-shaped neurite bundle of the telotroch (Fig. 2a; Fig. 4a). The telotroch is innervated by a meshwork of fine neurites (Fig. 2a; Table 1).
| Phoronis muelleri | Phoronis vancouverensis | Phoronis pallida | Phoronopsis harmeri | P. muelleri | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of tentacles | 10–28 | 0–2 | 2–4 | 4–6 | 10 | 0 | 6 primordial | 6 | 12–16 | 18 and more |
| Apical organ | ||||||||||
| U-shape | + | + | + | + | + | + | + | + | + | + |
| Flask-shaped cells | + | ? | ? | Spindle-shaped | + | + | + | + | + | + |
| Other cell type (bi-or multipolar cells) | + | − | − | + | + | + | + | + | + | + |
| Neuropil | + | + | + | + | + | + | + | + | + | + |
| Additional cells near apical organ | − | + | + | − | ? | + (2) | ? | ? | − | − |
| Anterior median neurite bundle | + | ? | ? | ? | + | − | − | + | + | + |
| Preoral hood | ||||||||||
| Oral ring neurites | ? | ? | ? | ? | ? | + | + | + | − | − |
| Perikarya in hood | + | − | − | − | ? | − | − | − | − | −* |
| Hood margin neurite bundle | − | − | + | + | + | + | Weak | + | + | + |
| Perikarya along marginal neurite bundle | + | − | − | − | ? | + | − | + | + | + |
| Mesosome | ||||||||||
| Ventral neurite bundle | ? | ? | ? | ? | ? | + | − | − | − | − |
| Perikarya along ventral neurite bundle | ? | ? | ? | ? | ? | + | ? | − | − | − |
| Neurites in mesosome | + | − | + | + | + | Vnc | − | − | + | + |
| Perikarya in mesosome | − | ? | ? | ? | − | Vnc | ? | − | − | − |
| Tentacle | ||||||||||
| Oral neurites (number) | ? | ? | ? | ? | − | N/A | − | − | + (1) | + (1) |
| Aboral neurites (number) | + (3) | ? | ? | ? | + (3) | N/A | + | + | + (2) | + (2) |
| Perikarya along tentacle | − | ? | ? | ? | − | N/A | − | − | + | + |
| Perikarya along tentacular ridge | − | ? | ? | ? | − | N/A | − | − | + | + |
| Tentacular neurite bundle | + | ? | ? | ? | + | + | + | + | + | + |
| Metasome | ||||||||||
| Neurites in trunk | + | ? | ? | ? | + | N/A | N/A | + | + | + |
| Perikarya in trunk | + | ? | ? | ? | + | − | − | − | ||
| Telotroch | ||||||||||
| Perikarya | − | ? | ? | − | − | N/A | N/A | − | − | − |
| Neurites | + | ? | ? | + | + | N/A | N/A | + | + | + |
- N/A, not applicable; Vnc, ventral nerve cord; ‘+’, present; ‘−’, absent; ‘?’, unknown; *, exception: frontal organ.
FMRFamide-lir nervous system
The apical organ consists of a U-shaped neuropil and a few bipolar perikarya on both sides (Fig. 3e; Fig. 5a, c). Neurites project in a dorso-lateral direction and give rise to the tentacular neurite bundle (Fig. 4b). The tentacular neurite bundle extends under the tentacles and projects into each of them on the median aboral side (Fig. 3b). A lateral part of the tentacular neurite bundle spreads over the mesosome and projects into the tentacles on the oral side (Fig. 3b; Fig. 5b). At the basis of each tentacle, the latero-oral processes are connected to each other via several commissures (Fig. 3b; Fig. 5b). Several bi- or multipolar perikarya are situated along the ventral side of the mesosome (Fig. 4b; Fig. 5a, b). In the anterior part of the apical organ, a median hood neurite bundle develops and runs towards the hood margin (Fig. 4b). Several perikarya are situated along the hood margin neurite bundle, and some perikarya are scattered along the entire preoral hood (Fig. 4b; Fig. 5a). Several neurites are scattered along the trunk region and are connected to the telotroch and its two ring-shaped neurite bundles (Fig. 4b; Fig. 5d; Table 2). In addition, perikarya with a granular appearance develop in the telotroch region (Fig. 4b; Fig. 5d, e). Some of them are connected to the nerve net of the trunk as well as to the ring-shaped neurite bundles and the fine interconnecting neurites of the telo-troch (Fig. 5d, e; Table 2).
