Multilevel studies on the two phenological forms of Large Blue (Maculinea arion) (Lepidoptera: Lycaenidae)
Abstract
enThe main goal of our research was to study comprehensively the differences between the two phenological forms of the socially parasitic and globally threatened Large Blue (Maculinea arion) in the Carpathian Basin using four character sets (mitochondrial sequences, allozymes, male genitalia and wing morphometrics). Comparative analyses of distance matrices, phylogenetic trees and ordination patterns have been applied. The genetic and morphometric patterns revealed by our studies were discordant. While we experienced a significant differentiation between the ‘spring’ and ‘summer type’ of M. arion in both wing and genital traits, the two phenological forms did not show any genetic differentiation on two mitochondrial loci and in allozymes. At the same time, all individuals were infected by Wolbachia. Although certain wing traits may not represent reliable tracers of phylogeny because of the particular adaptive significance, the wing characteristics involved in our research are probably determined genetically. Additionally, the significant differentiation of male genitalia also indicates incipient prezygotic isolation arising from phenological differentiation between the ‘spring and summer arion’. It is possible that all extant differences between the two forms are attributable to (1) different host-ant use, (2) incipient speciation, (3) cytoplasmatic incompatibility (CI) by Wolbachia or the combination of these factors. In addition, discordant results indicate that the combined use of different approaches and data sets is strictly necessary to clarify systematic and evolutionary relationships.
Zusammenfassung
deDer gefährdete sozialparasitische Bläuling Maculinea arion stand im Vordergrund dieser naturschutzbiologischen Untersuchungen. In der vorliegenden Arbeit werden die Unterschiede zwischen den phänologischen Formen von Maculinea arion aufgrund von vier verschiedenen Merkmalsgruppen (mitochondriale Sequenzen, Allozyme, Morphometrie der männlichen Genitalien und Flügelmuster) besprochen. Distanzmatrizes, phylogenetische Bäume und Ordinationsmuster werden verglichen. Wir haben gegensätzliche Ergebnisse in der genetischen bzw. morphometrischen Differenzierungen erhalten. Während in den morphometrischen Merkmalen der Flügel und Genitalien erhebliche Unterschiede zwischen den phänologischen ‘Frühjahrs-’ und ‘Sommer’-Formen gefunden wurden, zeigten die beiden mitochondrialen Loci und auch das Allozymmuster keine Differenzierung. Alle untersuchten Individuen waren jedoch mit Wolbachia infiziert. Obwohl die meisten Flügelmerkmale wegen ihrer adaptiven Plastizität phylogenetisch als irrelevant gelten, sollten die von uns gewählten Merkmale einen genetischen Hintergrund haben. Außerdem deuten die nachgewiesenen Unterschiede in den männlichen Genitalien auf eine anfängliche präzygotische Isolation zwischen den beiden phänologischen Formen hin. Als mögliche Gründe für eine solche Differenzierung könnten (1) die verschiedenen Wirtsameisen, (2) beginnende Speziation bzw. (3) zytoplasmatische Inkompatibilität durch Wolbachia oder die Kombination diese Faktoren gelten. Die gegensätzlichen Ergebnisse sollten die Notwendigkeit der kombinierten Anwendung verschiedener Methoden und Datensätze in der Untersuchung der systematischen und evolutionären Verhältnisse unterstreichen.
Introduction
In the past few decades, traditional morphology-based taxonomy has been increasingly replaced by DNA-based species identification. A short, standardized gene region of mtDNA (mitochondrial cytochrome c oxidase subunit I – COI) was proposed as a ‘DNA barcode’ for discriminating most animal species (Hebert et al. 2003; Dinca et al. 2010). The proposal to develop an identification system based on a single gene marker attracted early criticism (Ebach and Holdrege 2005). Additionally, performance tests have shown significant differences in identification success in the case of different animal groups (Hebert et al. 2004; Wiemers and Fiedler 2007). As a consequence, a great need remains for comprehensive studies and in-depth multidisciplinary assessments before any conclusion is drawn. Such a multilevel approach became necessary to study the distinctness of two phenological forms of the Large Blue (Maculinea arion).
The genus Maculinea Van Eecke, 19151 (Lepidoptera: Lycaenidae) is one of the most intensively studied insect groups in Europe (Settele et al. 2005). This is partly attributable to their very special social parasitic life cycle and partly to being umbrella species (Fleishman et al. 2005; Spitzer et al. 2009). Additionally, these butterflies face a serious conservation risk as their habitats have suffered severe decrease and fragmentation. Within the genus Maculinea, one of the most conspicuous declines was shown by the Large Blue – Maculinea arion (Linnaeus, 1758). This species became extinct in the Netherlands in 1964 (Tax 1989), in the UK in 1979 (Thomas 1995) and in Belgium in 1996 (although later the species was re-introduced into the UK and it also re-colonized in Belgium) (Goffart 1997). It shows a serious retreat all over Europe, especially on the northern border of the distribution area of the species (Wynhoff 1998); therefore, Large Blues are endangered on a European scale. The species is included in Annex IV of the European Habitats’ Directive, and it is listed in the IUCN Red List of Threatened Species as ‘near threatened’ and considered as ‘endangered’ (EN) in the European Red List of Butterflies (Munguira and Martin 1997; Van Swaay et al. 1998, 2010).
