Is Rifampin (Rifampicin) Essential for the Treatment of Rhodococcus equi Infections in Foals? A Critical Review of the Role of Rifampin
ABSTRACT
Rifampin is an enigma among antimicrobials. Blood and tissue compartment concentrations are a “moving target” along the treatment course due to the complex pharmacodynamic interactions within the body. Rifampin concomitant therapies are for the prevention and treatment of Rhodococcus equi infection in foals, for nearly 40 years. The necessity of rifampin concomitant therapies is based on beliefs that both antimicrobials (e.g., rifampin plus macrolide) penetrate into pulmonary abscesses and intracellular compartments above R. equi minimum inhibitory concentrations (MICs), as well as better efficacy, compared with other approaches, and limiting the rate of antimicrobial resistance to either single agent. However, rifampin acts as a perpetrator drug for many co-administered drugs. This critical review evaluates the available evidence for rifampin use in foals with R. equi, concerning pharmacokinetic/pharmacodynamic characteristics of rifampin in foals, in vitro microbiological studies and selection of antimicrobial resistance, as well as an analysis of randomized clinical trials. Rifampin is a nuclear pregnane X receptor activator, which results in strong negative drug interactions towards itself and other drugs, for drug-absorption routes either by upregulation of presystemic elimination mechanisms (e.g., intestinal and hepatic CYP3A4), or functional drug-absorption carriers (e.g., intestinal P-glycoprotein) and/or inhibition of intestinal and/or hepatic drug-uptake carriers (e.g., OATP1B1, OATP2B1, MRP2). Chronic rifampin administration results in decreases in the serum and target site/s concentrations of many parent drugs, including itself. Rifampin concomitant therapies do not demonstrate a significant advantage over monotherapy with macrolides, in randomized controlled blinded and double-blinded clinical trials for subclinical, and mild-to-moderate bronchopneumonia in foals with pulmonary abscesses, regardless of initial pulmonary abscess score. Efficacy of rifampin concomitant therapies for severe Rhodococcus equi pneumonia has not been fully investigated, but there is sufficient accumulated evidence in foals to raise major concerns about the incorrect use of rifampin in equine medicine. These concerns include rifampin as a bacteriostatic antibiotic against R. equi, with changing pharmacokinetics during treatment that decreases parent/coparent concentrations as well as the risk of selecting for multi-resistant R. equi.
1 Introduction
Rifampin is part of the rifamycin group of antimicrobials and further part of the ansamycin macrocyclic antibiotic class, which also includes streptovaricins and geldanamycin. There are seven molecules known as rifamycins, each characterized by different letters based on their chemical structure: rifamycin A, B, C, D, E, S, and SV, all isolated from the fermentation culture of the bacterium A. mediterranei. Rifampin is a semi-synthetic compound derived from rifamycin B. Another type is rifamycin sodium corresponding to the sodium salt of rifamycin SV, obtained through a chemical transformation of rifamycin B. The differences between rifampin and rifamycin sodium mainly concerns bioavailability, the later having poor systemic bioavailability and thus typically used topically (e.g., ophthalmology).
The name rifampin is used in North America, whereas it is called rifampicin in Europe and Australia. The structure of rifampin comprises two aromatic rings (containing a quinone), connected by a long chain, which confers a rigid character to the whole molecule. Depending on the bacteria, rifampin (rifampicin) is a broad-spectrum, concentration- or time-dependent, bactericidal and/or bacteriostatic antibiotic, with activity mainly against mycobacteria, Gram-positive, and facultative anaerobic organisms. For example, rifampin demonstrates bactericidal, concentration-dependent activity against Mycobacterium tuberculosis; whereas against R. equi, rifampin exhibits time-dependent, bacteriostatic activity (Giguere et al. 2012).
Rifamycins are classified by the World Health Organization as critically important antimicrobials for human medicine as part of the first-line treatment of mycobacterial infections (tuberculosis, leprosy and mycobacterium avium complex—MAC). Other clinical uses for rifamycins in human medicine include treatment of infections from Rhodococcus equi, invasive methicillin-resistant Staphylococcus aureus (MRSA), meningitis (Neisseria meningitidis or Haemophilus influenzae), and other pathogens.
