Woody species have stronger facilitative effects on soil biota than on plants along an aridity gradient
Funding information
National Natural Science Foundation of China [31901140, 31570453]
Abstract
Questions
Woody-shrub encroachment affects community structure and composition. However, most studies focus on their effects on understorey plant communities, and the relative importance of shrubs in affecting plants vs soil biota communities is poorly known.
Location
Inner Mongolian Steppe, China.
Methods
We examined the effect of shrubs on multiple community attributes, including plants, soil biota (bacteria, fungi and nematodes), and soil fertility, and quantified how these effects changed from semi-arid to hyperarid conditions (from 281 mm to 110 mm of mean annual precipitation). In addition, we assessed whether the effects of shrubs on plant communities were directly mediated by biotic filtering in seed germination and establishment, or indirectly mediated by plant biomass or soil fertility in the case of soil organisms.
Results
The effect of shrubs on soil biota was generally more positive than on plants, and it increased with aridity. We found that a larger proportion of below-ground taxa depended on shrub presence (36%) than plants (20%). Soil nematodes and soil bacteria were directly influenced by shrub presence whereas soil fungi were indirectly influenced by enhanced soil fertility. Shrubs also increased plant biomass under all conditions but only increased plant species richness in the most arid conditions. Despite the generally positive effect of shrubs, and the fact that they weakened the filtering effects of aridity on seed germination, aridity was a stronger predictor of changes in species composition than shrub presence was, particularly for plants.
Conclusions
Our results illustrate the variety of positive effects of shrubs and show that they are particularly important in supporting biodiversity in the most arid conditions. These strong and positive effects could partially buffer the impacts of increasing aridity on dryland soil biodiversity, but our study suggests that facilitative interactions may not be able to completely mitigate the impacts of increasing aridity on drylands.
1 INTRODUCTION
Increases in the density or cover of woody plants (woody encroachment or “thickening”) are occurring in grasslands worldwide (Eldridge et al., 2011). Woody plants affect the structure and composition of understorey plant communities and change microclimatic conditions and soil nutrient levels. For example, shrubs increase shading and enhance soil fertility, which can, in turn, enhance understorey plant richness and density (Segoli et al., 2012a; Lu et al., 2018). Due to these positive effects, shrubs can provide microrefugia, allowing more productive, but less stress-tolerant, plant species to occur in more arid conditions than they would otherwise (Bruno et al., 2003; Ballantyne & Pickering, 2015). Indeed, around 25% of plant species in drylands depend on the presence of shrubs (Soliveres & Maestre, 2014). Soil biota, such as nematodes and microbes, may also respond to the presence of shrubs (Jonathan et al., 2016; Ochoa-Hueso et al., 2018; Wang et al., 2020). In fact, the positive effects of shrub canopies on these soil organisms could be even stronger than those found for plants, due to the sensitivity of soil biota to high temperatures and soil desiccation (Blankinship et al., 2011). However, the relative importance of shrubs in affecting understorey plants vs soil biota communities is poorly known, as studies assessing the effects of shrubs on multiple organisms are uncommon.
Shrub effects on understorey communities change with environmental conditions (e.g., Holzapfel et al., 2006). Changes in plant–plant interactions across aridity gradients are related to the balance between facilitation and competition: it has been suggested that facilitation increases with environmental stress (and competition decreases) meaning facilitation should be strongest in the most arid environments (Callaway, 2007; Ploughe et al., 2019). However, extremely harsh environmental conditions can render facilitatory effects insufficient to ensure the survival of understorey plants, leading to a collapse of facilitatory effects under such conditions (Hacker & Gaines, 1997; Michalet et al., 2006; Berdugo et al., 2019). A large body of literature has focused on how plant–plant interactions respond to environmental gradients (see Zhang & Tielbörger, 2020 and Liancourt & Dolezal, 2021 for two of the latest reviews), yet few studies have addressed the same responses for plant–soil interactions (but see David et al., 2020; Wang et al., 2020) and we do not know how the response of soil biota to plant facilitatory effects may vary across environmental gradients. Therefore, it is not clear whether the patterns found for plant–plant interactions across environmental gradients are also found for plant–soil interactions or what mechanisms may drive variation in plant–soil interactions along environmental gradients.
Shrubs may affect plants or other organisms through various mechanisms, including microclimatic amelioration, improved soil conditions, seed filtering, enhanced pollinator visitation, herbivore protection, release of allelochemical compounds, or changes in interactions between understorey species (reviewed in Bruno et al., 2003; Filazzola & Lortie, 2014). Some of these mechanisms are only related to plants (e.g., attracting pollinators), whereas others (e.g., microclimatic amelioration, nutrient enrichment) are likely to benefit soil biota too. A better mechanistic knowledge of the effects of shrubs on their understorey communities, and how they interact with the environment, could improve our predictions regarding the role of facilitation in buffering the impacts of climate change (Brooker, 2006; Butterfield, 2009). However, we currently have a poor understanding of how the mechanisms behind shrub effects on community structure depend on environmental conditions or vary between organisms and ecosystem attributes.