Maculinea arion is a highly variable species morphologically. Nowadays there are at least twenty named forms listed (Verity 1940; Cheshire 2011) and three subspecies are commonly recognized in Europe (Higgins and Riley 1970; Thomas 1996; Tolman and Lewington 2009): (1) Maculinea arion arion (Linnaeus, 1758) is the most widespread nominate form, which is described from Germany (Nürnberg); (2) Maculinea arion ligurica (Wagner, 1904), which is described from Liguria region of north-western Italy originally as a varietas; (3) Maculinea arion obscura (Christ, 1878) is a high-mountain form in the Swiss Central Alps (Zermatt, Liestal), but phenotypically similar forms also occur in high mountains of the Balkan Peninsula. In the Carpathian Basin, the former two subspecies have been reported (Varga 2010), and there are numerous differences between them. The fast-flying, smaller-sized and dark violet-blue M. a. arion (referred to as ‘spring arion’ hereafter) flies from mid-May to mid-June and prefers short-grass dry swards with cushions of early-flowering Thymus species (Th. serpyllum L., Th. pannonicus All. and related species), which serve as initial food plants. The slower, larger and light silvery blue M. a. ligurica (referred to as ‘summer arion’) is on the wing from the end of June to mid-August and mostly occurs at xerothermic oak forest fringes, on woodland clearings and in fen-like habitats in hilly areas. Females oviposit among flower buds of late-flowering Thymus species (mostly Th. pulegioides L.) and/or Origanum vulgare L. (Varga 2010; : Annotation No. R23). Although differences in food plant use also imply some difference in habitat preference, there is only a weak ecological isolation between the two forms. Moreover, numerous syntopic occurrences of the ‘spring’ and ‘summer arion’ have been recorded in Aggtelek Karst region (Hungary) (Tóth & Bereczki, pers. obs.). Our knowledge on the host-ant use of the two subspecies is insufficient. Myrmica sabuleti Meinert, 1861 is known to be the main host ant of M. arion in Western Europe, and it was also found as a putative host ant in some M. arion habitats in Hungary (Tartally 2008). In Poland, M. arion is characterized by multiple host-ant use, which varies geographically (Sielezniew et al. 2003, 2010a,b,c; Sielezniew and Stankiewicz 2008). Hitherto, ant-nests infected by larvae of M. arion have not been found in Hungary probably because of the lack of intensive surveys. The phylogenetic studies focusing on the section Glaucopsyche show potential cryptic species within the predatory Maculinea, and high divergences were also found in M. arion (Als et al. 2004; Fric et al. 2007; Ugelvig et al. 2011b). Consequently, the two phenological forms of M. arion may be candidates for putative cryptic species.
At the same time, Sielezniew (2012) and Sielezniew et al. (2012) discovered Wolbachia infestation in M. arion and M. alcon populations, which may have influence on phylogeny of Maculinea species. Wolbachia infections are associated with a variety of phenotypic effects on the hosts: (1) cytoplasmatic incompatibility (CI); (2) male killing, the consequence of which is sex ratio distortion; (3) feminization of genetic males, which may also bias the sex ratio; and (4) parthenogenesis induction. The former three effects have been revealed in butterflies. CI, that is, when the sperm of infected males is incapable of fertilizing the eggs of uninfected females and females infected with a different Wolbachia strain, is the most frequently observed phenotype (Hoffmann and Turelli 1997; Werren et al. 2008). The spread of Wolbachia via CI can drive the spread of maternally inherited genetic elements, such as mtDNA. Specific mitochondrial genotypes may be associated with a Wolbachia CI strain surreptitiously. Consequently, the distribution of mtDNA variation in infected populations does not conform to the expectations of neutral theory (Narita et al. 2007; Gompert et al. 2008; Nice et al. 2009), which has been revealed not only in butterflies, but also in other invertebrate species (Turelli and Hoffmann 1991; Shoemaker et al. 2003; Shaikevich et al. 2005). Wolbachia may homogenize biological species for mtDNA following introgression of endoparasites, as reported in Acrea (Jiggins 2003) and Drosophila (Ballard and William 2000b), causing a ‘one barcode – two species’ phenomenon (i.e. two biological entities have the same mtDNA sequence). On the contrary, Wolbachia can make one species appear as two due to the high intraspecific mtDNA diversity associated with possession of different parasite strains, as reported in Adalia (Hurst et al. 1999a,b), leading to a ‘two barcodes – one species’ phenomenon. Additionally, divergent mitochondrial sequences may suggest the presence of cryptic species falsely (Hurst and Jiggins 2005).
Although Bereczki et al. (2011) have shown that the two putative subspecies cannot be differentiated based on allozyme loci, we intended to study the dissimilarities between the two forms in a multilevel research including DNA and morphological surveys. We also intended to explore whether the outcome of the different methods would be congruent or not. Besides, we were interested in discovering whether the influence of Wolbachia on mtDNA variability and selective sweep works in M. arion populations. Thus, the aims of our research were: (1) to reveal whether the two types of M. arion differentiate on the basis of mitochondrial sequences, (2) to establish the measure of the differentiation in allozymes, (3) to study the morphological differences between them on the traits of external genitalia and wings and (4) to compare the level of the genetic and morphometric differentiation.