Rifampin exerts antimicrobial effects by inhibiting DNA-dependent RNA polymerase (RNAP). Rifampin can inhibit RNAP at low concentrations (approx. 0.01 μg/mL) (Frank 1990). This inhibition occurs either by sterically obstructing the path of the elongating RNA at its 5′ end or by reducing the RNAP's affinity for short RNA transcripts. Rifampin enters bacteria and forms stable complexes with the β-subunit of bacterial RNAP. Rifampin uniquely targets microbial RNAP, effectively arresting ongoing RNA synthesis by preventing chain initiation. Rifampin penetrates the outer membrane of Gram-positive bacteria more easily than Gram-negative bacteria, as reflected by the lower MICs observed in Gram-positive compared with Gram-negative bacteria (Frank 1990). Resistance to rifamycins develops quickly by mutations, and therefore, rifampin is traditionally used concomitantly with other antimicrobials.
In veterinary medicine, all rifampin use is extra-label (off-label) with the likely highest consumption for the prevention and treatment of Rhodococcus equi infections in foals (also known as Prescottella equi, and Rhodococcus hoagie or Rattles). R. equi is a Gram-positive, facultative intracellular bacterium commonly found within the environment and the gastrointestinal tract of horses. R. equi infection in both foals and people is commonly characterized as a multifocal pyogranulomatous pneumonia, but extrapulmonary infections are also common (Giguère et al. 2017). Foals are typically infected from birth, whereby the insidious onset of bronchopneumonia does not result in the onset of clinical signs until between 1 and 5 months of age. Control of foal rhodococcosis is challenging due to the lack of an effective vaccine and relies on antimicrobial therapy and other control measures. Treatments are not only applied to clinically affected animals but also to prevent outbreaks (metaphylaxis) and presumptive cases identified by thoracic ultrasonography (Venner, Astheimer, et al. 2013). Treatments for Rhodococcus equi infections are prolonged, with several weeks to months required to achieve complete recovery (Giguère et al. 2017). The ability of R. equi to survive and replicate within macrophages is the basis for its pathogenicity, whereby strains unable to replicate intracellularly are typically avirulent for foals (Giguère et al. 2017). Although R. equi is susceptible to a variety of antimicrobials in vitro, many drugs are ineffective in vivo because of poor cellular uptake by lung alveolar macrophages and resulting low intracellular concentrations.
Since the introduction of macrolide–rifampin concomitant therapies in the late 1980s, foal mortality from R. equi was purported to be reduced. The commonly quoted reference is from Hillidge (1987) where survival rates increased from 20% to nearly 90%. This formed the basis for the traditional practice of macrolide–rifampin concomitant therapies for R. equi foals; however, there has not been a critical evaluation of the need for rifampin therapy for R. equi.
The objective of this review is to summarize the pharmacokinetic/pharmacodynamic (PK/PD) characteristics of rifampin in foals as well as critically appraise the need for rifampin treatment of R. equi in foals based on three types of studies: in vivo PK/PD studies, in vitro microbiological studies, and selection of antimicrobial resistance, as well as an analysis of randomized clinical trials.
2 Methods
- –
Description of randomization method, if provided.
- –
Specification if blinding or double blinding was part of the study design.
- –
Disease definition as inclusion criteria for the study.
- –
Mean abscess score and abscess number for control and treatment groups.
- –
Antimicrobial/s given to the treatment group (type, route of administration, dose).
- –
Observation period (days) of RCTs.
- –
Total number of foals in control and treatment groups.
- –
Number of foals that recovered without a change in antimicrobial therapy.
- –
Mean number of therapy days of foals that recovered without a change in antimicrobial therapy.
The PRISMA guideline was considered for the analysis of RCTs. Only RCTs including a placebo group were utilized for the analysis. The analysis of the treatment cure data (outcome measure) was performed with a random-effects model (R package “metafor” 1.9–8) (Viechtbauer 2010) using DerSimonian-Laird estimator as the method for estimation of τ2 (the true between-study variance). Heterogeneity between studies was identified by the I2 and τ2 statistics. Due to the heterogeneity between studies, the summary effect measure (in casu relative risk-RR) was calculated using random-effects meta-analysis with inverse-variance weights. RCTs treatment cure data were also assessed using the absolute risk differences between treatment and placebo groups.