The main aim of our study was to determine how shrubs affect multiple community attributes (richness and biomass of plants, diversity of soil bacteria, fungi and nematodes), and how these effects change with aridity. We endeavored to answer the following questions: (1) how do shrubs affect multiple above- and below-ground communities; (2) how do shrub effects on understorey plant and soil biotic communities vary along an aridity gradient; (3) what are the mechanisms (microclimate, soil, or plant-mediated) by which shrubs affect soil biotic communities; and (4) are shrub effects stronger than those of the environment in determining community composition?
2 METHODS
2.1 Study sites
We conducted our study in the Inner Mongolian Steppe in northern China. This region has a strong aridity gradient from the northeast to the southwest, along which their climates ranging from semi-arid to hyperarid zones (annual mean precipitation ranges from 281 to 110 mm; and the aridity index from 0.17 to 0.03; Table 1). We worked at four study sites: (1) Xilinhot at the wettest end of the arid zone; (2) Siziwang well within the arid zone; (3) Etuoke at the driest end of the arid zone; and (4) Alashanzuo, almost in the hyperarid zone (Table 1). We calculated the Aridity Index (AI = precipitation/potential evapotranspiration; FAO [Food and Agriculture Organization of the United Nations], 1977) for each site. Higher values of the AI correspond to more mesic sites (less arid).
| Site | Longitude (ºE) | Latitude (ºN) | Altitude (m) | Annual mean precipitationa (mm) | Annual mean temperatureb(℃) | Sunshine duration (h/year) | Aridity index (AI) | Moisture types (zones) |
|---|---|---|---|---|---|---|---|---|
| Xilinhaote | 115º55′19" | 44º28′31" | 990 | 281 | 2.35 | 2,932 | 0.174 | Semi-arid |
| Siziwang | 111º53′22" | 41º47′28" | 1,492 | 240 | 3.40 | 3,065 | 0.128 | Arid |
| Etuoke | 107º58′02″ | 39º07′02″ | 1,500 | 210 | 6.40 | 3,050 | 0.070 | “Dry” arid |
| Alashanzuo | 105°41′34" | 38º19′47" | 1561 | 110 | 7.80 | 3,200 | 0.034 | Hyperarid |
- Aridity index (AI) = precipitation/potential evapotranspiration (FAO, 1977).
- a Annual precipitation in 2015: 413 mm in Xilinhaote; 275 mm in Siziwang; 252 mm in Etuoke; 211 mm in Alashanzuo; annual precipitation from January to June in 2016: 114 mm in Xilinhaote; 187 mm in Siziwang; 96 mm in Etuoke; 86 mm in Alashanzuo.
- b Annual mean temperature in 2015: 3.89℃ in Xilinhaote; 4.89℃ in Siziwang; 7.98℃ in Etuoke; 9.57℃ in Alashanzuo; annual mean temperature in 2016: 3.41℃ in Xilinhaote; 4.38℃ in Siziwang; 7.55℃ in Etuoke; 9.50℃ in Alashanzuo.
We studied biotic and soil attributes beneath Caragana stenophylla shrubs and in open areas, in each of these four sites. Caragana stenophylla Pojark (Fabaceae), is a winter-deciduous, spiny shrub with relatively compact, cushion-like canopy. It is the most common and widely distributed shrub species in the Inner Mongolian Steppe with a distribution ranging from semi-arid to hyperarid conditions (Ma et al., 2013; Xie et al., 2015). It is economically valuable for fodder, green manure and honey production and it is important in providing several regulating ecosystem services related to the reduction of wind erosion, sand fixation, and water and soil conservation (Xie et al., 2016).
2.2 Vegetation and seed bank survey
In July 2016, we randomly selected five C. stenophylla shrubs (at a minimum distance of 100 m) within each study site and sampled 50 cm × 50 cm quadrats beneath the shrub canopy (inside the shrub) and more than 2 m from the edge of the shrub canopy (outside the shrub). In each quadrat, we harvested above-ground plant biomass, measured the number of plant individuals and identified all plant species. We dried plant biomass at 60℃ for about 72 h and weighed it to quantify standing above-ground biomass.
We measured the diversity of the soil seed bank using the seedling emergence method. We randomly selected five C. stenophylla shrubs (the same ones as above) and collected soil samples (30 [length] × 30 [width] × 3 cm [depth]) both inside and outside shrub canopies in September 2016 (the end of the growing season). Soil samples were taken to the lab and placed outdoors throughout the winter. Soil was sieved through a 2-mm mesh, then placed on a plastic plate (40 [length] × 28 [width] × 4 cm [depth]) and watered every three days. Seedlings germinated in March 2017. All seedlings were recorded and removed as soon as they could be identified. We stopped the germination trial after 5–6 weeks, when we had not recorded any further seedling emergence for more than one week.