Materials and methods
Sampling
DNA studies were based on sequences of 29 field-collected specimens (Appendix 1, Fig. 1), of which nine were ‘spring arion’ and 15 were ‘summer arion’ (the identification was carried out based on the date of sampling). We had four couples of ‘spring and summer arion’ from the same locality (see in boldface in Appendix 1, Fig. 1). We used only one M. arion individual from GenBank database (identifier: SPAus – accession number: HQ918148.1) because only its sequence overlapped completely with those of our samples. Maculinea alcon, M. teleius and M. nausithous specimens were used as outgroups.
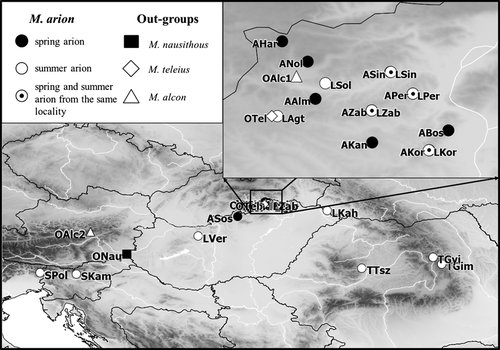
In our allozyme and morphometric studies, we used the same set of individuals (only males). Altogether 11 samples (143 individuals) were collected between 2002 and 2011 (Appendix 2, Fig. 1): four were ‘spring type’ and seven were ‘summer type’ M. arion (the identification was also carried out based on the sampling time). We possessed one pair of samples originated from the same syntopic population (see in boldface in Appendix 2). The samples from Kamnik and Polovnik (Slovenia) were unified because of the low number of individuals. M. nausithous (16 individuals) was included as outgroup. Images were collected at the end of the egg laying period and stored at −80°C until electrophoresis and morphometric analyses.
Molecular analyses
DNA studies
DNA was extracted by homogenizing either the head or thorax (Appendix 1) in 800 μl extraction buffer (Gilbert et al. 2007). The samples were incubated for 24 h at 56°C with gentle agitation and then centrifuged at 18 472 g for 1 min. The supernatant was washed twice with an equal volume of chloroform–isoamyl alcohol (24 : 1) to remove proteins. The DNA was precipitated by adding the mixture of 80 μl ammonium acetate (7.5 M) and an equal volume of ice-cold isopropanol and storing the samples at −20°C for 4 h. The DNA was pelleted by centrifugation at 18 472 g for 10 min at 4°C. After centrifugation, the supernatant was discarded and the DNA pellet was washed twice with 70% ice-cold ethanol. The pellet was air-dried for 1 h at room temperature and was redissolved in 50 μl elution buffer (10 mM Tris–HCl, pH 8.0 and 0.5 mM EDTA, pH 9.0). DNA aliquots were stored at 4°C.
The I subunit of the cytochrome c oxidase gene (COI), which is commonly used in barcoding animal life (Hebert et al. 2003; Wiemers and Fiedler 2007), offers an adequate tool to test whether the morphological and ecological differences between the two forms of M. arion manifest at the DNA level. We therefore sequenced this section of the mitochondrial genome together with subunit II (COII) to obtain insight into the phylogeny of taxa at the species level. These two mitochondrial genes were amplified by four modified universal primer pairs (COI: HybLCO and HybHCO, HybJerry and HybPat of Wahlberg and Wheat (2008); COII: Georges and Phyllis, Strom and BtLys of Monteiro and Pierce (2001)). Primers were modified at their 5′-end to include the universal sequencing primer T4 promoter. Amplification from 1 μl of DNA extracts was carried out in 25 μl final reaction volumes containing 10× PCR buffer, 2 mM MgCl2, 0.2 mM dNTPs, 0.02 units per μl of Taq DNA polymerase (Dream Taq Green, Fermentas) and 0.2 μM of each primer. Amplification was carried out in an ABI Veriti thermal cycler programmed for: initial denaturation for 3 min at 94°C; 35 cycles of 30 s at 94°C, 30 s at the locus-specific annealing temperature of 54/54/52/56°C (in the order of primer pairs, see above), 1 min at 72°C; final elongation of 10 min at 72°C. The success of PCR amplification was checked by running 2 μl of product on 1% agarose gels stained with ethidium bromide. PCR products were sequenced by commercial service provider Macrogen Inc. (Seoul, South Korea). Sequences were edited and revised manually by chromas lite v. 2.01 and aligned by mega v. 4.0 (Tamura et al. 2007).
The presence of Wolbachia was checked by the amplification of the 16S ribosomal region with Wolbachia-specific primers W-Spec of Werren and Windsor (2000) following their guidelines of amplification. Infected accessions (Appendix 1) were tested further with the strain-specific ftsZ primers and PCR conditions of Werren et al. (1995) and Sasaki et al. (2002).