| Number of tentacles | Phoronis muelleri | Phoronis vancouverensis | Phoronopsis harmeri | Phoronis muelleri | ||||
|---|---|---|---|---|---|---|---|---|
| 10–28 | 0–2 | 4–6 | 0 | 6 primordial | 6 | 12–16 | 18 and more | |
| Apical organ | ||||||||
| U-shape | + | − | ? | + | + | + | + | + |
| Monopolar cells | + | − | − | + | + | + | − | − |
| Other cell type (bi-or multipolar cells) | + | +* | − | + | + | + | + | + |
| Neuropil | + | + | + | + | + | + | + | + |
| Anterior median neurite bundle | + | − | + | − | ? | + | + | + |
| Preoral hood | ||||||||
| Oral ring neurites | ? | ? | ? | + | + | + | − | − |
| Perikarya in hood | + | − | − | + | ? | + | + | + |
| Hood margin neurite bundle | + | + | + | + | + | + | + | + |
| Perikarya along marginal neurite bundle | − | − | − | − | ? | ? | + | + |
| Mesosome | ||||||||
| Ventral neurite bundle | ? | ? | ? | + | + | + | − | − |
| Perikarya along ventral neurite bundle | ? | ? | ? | + | + | + | − | − |
| Neurites in mesosome | + | + | + | + | + | + | + | + |
| Perikarya in mesosome | + | ? | ? | + | + | + | + | + |
| Tentacle | ||||||||
| Oral neurites (number) | + | ? | ? | N/A | ? | ? | + (2) | + (2) |
| Aboral neurites (number) | − | ? | ? | N/A | ? | ? | + (1) | + (1) |
| Perikarya along tentacle | + | ? | ? | N/A | ? | ? | − | − |
| Perikarya along tentacular ridge | + | ? | ? | N/A | ? | ? | − | − |
| Tentacular neurite bundle | − | − | − | + | + | + | + | + |
| Metasome | ||||||||
| Neurites in trunk | + | ? | + | N/A | ? | + | + | + |
| Perikarya in trunk | − | ? | − | N/A | ? | − | − | − |
| Telotroch | ||||||||
| Perikarya | ? | ? | − | N/A | ? | − | + | − |
| Neurites | ? | ? | + | N/A | ? | + | + | + |
- N/A, not applicable; ‘+’, present; ‘−’, absent; ‘?’, unknown; *, behind apical organ.
18 and more tentacles
Serotonin-lir nervous system
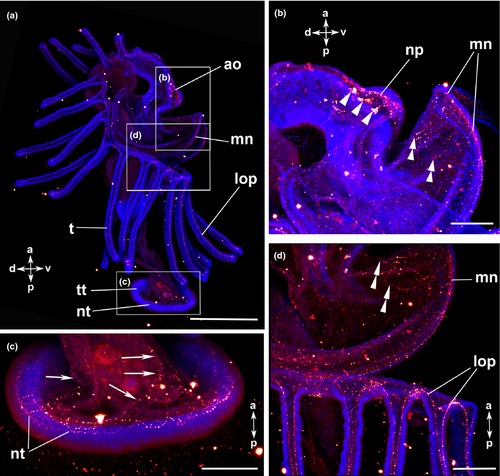
The serotonin-lir nervous system is largely similar to that of the previous stage (Fig. 4a; Fig. 6a, b; Fig. 7a, b). The newly formed frontal organ, a sense organ situated on the preoral hood, contains several, probably bipolar, perikarya that project into the median neurite bundle of the preoral hood (Fig. 6c). It is connected to the apical organ as well as to some thinner neurites of the preoral hood (Fig. 6c). The number of flask-shaped cells in the apical organ has increased to up to 37 in the 22-tentacle stage (Fig. 6c, d). Nodes are visible along the processes of the flask-shaped cells that project into the neuropil (Fig. 6e; Table 1).

FMRFamide-lir nervous system
The general organization of the FMRFamide-lir nervous system is similar to that described for the previous stage (Fig. 4b; Fig. 7c, d; Fig. 8a). At the tentacle tips, the oral and the aboral tentacular processes are connected to each other (Fig. 8c).The signal of the apical organ increases in intensity (Fig. 8d). The perikarya in the telotroch region disappear, while the two ring-shaped neurite bundles in the telotroch and its meshwork of fine neurites remain (Fig. 8b; Table 2).