2.3 Soil nutrients, fauna and microbial analyses
In July 2016, we randomly selected three C. stenophylla shrubs and collected soil samples of 300 g at three depths (0–10 cm, 10–20 cm and 30–40 cm) both inside and outside shrub canopies. The roots of C. stenophylla are distributed across these depths, and we chose to analyse the depths separately because soil nutrients and biota responses to environmental changes could vary depending on the soil depth (e.g., Wang et al., 2020a,b; Zhao et al., 2017). We stored soil samples at 4℃ and brought them back to the lab as soon as possible. For each soil sample, at the different depths, we measured soil properties and analyzed nematode and microbial diversity.
We extracted nematodes from approximately 20 g of fresh soil using a modified Baermann method (Ruess, 1995). After 24 hr of extraction, we preserved nematodes in 4% formaldehyde, then counted them and identified them to genus level. Nematode numbers were calculated as density per gram of dry soil.
We assessed the diversity of soil microbial communities using an Illumina Hiseq 2,500 platform at Guangdong Magigene Biotechnology Co., Ltd. Guangzhou, China. Soil DNA was extracted from 0.5 g of defrosted soil samples using the DNA extraction kit following the manufacturer's instructions (Sangon Biotech Co., Ltd. Shanghai, China). 16S rRNA/ITS genes of distinct regions (e.g., bacterial 16S: V3–V4; fungal ITS: ITS2) were polymerase chain reaction (PCR)-amplified with the bacteria-specific primers 338F (5'-ACTCCTACGGGAGGCAGCA-3') and 806R (5'-GGACTACHVGGGTWTCTAAT-3'), and the fungal-specific primers ITS3-F (5'-GCATCGATGAAGAACGCAGC-3') and ITS4-R (5'-TCCTCCGCTTATTGATATGC-3'). We used Trimmomatic (V0.33, http://www.usadellab.org/cms/?page=trimmomatic) for quality filtering. Reads shorter than 50 bp or with quality scores below 20 were removed and then paired reads that overlap more than 10 bp were merged into one sequence according to the overlap between pair-end reads using FLASH (Fast Length Adjustment of SHort reads, V1.2.11, https://ccb.jhu.edu/software/FLASH/). Sequences were assigned to each sample based on their unique barcode and primer using MOTHUR software (V1.35.1, http://www.mothur.org), after which the barcodes and primers were removed and got the effective Clean Tags. Valid sequences without chimeras were subsequently clustered into different OTUs (Operational Taxonomic Units) by using USEARCH software (V10, http://www.drive5.com/usearch/) at a 97% similarity cut-off. The taxonomy of each representative OTU was analysed against the SILVA (16S, https://www.arb-silva.de/) and UNITE (ITS, http://unite.ut.ee/index.php) databases at a default confidence threshold (setting the confidence threshold to the default ≥0.5).
We measured soil organic matter (SOM), nitrate (NO3−), ammonium (NH4+), available phosphorus and available potassium at the three different soil depths. We quantified soil organic matter concentrations using colorimetry, after oxidation with a mixture of potassium dichromate and sulfuric acid. We measured nitrate and ammonium by using a continuous flow analyzer and available phosphorus by using the sodium hydrogen carbonate solution–Mo–Sb spectrophotometric method. We measured available potassium using ammonium acetate extraction flame photometry.
2.4 Data analysis
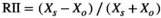
Shrubs can act as environmental filters for understorey plant communities via two different mechanisms: (1) by affecting seed dispersal (capturing different seeds due to wind or animal dispersion beneath the canopy vs outside; Segoli et al., 2012a); or (2) by providing contrasting micro-environmental conditions beneath their canopies (Tewksbury & Lloyd, 2001; Filazzola & Lortie, 2014). To evaluate the effect of these two mechanisms on plant understorey composition, we analyzed the dissimilarity in seed bank composition inside and outside shrub canopies (seed dispersal filter), and the dissimilarity between seed bank and vegetation (inside and outside shrub canopies; micro-environment filter). To do so, we calculated beta diversity using the Jaccard dissimilarity index (JI). JI ranges from 0 to 1, with 0 indicating that the two microsites (inside and outside shrub canopies), or the seed bank and vegetation, have identical species composition and 1 indicating they have no species in common. We used linear mixed models (fitting a linear or quadratic term in the regression) to test for effects of aridity, shrub presence, and their interaction, on all JI, with site as a random effect.