Based on the concatenated COI and COII sequences, phylogenetic tree reconstruction methods of three different search criteria were applied to demonstrate phylogenetic relationship between the ‘spring’ and ‘summer arion’. Heuristic searches were run in paup v. 4.0b10* (Swofford 2003) under both maximum parsimony (MP) and maximum-likelihood (ML) criterion separately, while Bayesian phylogenetic relationships were assessed in mrbayes v. 3.2.1 (Ronquist et al. 2012). The MP search used TBR search algorithm holding 10 trees at each iteration step with ‘MulTree’ option in effect, while ‘steepest descent’ not in effect. The ML search utilized an evolutionary model of TVM+I as selected by modeltest v. 3.7 (Posada and Crandall 1998) with default settings. For the Bayesian analysis, we specified the evolutionary model HKY+I+G as selected by mrmodeltest v. 2.3 (Nylander 2004), and two separate runs were conducted for 2 million generation samplings every 1000th generation. Resulting probability files were checked by tracer v. 1.5 (Drummond and Rambaut 2007) for convergence and effective sample sizes of the runs, then were combined and a maximum clade credibility tree was computed after discarding first 25% of trees as ‘burn-in’. All analyses were run on Bioportal (Kumar et al. 2009).
Allozyme studies
Allozyme polymorphism was studied at 12 loci by vertical polyacrylamide gel electrophoresis. Thoraxes homogenized in 300 μl of extraction buffer were used to study Gpdh, G6pgdh, Hk, Idh, Mdh, Pgi, Pgm and Sod. Abdomens homogenized in 200 μl of extraction buffer were used to analyse Acon, Acph, Aox and Est. The extraction buffer, the electrophoresis buffer systems and running conditions, together with the staining solutions, were applied as described in Bereczki et al. (2005). Genotypes of the individuals were scored according to their enzyme pattern.
Since Bereczki et al. (2011) has involved a detailed study about the allozyme variability of M. arion, the recent study has been based on a reduced data set. Therefore, this paper only includes the analyses that have been carried out in parallel with the morphometric surveys. Allele frequencies were used to estimate Nei's genetic distances (Nei 1975), and an UPGMA dendrogram (Sneath and Sokal 1973) was constructed on the basis of the distance matrix using Past v. 2.17 (Hammer et al. 2001). Pairwise FST values were also established by genalex v. 6.4 (Peakall and Smouse 2006).
Morphometric analyses
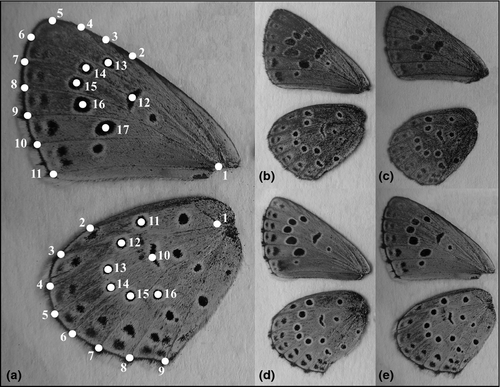
Prior to electrophoresis, wings and the terminal segments of the abdomen were cut. Wings were fixed on transparency films and photographed by Sony DSC-H2 digital camera. Landmark-based geometric morphometric approach was used to quantify the variation in the shape of wings and the pattern of black spots on the underside. We recorded 17 landmarks on fore-wing and 16 on hind-wing (only one side was measured in both cases) by tpsdig v. 2.1 (Stony Brook University, New York, USA) (Fig. 2a). Procrustes generalized least squares (GLS) method was applied to get the superimposed coordinates for the statistical analyses.
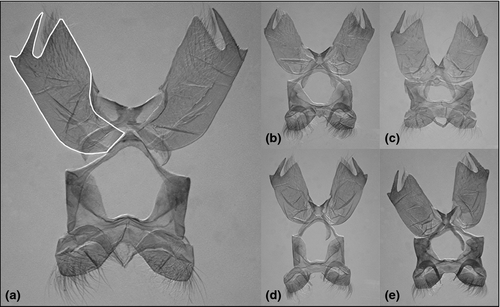
The preparation procedure of male external genitalia was slightly modified after Robinson (1976). The sclerotized genitalia were separated from the body tissues by keeping the terminal segments of the abdomen in 15% KOH overnight, followed by heating for 30 min at 75°C before preparation. The genitalia were cleaned and dehydrated with 96% ethanol and mounted in Euparal fixative (mounting medium) on microscope glass. Slides were digitalized by combining an Olympus camera and a Nikon 102 stereomicroscope. Since we found only few real landmarks on valvae, we recorded a close curve on them using tpsdig v. 2.1 (Fig. 3a). For the analysis of the outlines, elliptic Fourier analysis (Giardina and Kuhl 1977; Kuhl and Giardina 1982) was used. The algorithm fits Fourier series on x- and y-coordinates as functions of the curvilinear abscissa (Claude 2008). The statistical analyses of the two morphometric approaches were similar.