Small cardioactive peptide B-like immunoreactivity
The U-shaped apical organ is situated in the preoral hood and contains several, probably bipolar, perikarya on both sides (Fig. 3f; Fig. 7e, f; Fig. 9a, b). There are several fine neurites along the entire preoral hood (Fig. 7e, f; Fig. 9b). A main hood neurite bundle runs along the margin of the preoral hood (Fig. 9b, d). A few neurites are located in the mesosome. Two oral processes project into each tentacle (Fig. 3c; Fig. 7e; Fig. 9a, d). A few neurites are situated in the trunk and some of them are connected to the ring-shaped neurite bundles of the telotroch. In addition, the telotroch is innervated by several fine neurites that form a meshwork together with the two ring-shaped neurite bundles (Fig. 9a, c; Table 3).
| Phoronis muelleri (18 and more tentacles) | |
|---|---|
| Apical organ | |
| U-shape | + |
| Monopolar cells | − |
| Other cell types (bi- or multipolar cells) | + |
| Neuropil | + |
| Anterior median neurite bundle | − |
| Preoral hood | |
| Oral ring neurites | ? |
| Perikarya in hood | − |
| Hood margin neurite bundle | + |
| Perikarya along marginal neurite bundle | − |
| Mesosome | |
| Ventral neurite bundle | − |
| Perikarya along ventral neurite bundle | − |
| Neurites in mesosome | + |
| Perikarya in mesosome | − |
| Tentacles | |
| Oral neurites | + |
| Aboral neurites | − |
| Perikarya along tentacles | − |
| Perikarya along tentacular ridge | − |
| Tentacular neurite bundle | − |
| Metasome | |
| Neurites in trunk | + |
| Perikarya in trunk | − |
| Telotroch | |
| Perikarya | − |
| Neurites | + |
- ‘+’, present; ‘−’, absent; ‘?’, unknown.
Discussion
Comparison of the larval nervous system of Phoronida
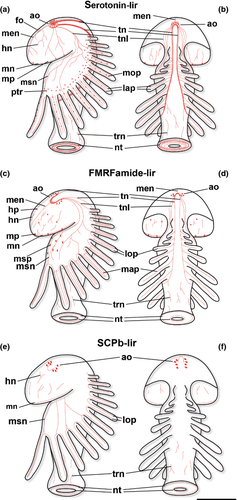
Our data on the serotonin-lir and FMRFamide-lir nervous system of P. muelleri show several significant differences to that of an earlier study on the same species (cf. Hay-Schmidt 1990a). In the previous study, the serotonin-lir marginal neurite bundle of the preoral hood and the serotonin-lir perikarya along the tentacular ridge were not observed (cf. Tables 1, 2) and the innervation of the tentacles differs between the two studies with respect to the position of the three serotonin-lir processes. In the previous study, FMRFamide-lir perikarya were present and serotonin-lir perikarya were absent along the tentacular ridge, while in our study, only serotonin-lir perikarya along the tentacular ridge were detected. Moreover, the FMRFamide-lir components of the apical organ differ between the two studies. In the previous study, five to seven monopolar cells were found in the antero-lateral part of the apical organ and about 20 additional bipolar cells were identified behind the apical organ, while we found only a few bipolar FMRFamide-lir perikarya in the lateral part of the apical organ. In addition, we found that the FMRFamide-lir tentacular neurite bundle extends under the tentacles and projects into each tentacle on the aboral side, while in the previous study, such a structure was not detected (Hay-Schmidt 1990a). We also identified several perikarya along the hood margin neurite bundle that had not been noticed previously. A ‘minor nerve ring’, which interconnects neural processes of neighbouring tentacles, was described previously (Hay-Schmidt 1990a). In the present study, the innervation of the tentacles was more complex, since the tentacular processes were not only connected to the processes of the neighbouring tentacles, but also to the tentacular neurite bundle and to the neurites of the mesosome. Contrary to the previous study, the telotroch was innervated by two FMRFamide-lir ring-shaped neurite bundles in our specimens. The FMRFamide-lir perikarya, which are connected to the ring-shaped neurite bundle of the telotroch, were only detectable in the 12–16 tentacle stage in the present study.
The mentioned differences between these two studies on P. muelleri might result from different epitopes recognized by the antibodies used. Moreover, different technical equipment was used, whereby the specimens were analysed by epifluorescence microscopy in the previous study, while a confocal laser scanning microscope was used herein, which allows for more detailed data acquisition. Nevertheless, the striking differences, which are also manifested by components identified previously that could not be confirmed by us, strongly argue for a certain degree of plasticity in the presence of neuroactive compounds in P. muelleri larvae.