We used Generalized Dissimilarity Modeling (GDM) to evaluate the relative importance of aridity and shrubs in driving the turnover in composition of the different organisms (plants, nematodes, bacteria and fungi). Differences in aridity between sites were used to explain the beta diversity between them. For the shrub effect, two samples were coded as 0 if they both came from below the shrub or both from the open, and as 1 if one sample came from below the shrub and one from the open. GDM is a statistical technique for analyzing turnover in community composition across environmental gradients (Ferrier et al., 2007). GDM uses maximum-likelihood estimation and flexible I-splines to transform each of the predictor variables. The maximum height of each spline indicates the total amount of compositional turnover associated with that variable and therefore corresponds to the relative importance of that variable in explaining beta diversity (in this case the Jaccard dissimilarity index). The shape of the line indicates how the effect of environmental differences (e.g., differences in aridity) on beta diversity changes with the level of the variable (e.g., change along the aridity gradient). We fitted the GDMs with shrub presence and aridity (AI) as predictors using the gdm package in R version 3.3.3 (Fitzpatrick et al., 2013; R Development Core Team, 2017).
We next carried out a series of multiple stepwise regression analyses to infer the underlying mechanisms behind the effects of shrubs on soil nematode, bacterial and fungal diversity. All the models included “site” (four levels) and “open–shrub” pair (12 levels) as random effects. We compared three models to determine the mechanisms behind the effect of shrubs on soil biota: the first testing direct effects, the second indirect effects mediated by plant biomass, and the third indirect effects mediated by soil fertility. The first pathway (model 1: soil biota = shrub presence × aridity) refers to the amelioration of abiotic stress, such as through buffering of temperature extremes, higher water contents and reduced wind speed under the shrub canopies (Segoli et al., 2012a,b; Lu et al., 2018). This is what we considered the direct shrub effect. Shrubs generally enhance the levels of soil nutrients, which can indirectly affect soil biota (Ochoa-Hueso et al., 2018). Shrubs often also increase plant productivity, which can provide more plant detritus or produce more root exudates, resulting in more resources for soil biota (Milchunas & Noy-Meir, 2002). Thus, the remaining two pathways by which the shrub may affect soil biota are those indirectly mediated by soil fertility (model 2: soil biota = (shrub presence ×aridity) + soil fertility), or plant biomass (model 3: soil biota = (shrub presence × aridity) + plant biomass; Figure 1). We performed the multiple regressions in R version 3.3.3 (Graham, 2003). We acknowledge that the relative importance of all these mechanisms could be simultaneously tested via more data-intensive techniques (such as structural equation models). However, our limited sample size prevented us from fitting such models, and we therefore opted for a series of simplified analyses to compare the importance of these different mechanisms (comparing the Akaike information criterion [AIC] for models 1, 2 and 3 for each soil organism).
Finally, in order to compare the potential niche expansion (sensu Bruno et al., 2003) provided by shrubs to different organisms, we used species indicator analyses (Dufrêne & Legendre, 1997). With these analyses, we could identify “shrub- or open-selective” species (i.e., those found more commonly either below a shrub or in the open, respectively) and “non-selective” species (i.e., those with similar abundance below a shrub and in the open). We did so by using the labdsv package in R version 3.3.3 (Roberts, 2007). We chose different Indicator values for plants vs soil because of their different abundances. For plant communities, if the Indicator value (IndVal) index is equal to or bigger than 0.5, the species was classified as a shrub-selective species (Li et al., 2009); if the Indicator value (IndVal) index is smaller than 0.5, the species was classified as a non-selective species. For soil biota, if the Indicator value (IndVal) index is equal to or bigger than 0.7, the species was considered a shrub-selective species (McGeoch et al., 2002); otherwise, the species was considered a non-selective species. Then we calculated the proportion of shrub-selective vs non-selective species, which provided us with an estimation of the proportion of species of each organism group that depends on shrubs to thrive under local environmental conditions.
3 RESULTS
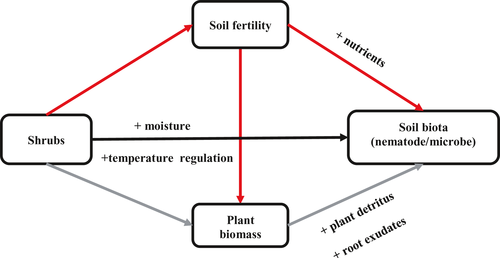
3.1 Effect of shrubs on soil nutrients: the fertile-island effect
The RII indices for soil attributes were above zero in most cases (Figure 2), which indicates that shrubs enhanced soil fertility in general. The RII indices for each soil attribute changed along the aridity gradient, although the shape of this change depended on the nutrient. The RII for P, SOM, and nitrate did not change significantly with aridity, whereas RIIs for potassium and ammonium showed a quadratic relationship with aridity (Figure 2a–e). Shrubs generally increased the soil fertility index, and this effect tended to increase (although not significantly) with aridity (Figure 2f).