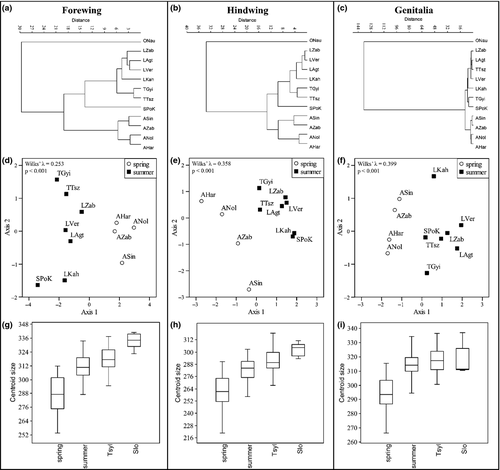
Repeatability was calculated by the following formula: 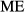
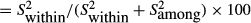 (Lessells and Boag 1987), where
(Lessells and Boag 1987), where  is the within-measurement component of variance and
is the within-measurement component of variance and 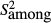 is the among-measurement component. anova was used to calculate these values (Bailey and Byrnes 1990; Yezerinac et al. 1992) in R programme package (R Development Core Team 2010). Principal component analyses (PCAs) were applied to reduce the number of variables. The scores of the PC axes that could explain more than 1% of the total variance were used in canonical variates analysis (CVA) and multivariate analysis of variance (manova) (see: Dapporto et al. 2009, 2011; Dinca et al. 2011). Jack-knifed grouping and Wilks’ lambda were applied to quantify the validity of the visible pattern.
is the among-measurement component. anova was used to calculate these values (Bailey and Byrnes 1990; Yezerinac et al. 1992) in R programme package (R Development Core Team 2010). Principal component analyses (PCAs) were applied to reduce the number of variables. The scores of the PC axes that could explain more than 1% of the total variance were used in canonical variates analysis (CVA) and multivariate analysis of variance (manova) (see: Dapporto et al. 2009, 2011; Dinca et al. 2011). Jack-knifed grouping and Wilks’ lambda were applied to quantify the validity of the visible pattern.
UPGMA tree was constructed based on Mahalanobis distances. The differences in centroid sizes (the square root of the summed squared distances of each landmark from the centre of the form) were analysed by univariate anova. All morphometric analyses were carried out using past 2.17 (Hammer et al. 2001).
Results
Molecular studies
Four bits of mitochondrial (mt) cytochrome c sequences were concatenated into one ‘contig’ sequence for all studied taxa containing full mtCOI and partial mtCOII sequences. Final concatenated sequences were aligned without the need of introducing gaps into a matrix of 2217 base pairs (bp), of which 1361 bp were from COI and 856 bp were from COII. Altogether, there were 10 variable positions in the alignment of M. arion sequences and each was parsimony informative. All three different phylogenetic tree reconstruction methods yielded the same topology of phylogenetic trees (Fig. 4a,b). The ‘spring’ and the ‘summer type’ of M. arion were not differentiated on the basis of these mitochondrial sequences. Moreover, the target sequences did not show any geographical pattern. Only the two Slovenian individuals from Kamnik have been found on a well-supported sub-branch (bootstrap ML: 80%; bootstrap MP: 85%; Bayesian posterior probability: 0.99) although it should be noted that the differentiation of these specimens is attributable to altogether six mutations on 2217 sites. Outgroups were clearly separated from M. arion individuals. This lack of resolution within M. arion can be explained by infection with Wolbachia, which was proven by appropriate tests. All M. arion individuals were infected by Wolbachia supergroup A (Appendix 1), and M. alcon specimens were infected with B supergroup. M. nausithous was uninfected and we got a weak band indicating the infection by B supergroup in M. teleius.
The results of our previous allozyme study (Bereczki et al. 2011) have been confirmed by the recent analysis based on a reduced data set. The two types of M. arion were not separable at the enzyme level. All M. arion samples were located in the same branch of the dendrogram and they separated clearly from the outgroup (Fig. 4c). The samples from the same locality (AZab and LZab) did not cluster together. Pairwise FST values indicated small differences among populations even between the samples which are located in different geographical regions at long distances apart. The separation of the Slovenian sample from all the other ones was the highest extent (Fig. 4d).
Morphometric studies
The repeatability of measurements was satisfactory (ME < 5%) in most cases. The error was <9% in the case of each hind-wing and genitalia coordinate. We excluded the landmarks of fore-wing from our analyses that have more than 20% measurement error.
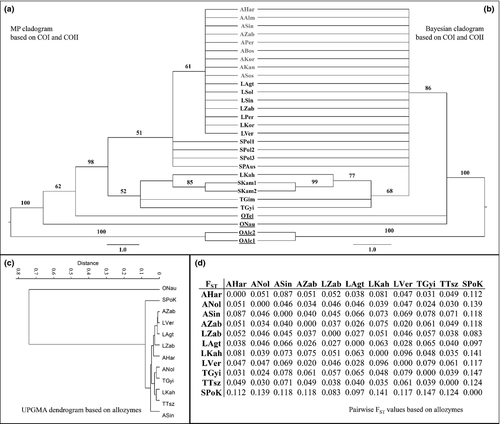
The results of our morphometric survey were very similar in cases of wings and genitalia alike. The ‘spring’ and the ‘summer arion’ differentiated significantly (fore-wing: Wilks’ λ=0.253, p < 0.001; hind-wing: Wilks’ λ=0.358, p < 0.001; genitalia: Wilks’ λ=0.399, p < 0.001). More than 85% of the individuals were correctly classified by a cross-validated method in both wings and genitalia (Appendix 3A–C). The best classification was obtained in the case of fore-wing (the lowest Wilks’ λ with the highest classification value −93.0%).