Compared with the larval nervous system of P. harmeri, the serotonin-lir and FMRFamide-lir nervous system of P. muelleri shows important differences (cf. Temereva and Wanninger 2012). In early larval stages of P. harmeri a serotonin-lir and FMRFamide-lir ventral neurite bundle with associated oral ring neurites is present. This resembles a common feature in many other lophotrochozoans (Wanninger 2009). Such a ventral neurite bundle was not detected in the current study. Moreover, the innervation of the tentacles differs slightly between the two species. In P. harmeri, a serotonin-lir abfrontal loop of processes projects into each tentacle, while in P. muelleri two serotonin-lir aboral and a serotonin-lir oral process as well as one FMRFamide-lir aboral and two FMRFamide-lir oral processes are present.
During early development of P. harmeri, the first serotonin-lir flask-shaped cells in the apical organ differentiate in the mid-gastrula stage (Temereva and Wanninger 2012). Subsequently, they increase to about 25 in the 6-tentacle stage. In the apical organ of P. muelleri, there are approximately 22 flask-shaped cells in the 12-tentacle stage and about 37 flask-shaped cells when the larva has 22 tentacles (Fig. 2c, d; Fig. 6c, d). The apical organ of P. harmeri contains monopolar FMRFamide-lir perikarya and two dorso-lateral groups of several bi- or multipolar FMRFamide-lir perikarya (Temereva and Wanninger 2012). Only two groups of several bipolar FMRFamide-lir perikarya are present in the apical organ of P. muelleri. Serotonin-lir and often also FMRFamide-lir components are present in the apical organ of many other lophotrochozoans such as annelids and molluscs, supporting the notion that the last common ancestor of the lophotrochozoans already had an apical organ with serotonin-lir components (Wanninger 2008). Compared with the serotonergic nervous system of competent larvae of Phoronis pallida, the neural organization is similar to the one described in the present study, except for the innervation of the tentacles (Santagata 2002). The larval tentacles of P. pallida are innervated by three aboral serotonin-lir neurites, while one oral and two aboral serotonin-lir neurites are present in the larva of P. muelleri. Serotonin-lir perikarya along the tentacular ridge and along the tentacles are present in the larva of P. muelleri, but are absent in the larva of P. pallida (Table 1). Moreover, serotonin-lir perikarya were found around the opening of the trunk sac in metamorphic competent larvae of P. pallida (Santagata, 2002), but were not detected in our study.
Comparative larval neuroanatomy of lophotrochozoans
Most lophotrochozoans, for example, annelids, ectoprocts, sipunculans, platyhelminths and some entoprocts, have a larva that possesses a simple apical organ with up to four associated serotonin-lir flask-shaped cells (Voronezhskaya et al. 2003; Fuchs and Wanninger 2008; Wanninger 2008; Nielsen and Worsaae 2010). A simple apical organ with only a few serotonin-lir flask-shaped cells is therefore considered as part of the ground pattern of Lophotrochozoa (Wanninger 2009). However, a more complex apical organ containing six to eight serotonin-lir flask-shaped cells and several additional peripheral cells is present in the larva of polyplacophoran molluscs and in the creeping-type larva of entoprocts (Voronezhskaya et al. 2002; Wanninger et al. 2007). This derived complexity is viewed as an apomorphy of a putative mollusc-entoproct-clade, the Tetraneuralia (Wanninger 2008). A simple apical organ with one or two sets of four serotonin-lir flask-shaped cells is present in the larvae of craniiform and rhynchonelliform brachiopods (Altenburger and Wanninger 2010; Altenburger et al. 2011). In contrast, the apical organ of the ‘paralarva’ of planktotrophic linguliform brachiopods contains considerably more serotonin-lir cells (Hay-Schmidt 1992) and resembles more closely the apical organ found in phoronids, although the typical flask-shape of the serotonin-lir cells was not unambiguously determined in the linguliform paralarvae. While in some ectoprocts, the short-lived lecithotrophic coronate larvae have an apical organ that consists of two serotonin-lir flask-shaped cells, others show a serotonin-lir apical commissure without serotonin-lir or FMRFamide-lir cell bodies (Shimizu et al. 2000; Wanninger et al. 2005b). However, the long-lived planktotrophic cyphonautes larva possesses a simple apical organ that contains two flask-shaped serotonin-lir cells and some additional serotonin-lir perikarya (Nielsen and Worsaae 2010). It is still debated whether the corona-type or the planktotrophic cyphonautes larva belongs to the ground pattern of ectoprocts, and therefore, it is difficult to interpret these results phylogenetically.