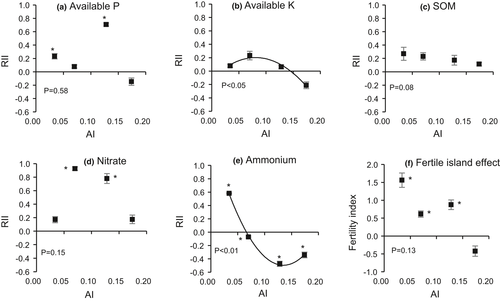
3.2 Effects of shrubs on plant communities
The effect of shrubs on plant biomass was positive across all four aridity levels (Figure 3a). This indicates that C. stenophylla has facilitative effects on plant biomass across most of its range on the Inner Mongolian Plateau. Aridity significantly reduced plant biomass (p < 0.01) and moderated the facilitative effects of shrubs. The RII for plant biomass increased in the range AI = 0.174–0.070 but did not change at higher aridity levels (Figure 3a). In contrast to plant biomass, the effect of shrubs on plant species richness was mostly negative (Figure 3b), except in the hyperarid zone (AI = 0.034).
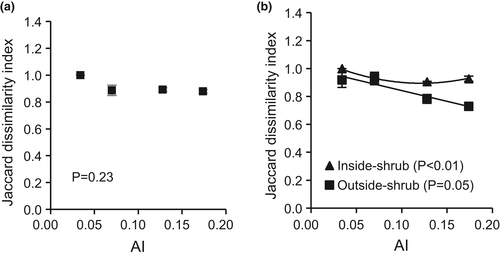
Seed bank composition differed between areas beneath shrub canopies and outside them (Figure 4a). The dissimilarities between the composition of the seed bank and the realized vegetation were also very high in general and were even higher inside shrub canopies than outside them (Figure 4b). The difference between the seed bank and vegetation beneath shrub canopies remained consistent in the range AI = 0.174–0.070 but increased in the hyperarid zone (AI = 0.034, p < 0.01). The difference between the seed bank and vegetation in open areas, instead, increased from AI = 0.174 to 0.07, but slightly decreased in the hyperarid zone (AI = 0.034, p = 0.05). There was a significant interaction between shrubs and aridity regarding the dissimilarity between the composition of the seed bank and the realized vegetation (p < 0.01). This shows that shrubs could weaken the difference between the potential (seed bank) and realized plant communities driven by aridity, and therefore partially buffer the abiotic filter in plant recruitment.
3.3 Effects of shrubs on soil communities
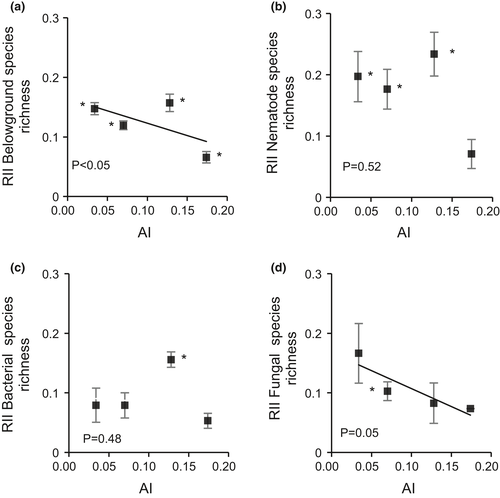
Shrubs increased soil biodiversity (the diversity of bacteria, fungi and nematodes) across all four aridity zones, and these effects were more positive than those found for plant richness (Figure 3b vs Figure 5). Consistent with these results, the proportion of “shrub-selective” species (i.e., those more commonly found under the canopies of shrubs) was higher for below-ground biota than for plant species (Table 2). The changes in RII values across the aridity gradient were similar to those found for plant biomass, with stronger facilitation with increasing aridity (p < 0.05, Figure 5a), indicating that shrubs most strongly increased below-ground diversity in the most arid areas.
| Climatic aridity | Above-ground | Below-ground |
|---|---|---|
| Semi-arid | 25.00% | 28.77% |
| Arid | 16.67% | 40.00% |
| “Dry” arid | 15.38% | 46.65% |
| Hyperarid | 23.08% | 29.42% |
- Shrub-selective species: species found more commonly either below a shrub or in the open, respectively. Non-selective species: species with similar abundance below a shrub and in the open. Proportion of shrub-selective species = [number of shrub-selective species /(number of shrub-selective species + non-selective species)] × 100%.
Moreover, the effect of shrubs on the diversity of soil nematodes, bacteria and fungi across the aridity gradient was different. Shrub effects were positive in all four climatic zones for soil nematodes and bacteria and there were no significant changes across the aridity gradient (Figure 5b, c). In contrast, for fungal diversity, shrub effects were also positive but became weaker with increasing aridity (p = 0.05, Figure 5d).