The samples of the ‘spring’ and the ‘summer arion’ clustered separately both on the phenogram of wings (Fig. 5a,b) and on that of genitalia (Fig. 5c). The samples from the same locality (AZab and LZab) clustered separately according to the phenology. In cases of wings, a geographical pattern can be recognized. The Slovenian sample differentiated from the other ones at the highest extent in wings, but it is clustered together with one of the Transylvanian samples on the basis of genital traits. The group centroids of the two different types of M. arion samples also differentiated from each other at the first axis (Fig. 5d–f). Moreover, we experienced significant differences (p < 0.001) in size between the ‘spring’ and ‘summer arion’. The Transylvanian and Slovenian samples were clearly grouped into the ‘summer arion’ on the basis of size (Fig. 5g–i).
Discussion
The genetic and morphometric patterns revealed by our studies are discordant. While we experienced significant differences in morphology between the ‘spring’ and ‘summer type’ of M. arion, the two phenological forms show genetic differentiation neither on the mitochondrial sequence nor at the allozyme level.
The variability of the studied mitochondrial regions was highly reduced. Only the Slovenian specimens from Kamnik formed a highly supported clade nested within the otherwise unresolved clade of M. arion. At the same time, all M. arion specimens proved to be infected by Wolbachia A. M. alcon individuals, used here as outgroups, were also infected but with Wolbachia B. Similar to our results, Sielezniew (2012) and Sielezniew et al. (2012) reported the infection of the same types of Wolbachia and a reduced mtDNA diversity compared to nuclear variation in the populations of M. arion and M. alcon in Poland and Lithuania. Therefore, the lack of resolution within M. arion found here is presumably the consequence of indirect selection mediated by Wolbachia (Ballard and William 2000a; Jiggins 2003; Hurst and Jiggins 2005) rather than that of population dynamic events. Thus, the usage of additional nuclear markers and regular assays for Wolbachia presence as well as multilocus sequence typing (MLST) should be applied in phylogenetic and phylogeographical studies despite the fact that Wolbachia COI was only present in 0.16% of >2 million insect COI sequences (Smith et al. 2012). Although detailed population dynamic studies (mark–release–recapture) were carried out only in one population from Vérteskozma (‘LVer’) in Hungary (Kőrösi et al. 2005) and sex ratio distortion was not recorded (Kőrösi, pers. comm.), further studies are needed to elucidate the possible effect and phenotype of Wolbachia in M. arion populations in the Carpathian Basin. Besides, it would be worth to use nuclear markers mostly in the couples of samples originated from the same locality.
On the basis of our allozymes studies, genetic differentiation among M. arion populations is low. The isolation of the Slovenian sample was the highest. Our results coincide with those of Polish authors based on microsatellites (Rutkowski et al. 2009; Sielezniew and Rutkowski 2012) who also experienced small and moderate differentiation among M. arion samples.
This pattern is probably in relation to the biological characteristics of this species. Only one or two larvae of M. arion may survive in an ant-nest, because one predatory caterpillar requires on average 350 ant workers to be raised compared with 50 for a ‘cuckoo’ larva (Elmes et al. 1991; Thomas and Wardlaw 1992). In addition, the majority of Myrmica species live in small colonies of 200–500 workers (Elmes et al. 1998) such as M. sabuleti, the primary host ant of M. arion in Western Europe. Therefore, only few Myrmica colonies are large enough to support just a single M. arion larva, and small colonies (or multiple infected large ones) are probably to be completely exploited by caterpillar(s) in the spring (Thomas and Wardlaw 1992), because they experience dramatic reductions in colony fitness by infection (Thomas et al. 1989). Consequently, the depleted colonies may be less suitable as a host for M. arion in the following year although the ant colony may be replaced by another one (Tolman and Lewington 2009). This intimate butterfly–ant relationship may lead to strong oscillations in population census size such as reported in the northernmost Finnish population (Kolev 1998) and in Vértes population in Hungary (LVer). In the latter population, approximately 300 individuals were marked in a two-ha area in 2002, but mark–release–recapture studies failed due to the absence of M. arion in 2004–2005 (Kőrösi et al. 2005). Consequently, the genetic diversity may primarily be maintained by gene flow among local low-density populations.
Additionally, M. arion has high dispersal ability, which has been revealed by capture–recapture studies (Pajari 1992). At the same time, molecular studies indicated that gene flow occurs over distances 15 times longer than the maximum distance recorded from mark–recapture studies, and M. arion can maintain fully functional metapopulations where the suitable habitat patches are not further apart than approximately 10 km (Ugelvig et al. 2011a, 2012). Therefore, the high potential of gene flow may also contribute to the low genetic differentiation found among M. arion populations.
Our morphometric research showed significant differences between the ‘spring and summer arion’ both in wings and in genitalia. In the case of wings, a geographical pattern was observed. Numerous studies revealed that certain wing traits – for example size, melanization level – may be determined environmentally along climatic (latitudinal or altitudinal) gradients (Dennis 1977; Dennis and Shreeve 1989; Smyllie 1992) and greatly exposed to environmental stress (Talloen et al. 2009). Sielezniew and Dziekańska (2011) found that the melanization level was higher in the north-eastern part of Poland than in the South, and the mid-eastern region showed intermediate characteristics. At the same time, other wing traits are probably not exposed to environmental effects considerably, but determined rather by genetic factors (Talloen et al. 2009), for example the position of spots on the underside, which is used for the identification in Maculinea taxonomy (Sibatani et al. 1994). Since we experienced clear geographical pattern hardly explained by climatic factors, we suppose that the wing traits involved in our study are probably determined genetically rather than environmentally. Nonetheless, this pattern may also be in relation to the different host-ant use (Gadeberg and Boomsma 1997; Sielezniew and Dziekańska 2011; Sielezniew and Rutkowski 2012).