Interestingly, in the mid-gastrula stage of P. harmeri, four serotonin-lir flask-shaped cells differentiate simultaneously in the apical plate and subsequently increase in number (Temereva and Wanninger 2012). The synchronous formation of these four flask-shaped cells in the early development of the actinotroch larva resembles a typical simple apical organ similar to most spiralian larvae. Thus, the complex apical organ of the actinotroch larva may be interpreted as a secondary condition that evolved from a simple apical organ of the last common lophotrochozoan ancestor.
Many adult lophotrochozoans such as annelids, molluscs, entoprocts and platyhelminths possess one or more ventral nerve cord(s) (see, e.g. Wanninger 2009 for review). Even in brachiopods that have a little concentrated, plexus-like nervous system as adults, two ventral neurite bundles with three commissures are present in the juvenile stage (Altenburger and Wanninger 2010). Such a ventral nervous system is absent in adult phoronids. Interestingly, however, a recent study on neurogenesis in P. harmeri showed such a transitory, paired ventral neurite bundle in the actinotroch larva. In our study, no ventral neurite bundle was found by any of the antibodies applied (Table 1, 2). It remains unknown whether or not a ventral neurite bundle is present in other phoronid species, since only few developmental data on early phoronid neurogenesis are available. Nevertheless, the presence of a ventral neurite bundle during early development of P. harmeri indicates that such a neural feature may have also been part of the ancestral phoronid bodyplan.
Serotonin-lir neurites that underlie ciliary bands are common for most lophotrochozoan larvae (Hay-Schmidt 1990c, 2000; Friedrich et al. 2002; Page 2002; Voronezhskaya et al. 2002, 2003; Nielsen 2005; Rawlinson 2010) and are considered as part of the lophotrochozoan ground pattern, although this feature was lost multiple times, for example, in sipunculans, echiurans and cycliophorans (Hessling and Westheide 2002; Wanninger 2005; Wanninger et al. 2005a). The actinotroch larvae of phoronids have a preoral ciliated band that is situated along the edge of the preoral hood, a postoral ciliated band that runs along the tentacles and a posterior telotroch (Nielsen 1987). In P. muelleri, each of these ciliated bands is innervated by serotonin-lir and FMRF-amide-lir neurite bundles. This supports the notion that serotonergic neurite bundles associated with ciliary bands are part of the lophotrochozoan groundplan, although homology of the ciliated structures themselves between different phyla remains uncertain (Wanninger 2008, 2009).
Our study provides the first evidence of small cardioactive peptide B distribution in the nervous system of an actinotroch larva (Fig. 7e, f; Fig. 9; Table 3). The staining results in a weak signal where the apical organ, the marginal neurite bundle of the preoral hood, the innervation of the tentacles and the telotroch neurites are the most prominent neural structures. Although it is hypothesized that small cardioactive peptide B plays a role in the regulation of the heart beat rate, muscle modulation and ciliary activity in molluscan larva, little is known concerning potential additional functions of this neuropeptide (Ellis and Kempf 2011). The positive signal in phoronids and a wide range of other invertebrates such as molluscs, annelids, gastrotrichs, rotifers and arthropods corroborates the assumption that this compound was also present in the protostome groundplan (cf. Lloyd et al. 1984, 1987; Kempf et al. 1987; Candelario-Martinez et al. 1993; Fox and Lloyd 1997; Gainey et al. 1999; Perry et al. 1999; Ohsuga and Kurokawa 2000; Willows et al. 2000; Kempf and Page 2005; Settembrini and Villar 2005; Kanda and Minakata 2006; Hochberg 2007; Ellis and Kempf 2011; Hochberg and Atherton 2011).
Acknowledgements
We are grateful to Elena Temereva (University of Moscow, Russia) for useful discussions and help with species identification. B. Masinovsky, A.O.D. Willows and S.C. Kempf developed the small cardioactive peptide B antibody used in this study. The staff of the Sven Lovén Centre for Marine Sciences (Kristineberg, Sweden) is acknowledged for providing facilities for species collection. AW is grateful for generous support by the Faculty of Life Sciences, University of Vienna.