The multiple regression analysis revealed that the effects on nematode diversity and bacterial diversity were directly driven by shrub presence (Table 3), whereas those on the diversity of soil fungi were driven by soil fertility, after correcting for aridity and shrub presence (Table 3). These results suggest that shrub effects on soil nematodes and bacterial diversity are driven by microclimatic amelioration, but those on soil fungi are driven by soil amelioration. Despite the similar patterns found for the responses of plant biomass and soil biota to shrub presence, plant biomass did not seem to mediate any of the effects of shrubs on soil biota, as plant biomass never had a significant effect on soil biota after correcting for shrubs and soil conditions (Table 3).
| Predictors | Nematode diversity | Bacterial diversity | Fungal diversity |
|---|---|---|---|
| Shrub | 3.255** | 3.261** | 1.143 |
| Aridity | 3.042** | 0.650 | −0.237 |
| Plant biomass | −1.964 | −0.633 | 1.779 |
| Soil condition | −0.439 | −0.801 | 2.760* |
- *, p < 0.05; **, p < 0.01.
3.4 The relative importance of shrubs vs aridity in driving compositional turnover
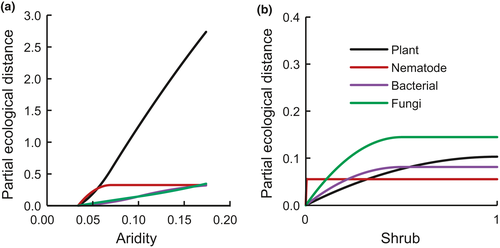
The GDMs consistently showed that aridity had a larger effect on compositional turnover (beta diversity) than shrubs did. The maximum effect of differences in aridity (maximum height of the aridity splines) was higher than the effect of differences between shrubs and open areas, for all organisms (Figure 6). The influence of aridity vs shrubs was particularly strong for plants, and the relative importance of both aridity and shrubs was more balanced for soil organisms, consistent with the larger positive effects found when using the RII scores.
4 DISCUSSION
4.1 Shrub effects on plant communities
We found that the effect of shrubs on both plant species richness and biomass changed across the aridity gradient, although the shape of these responses differed. Shrubs always increased plant biomass and they had the largest positive effect on biomass in the most arid sites. The positive effect of shrubs on plant biomass could, at least partly, be driven by a fertile-island (nutrient enrichment) effect (Figure 2f). Nutrient enrichment has been suggested as one of the most important positive effects of leguminous trees and shrubs on herbaceous biomass, even in drier environments (Ludwig et al., 2004; Mazía et al., 2016). We found more nutrient enrichment under shrub canopies in the hyperarid zone (Figure 2f), which could explain why shrubs increased plant biomass (and richness) most strongly in arid conditions. In addition to atmospheric nitrogen fixation, deciduous nurse plants, such as C. stenophylla, can enhance the absorption of nitrogen by grasses growing beneath them and can increase mineralization of organic matter (Mazía et al., 2016), which in turn increases productivity in understorey plant communities.
In contrast to their effects on biomass, shrubs reduced species richness when AI was between 0.128 and 0.07. Our findings are inconsistent with a study in the Iberian Peninsula, which found that the effect of shrubs on species richness was positive in the arid sites (Armas et al., 2011). The different findings may be due to differences in climatic conditions (AI was 0.3 in Europe), or might indicate that facilitative effects of C. stenophylla differ from those of other shrubs. In the most arid conditions, C. stenophylla has generally larger and more compact cushion-like canopies (Ma et al., 2013). These changes in shrub morphology could have reduced its positive effects on understorey richness, either through an increase in shading, which can reduce plant performance (e.g., Soliveres et al., 2013) and hamper seed germination (Segoli et al., 2012b), or via a stronger competitive effect of compact shrub canopies on understorey plants (Al Hayek et al., 2015). Alternatively, shrubs may have facilitated larger and more productive plant species (Figure 3a) and larger individuals (e.g., the individual biomass of Salsola ruthenica, an abundant and competitive plant in our study region, was 0.58 g beneath the shrub, but 0.09 g in open areas, when AI=0.07; not shown). Larger individuals could mean an increase in overall biomass but also indicate stronger competition amongst facilitated species under shrubs (Bonser & Reader, 1995; Guo & Berry, 1998). However, under hyperarid conditions, shrubs increased plant species richness. This was in line with the previous studies showing that the effect of shrubs on species richness was positive under the most arid conditions (Holzapfel et al., 2006). This effect is partially explained by the stronger soil amelioration by shrubs under these conditions, and an additional mechanism could be that extreme water stress reduces seedling survival, allowing only a few highly drought-adapted species to survive. An increase in water availability under shrubs could relax this extreme environmental filter and result in higher seed germination and seedling survival beneath the shrubs (see also Xie et al., 2015). This would explain why, under hyperarid conditions, we found the highest dissimilarity in species composition between the seed bank and standing vegetation (Figure 4a), and the strongest dissimilarity between open areas and shrub canopies (Figure 4b). Overall, our results suggest that shrubs have generally positive effects on understorey plant communities even under some of the most arid conditions sampled so far, and one of the mechanisms underlying these effects may be by relaxing the strong environmental filter on seed germination and establishment. However, in less extreme conditions shrubs might increase biomass but not species richness, perhaps due to increased competition between facilitated species or due to changes in nurse plant morphology.