At the same time, good agreement has been revealed between the outcome of the molecular studies and that of male genitalia morphometrics in numerous analyses (Cesaroni et al. 1989, 1994; Garnier et al. 2005). This suggests that selective pressures controlling genital structures are relatively homogeneous across taxa and the patterns of divergence in genital morphology may reflect overall genetic divergence rather than differential adaptive responses. Consequently, the quantitative traits of male genitalia may be good estimators of the overall divergence among populations and closely related species and generally considered reliable taxonomic characteristics for traditional systematic work at the species level.
Our molecular and morphometric analyses lead to discordant results, but we have to consider some basic facts. First, the diversity of the barcoding gene is proved to be reduced presumably due to Wolbachia infection. Second, the allozymes of Maculinea species are generally less polymorphic than those of other European lycaenid species (Schmitt and Seitz 2001, 2002a,b; Aagaard et al. 2002; Schmitt et al. 2002, 2003, 2005; Schmitt and Hewitt 2004). Therefore, it is possible that allozyme and mitochondrial DNA studies are not suitable for the detection of the divergence between the ‘spring and summer arion’. Nevertheless, our morphometric studies have revealed significant differences between M. a. arion and M. a. ligurica. Although certain wing traits may not represent reliable tracers of phylogeny because of their particular adaptive significance, the wing characteristics involved in our research are probably determined genetically. Additionally, the significant differentiation of male genitalia also indicates incipient prezygotic isolation arising from phenological differentiation between the two types of M. arion.
In summary, our study clearly indicates that the combined use of different approaches and data sets is highly necessary to clarify systematic and evolutionary relationships among taxa despite the fact that molecular data often tend to receive more emphasis than morphological ones. Although we did not find differences between the two forms of M. arion on the basis of molecular data, it is not at all unlikely that our markers are not suitable for the detection of the divergence between them. It is possible that all extant differences of the two forms are attributable to (1) different host-ant use, (2) incipient speciation, (3) CI by Wolbachia or a combination of these factors. Further molecular and ecological studies are needed to elucidate the pattern of variation.
Acknowledgements
The study was supported by the OTKA (K-84071), TÁMOP-4.2.2/B-10/1-2010-0024 and TÁMOP 4.2.4.A/2-11-1-2012-0001 projects. Grateful acknowledgements are due to László Peregovits and Sándor Szabó for collecting samples. The support of the Nature Conservation Authorities of Hungary is also greatly appreciated.
Note
Appendix 1: Information on the specimens used in the DNA studies
| Taxa | Locality | Abbr. | Sampling time | DNA extraction | Accession number | Wolbachia supergroup |
|---|---|---|---|---|---|---|
| Spring arion (Hungary) | Haragistya | AHar | 2011-05-31 | Thorax | HG326619 | A |
| Almás-tető | AAlm | 2011-05-26 | Thorax | HG326620 | A | |
| Szin | ASin | 2011-05-25 | Thorax | HG326621 | A | |
| Zabanyik | AZab | 2011-05-24 | Thorax | HG326622 | A | |
| Perkupa | APer | 2011-05-28 | Thorax | HG326623 | A | |
| Boszorkányvölgy | ABos | 2011-05-25 | Thorax | HG326624 | A | |
| Korlát-hegy | AKor | 2011-05-25 | Thorax | HG326625 | A | |
| Kánó | AKan | 2011-05-24 | Thorax | HG326626 | A | |
| Sóshartyán | ASos | 2002-05-19 | Head | HG326627 | A | |
| Summer arion (Hungary) | Aggtelek | LAgt | 2002-08-06 | Head | HG326628 | A |
| Szőlőhegy | LSol | 2011-07-14 | Thorax | HG326629 | A | |
| Szin | LSin | 2011-08-02 | Thorax | HG326630 | A | |
| Zabanyik | LZab | 2011-08-03 | Thorax | HG326631 | A | |
| Perkupa | LPer | 2011-08-04 | Thorax | HG326632 | A | |
| Korlát-hegy | LKor | 2011-08-05 | Thorax | HG326633 | A | |
| Kaszonyi hegy | LKah | 2002-07-23 | Head | HG326634 | A | |
| Vérteskozma | LVer | 2002-07-10 | Head | HG326635 | A | |
| Summer arion (Transylvania) | Gyimesbükk | TGim | 2011-07-16 | Thorax | HG326636 | A |
| Gyilkos tó | TGyi | 2002-07-02 | Head | HG326637 | A | |
| Summer arion (Slovenia) | Kamnik | SKam1 | 2003-07-04 | Head | HG326638 | A |
| SKam2 | 2003-07-04 | Head | HG326639 | A | ||
| Polovnik | SPol1 | 2002-07-12 | Head | HG326640 | A | |
| SPol2 | 2002-07-12 | Head | HG326641 | A | ||
| SPol3 | 2003-07-04 | Head | HG326642 | A | ||
| M. teleius | Aggtelek | OTel | 2005-07-29 | Thorax | HG326643 | ? |
| M. nausithous | Kétvölgy | ONau | 2003-08-01 | Head | HG326644 | - |
| M. alcon | Tohonya-hát | OAlc1 | 2011-06-23 | Thorax | HG326645 | B |
| Hochschwab | OAlc2 | 2003-07-11 | Head | HG326646 | B |
- Abbr.: the abbreviations of localities (Fig. 1). See in boldface the couples of ‘spring and summer arion’ from the same locality. Wolbachia supergroup: the type of Wolbachia infection.