However, our results should be interpreted with caution, as we only have four sites along the aridity gradient, making it challenging to robustly detect the non-linear relationships that would characterize facilitation collapse under extreme environments. Indeed, sampling gradients with a low number of points could maximize the chances of finding strong and positive relationships between facilitation and water stress (Soliveres & Maestre, 2014). In addition, our space-by-time approach does not allow us to study how different climatic conditions across years may modulate effects of shrubs on their understorey communities (Butterfield, 2009), or to consider the degree of adaptation of the different species to the local conditions (Metz & Tielbörger, 2016), something that deserves further attention, particularly for shrub effects on soil biota. Further, without manipulative experiments it is not possible to be sure that variation in aridity is the driver of the patterns found. However, manipulative experiments at this scale would be unfeasible and our observational approach is able to identify key differences in the association between shrubs and soil biota and plants along the aridity gradient.
4.2 Shrubs have more positive effects on soil biota than on plant communities
Shrubs had facilitative effects on soil communities, and these effects were bigger than those found for plant richness (Figure 3b vs Figure 5). We found that a higher proportion of soil biota (36%) than plants (20%) were more associated to shrubs than expected by chance. The role of facilitation in enhancing biodiversity has been highlighted before (e.g., Bruno et al., 2003; Brooker, 2006; Soliveres & Maestre, 2014); our study extends these findings to show that the influence of facilitation on soil biota can be even more important than that previously shown for plants (see also Wang et al., 2020). This may be because soil organisms are very sensitive to heat and drought and therefore benefit more strongly from the soil amelioration and shading effects of shrubs (Blankinship et al., 2011). The positive effects on soil organisms were similar to those found for plant biomass and could be due to a shared response to the benign micro-environmental conditions provided by shrubs. Our results indicate that a shared response, between plant biomass and soil organisms, is more likely than an effect of biomass on soil microbes, as plant biomass did not affect soil microbes, after correcting for the effect of shrubs (Table 3).
The positive effect of shrubs on below-ground communities (specifically on fungi) did not collapse even at extreme aridity levels (Figure 5). Thus, contrary to patterns often found in plant–plant interactions (e.g., Berdugo et al., 2019; Michalet et al., 2006), facilitation is less likely to collapse under very dry conditions for plant–soil interactions, perhaps because direct competition is less apparent between plants and soil biota than it is among plants. Interestingly, effects of soil biota on plant performance are also positive, and become stronger under hasher environmental conditions, as shown by a recent study reporting stronger and more positive effects of soil microbes on plant germination with increasing environmental stress (David et al., 2020). Considered together, this evidence suggests that bidirectional interactions (between nurses and their facilitated communities) are positive for plant–soil organisms (plants and soil biotas enhance each other's performance), which is not necessarily the case for plant–plant interactions, which may suffer from trade-offs (facilitated plants competing with their nurses; Kéfi et al., 2008; Schöb et al., 2014).
What mechanisms underlie interactions between shrubs and soil biota? One possible mechanism may be that aridity increased the fertile- island effect (Figure 2f) and this increase in fertility increased soil biodiversity, leading to the strongest facilitation in the most arid environmental conditions. Our series of multiple regressions showed that this soil fertility-mediated effect is the main mechanism behind the positive effects of shrubs on soil fungi. A recent global analysis recently showed a maximum fertile-island effect under semi-arid conditions rather than under the most arid ones (Ochoa-Hueso et al., 2018). These results contrast with the patterns we found, which could be explained by the lack of hyperarid sites in the previous study, or a species-specific facilitatory effect of our studied shrub. Regardless of the reasons, one would expect that shrub facilitatory effects on soil fungi peak at the same point as their effects on soil fertility. Indeed, the diversity of soil fungi, not bacteria, was associated with stronger soil fertility island effects in our case (r2 = 0.42; p < 0.05; see also She et al., 2018). For soil bacteria, direct effects of shrubs were the main mechanism leading to positive effects. This suggests that, besides soil fertility, other factors offered by shrubs, such as soil water content or pH, might influence soil bacteria communities. We found the diversity of soil bacteria (r2 = 0.43; p < 0.05), not fungi (r2 = 0.13; p = 0.55), was associated with soil water content in our case. And previous reports also showed that bacterial communities would be more strongly influenced by pH than fungal communities (Rousk et al., 2010). Collectively, these results highlight that fungi and bacteria are affected in different ways by the presence of shrubs.