Appendix 2: Information on the samples used in the allozyme and morphometric analyses
| Taxa | Locality | Abbr. | Sampling time | N |
|---|---|---|---|---|
| Spring arion (Hungary) | Haragistya | AHar | 2005-06-06/07 | 16 |
| Nagyoldal | ANol | 2003-05-28; 2004-06-09 | 19 | |
| Szin | ASin | 2011-05-25 | 9 | |
| Zabanyik | AZab | 2005-05-30; 2005-06-05/06; 2011-05-24 | 16 | |
| Summer arion (Hungary) | Zabanyik | LZab | 2005-07-28, 29; 2011-07-14 | 9 |
| Aggtelek | LAgt | 2005-07-28/29 | 18 | |
| Kaszonyi hill | LKah | 2003-07-21; 2003-08-05 | 15 | |
| Vérteskozma | LVer | 2002-07-01/03; 2003-07-02/07 | 12 | |
| Summer arion (Transylvania) | Lake Gyilkos | TGyi | 2004-07-24 | 13 |
| Torockószentgyörgy | TTsz | 2004-07-22/23 | 11 | |
| Summer arion (Slovenia) | Polovnik, Kamnik | SPoK | 2002-07-12; 2003-07-04 | 5 |
| M. nausithous (outgroup) | Kétvölgy | ONau | 2004-08-05 | 16 |
| Total | 159 |
- Abbr.: the abbreviations of localities (Fig. 1). See in boldface the pair of samples from the same syntopic population. N: number of individuals.
Appendix 3: Classification results of the canonical variates analysis (CVA)
| Classification resultsa, b | ||||
|---|---|---|---|---|
| Type | Predicted group membership | |||
| 1 | 2 | Total | ||
| (A) Fore-wing | ||||
| Original | ||||
| Count | Spring | 56 | 4 | 60 |
| Summer | 4 | 79 | 83 | |
| % | Spring | 93.3 | 6.7 | 100.0 |
| Summer | 4.8 | 95.2 | 100.0 | |
| Cross-validatedc | ||||
| Count | Spring | 55 | 5 | 60 |
| Summer | 5 | 78 | 83 | |
| % | Spring | 91.7 | 8.3 | 100.0 |
| Summer | 6.0 | 94.0 | 100.0 | |
| Classification resultsd, e | ||||
|---|---|---|---|---|
| Type | Predicted group membership | |||
| 1 | 2 | Total | ||
| (B) Hind-wing | ||||
| Original | ||||
| Count | Spring | 51 | 9 | 60 |
| Summer | 5 | 78 | 83 | |
| % | Spring | 85.0 | 15.0 | 100.0 |
| Summer | 6.0 | 94.0 | 100.0 | |
| Cross-validatedf | ||||
| Count | Spring | 48 | 12 | 60 |
| Summer | 8 | 75 | 83 | |
| % | Spring | 80.0 | 20.0 | 100.0 |
| Summer | 9.6 | 90.4 | 100.0 | |
| Classification resultsg, h | ||||
|---|---|---|---|---|
| Type | Predicted group membership | |||
| 1 | 2 | Total | ||
| (C) Genitalia | ||||
| Original | ||||
| Count | Spring | 53 | 7 | 60 |
| Summer | 13 | 70 | 83 | |
| % | Spring | 88.3 | 11.7 | 100.0 |
| Summer | 15.7 | 84.3 | 100.0 | |
| Cross-validatedi | ||||
| Count | Spring | 53 | 7 | 60 |
| Summer | 14 | 69 | 83 | |
| % | Spring | 88.3 | 11.7 | 100.0 |
| Summer | 16.9 | 83.1 | 100.0 | |
- a 94.4% of original grouped cases correctly classified.
- b 93.0% of cross-validated grouped cases correctly classified.
- c Cross-validation is carried out only for those cases in the analysis. In cross-validation, each case is classified by the functions derived from all cases other than that case.
- d 90.2% of original grouped cases correctly classified.
- e 86.0% of cross-validated grouped cases correctly classified.
- f Cross-validation is carried out only for those cases in the analysis. In cross-validation, each case is classified by the functions derived from all cases other than that case.
- g 86.0% of original grouped cases correctly classified.
- h 85.3% of cross-validated grouped cases correctly classified.
- i Cross-validation is carried out only for those cases in the analysis. In cross-validation, each case is classified by the functions derived from all cases other than that case.