We found that soil nematodes were also directly affected by the micro-environment offered by the shrubs. Nematodes are sensitive to precipitation (Chen et al., 2015) and shrubs could intercept water from surface run-off after rainfall events, leading to the higher soil water content beneath the shrubs (e.g., the soil water content was 6.8% beneath the shrub, but 4.9% in open areas; data from AI = 0.128, not shown). In the desert, subjected to low precipitation, water availability was the primary limiting factor. Thus, the direct effects of shrubs are likely to be mediated via their effects on water availability, and these are more important than changes in nutrients or plant biomass in affecting nematodes. These results align with studies showing that abiotic factors (precipitation) explained more of the variation in soil nematode communities than biotic factors (plant biomass and species richness; Chen et al., 2015). In addition, shrubs are likely to have affected the key nematode feeding groups. Shrubs can provide C-rich root exudates and litter inputs, both of which are known to stimulate fungal activity (Kaiser et al., 2015) and may therefore indirectly provide food for fungal-feeder nematodes. This suggests that plants could indirectly influence nematode communities through microbial communities (Wang et al., 2019). In support of this, we found that the diversity of soil nematodes was associated with soil microbes (r2 = 0.55; p < 0.01). Considered together, this might suggest that soil fungal communities were strongly controlled via soil fertility, and that the nematode community was strongly controlled via food resources.
As a whole, shrubs generally facilitated above-ground plant biomass and this increase was mainly associated with the increase in soil fertility. This also corresponds to changes in microbial communities caused by soil fertility, and has knock-on effects on the nematode communities. On the longer term, due to the ubiquitous presence of shrubs, the effects of shrubs via changes to the plant community will play an important role in reshaping the microbial communities, with potentially important consequences for nematode communities. Alterations to these processes may in turn feed back to plant community composition.
4.3 Relative importance of shrub presence vs aridity as drivers of the composition of dryland species
Numerous studies in deserts have shown positive interactions between shrubs and plant communities (Filazzola & Lortie, 2014). Such facilitation can potentially buffer desert communities against current and future environmental change, which may include increased aridity (Lucero et al., 2020). Accordingly, positive interactions mediated by shrubs have been invoked as an insurance that may sustain biodiversity under the harsher conditions expected in the future (Brooker, 2006; Cavieres et al., 2016). In addition to plants, we showed that this “insurance effect” could extend to soil nutrients, seed bank composition and the diversity of soil organisms. But were these positive effects enough to override the influence of aridity on the species composition of dryland communities? Our results showed that, although shrubs strongly influenced community composition, aridity had an even stronger effect on species turnover for most taxa (plants, soil nematodes and soil microbes). This highlights the sensitivity of dryland environments to the forecasted increases in aridity and suggests that nurse plants will not be able to fully buffer these impacts, particularly for plants (see also Berdugo et al., 2017).
5 CONCLUSIONS
Our study examined the effect of shrubs on the composition and diversity of plant and soil communities. We found that shrubs have more positive effects on soil than plant communities, and that these effects are less likely to collapse under very arid conditions. The effects of shrubs, and their interactions with environmental conditions, are highly dependent on different plant attributes (biomass vs richness) and the different groups of soil biota (nematode vs microbial communities). Soil bacteria and nematodes were directly influenced by shrub presence, whereas soil fungi were indirectly influenced by enhanced soil fertility. We also showed that shrubs could influence community structure through a biotic filtering effect (both at the seed and adult stages), but that these effects were not strong enough to override the influence of increasing aridity, especially for plants. Our study extends our knowledge of the facilitatory effects of shrubs to soil organisms and provides the basis for more extensive studies to address the generality of our results and the potential mechanisms that may be operating. It also provides a comprehensive view of the potential role of nurse plants in partially buffering climate change effects on dryland ecosystems.
ACKNOWLEDGMENTS
We thank Xilinhot Grassland Management office, Siziwang Grassland Management office, Etuoke Grassland Management office and Alashanzuo Grassland Management office for their logistical support to this study. This research was funded by the National Natural Science Foundation of China [31901140, 31570453] and the China Scholarship Council. SS was supported by the Spanish Government under a Ramón y Cajal contract (RYC-2016- 20604).
AUTHOR CONTRIBUTIONS
XLN, ZGG and MCC designed the experiment. XLN, MXF, LY, WYT and MCC carried out the experiment. ML identified plant species at species level. XLN analyzed the data with insight from SS and EA. XLN, SS and EA wrote the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available from “Figshare” at http://doi.org/10.6084/m9.figshare.14431175