Potential risk factors (e.g., treatment group, mean abscess score, therapy days) affecting the outcome measure were further analyzed with a mixed-effects meta-regression model. Interactions between relevant risk factors were further explored with the model.
3 Pharmacokinetics (PK)
Rifampin disposition is exceedingly complex and its pharmacokinetics differ markedly between species (e.g., humans, dogs, horses). Early PK studies of rifampin in horses and foals were mainly carried out as single-dose studies, without accounting for hepatic or gastrointestinal enzyme induction, age, breed, or sex effects. Those studies used relatively insensitive methodology (i.e., microbiological assays) and did not evaluate concomitant treatment with macrolides (Burrows et al. 1985; Burrows, MacAllister, Ewing, Stair, and Tripp 1992; Wilson et al. 1988). Modern study designs and analytical methods have provided a greater understanding of rifampin PK/PD at target sites of infection as well as drug–drug interactions (DDIs) with co-administered antimicrobials. This has led to a re-evaluation of rifampin given to foals in chronic dosage regimens and with concomitant macrolides.
Orally administered rifampin has moderate-to-low bioavailability in horses and foals, with studies reporting values ranging from 39.5% to 68% (Kohn et al. 1993; Wilson et al. 1988; Burrows et al. 1985). Concurrent feeding can significantly reduce the rate and extent of rifampin absorption, potentially delaying the time to reach peak concentrations (Burrows, MacAllister, Ewing, Stair, and Tripp 1992).
Rifampin is lipid soluble, resulting in moderate to high values of volume of distribution (Vd) in adult horses (0.64–0.93 L/kg) (Burrows et al. 1985; Wilson et al. 1988; Kohn et al. 1993) and foals (0.85 L/kg) (Berlin et al. 2017). A single-dose oral study in adult horses showed rifampin mean maximal plasma concentrations (Cmax) of 3.86 μg/mL (range: 2.4–5.86) (Kohn et al. 1993), and a repeated oral dosing study for 10 days in foals resulted in a Cmax of 5.50 ± 1.54 μg/mL (Berlin et al. 2017), which is low compared to other species (humans, dogs) due to low bioavailability in horses (Frank 1990). From a repeated-dose study in foals, increasing the oral dose from 10 mg/kg to 20 mg/kg resulted in a dose proportional increase in mean area-under-the-curve (AUC0_24h: 72.3 ± 21.0 vs. 161 ± 18.4 μg × h/mL) (Berlin et al. 2017).
Rifampin clearance is a complex process involving both hepatic and renal pathways, with hepatic metabolism and bile excretion as the predominant route. The clearance rate is influenced by several factors, including age, liver function, and DDIs. The hepatic extraction ratio of rifampin is generally considered to be low, meaning that a relatively small proportion of the drug is removed from the blood as it passes through the liver. Total body clearance in adult horses is between 1.14 mL/min/kg (range: 1.02–1.19) (Kohn et al. 1993) and 1.34 ± 0.22 mL/min/kg (Burrows et al. 1985), and 0.87 ± 0.07 mL/min/kg in foals (Berlin et al. 2017).
Rifampin binds to the nuclear pregnane X receptor (PXR) and the constitutive androstane receptor (CAR). PXR and CAR in turn activate a set of target genes, including Phase I enzymes such as CYP2B6, CYP2C8, CYP2C9, and CYP3A (analogue in horses: CYP3A89), Phase II enzymes including UDP-glucuronosyltransferases (UGTs), glutathione-S transferases (GSTs), and the Phase III detoxification systems, including efflux carriers of the ATP-binding cassette (ABC) transporter family, including P-glycoprotein (ABCB1) and multidrug resistance-associated protein 2 (MRP2 or ABCC2) transporters, and uptake carriers including the organic anion transporters (OAT) (Chang et al. 2017) (Figure 1). Rifampin can induce the metabolic enzymes and transport proteins involved in its own disposition (auto-induction). Rifampicin-mediated PXR induction processes are both time- and concentration-dependent, which may explain differences in the extent of induction in different target tissues (e.g., enterocytes, epithelial lining fluid, bronchoalveolar lavage cells) in foals (Peters et al. 2011, 2012). Significant enzyme induction can occur by 2.5 days of therapy (Peters et al. 2012) and once induction occurs, the increase in enzyme activity may last for more than 2 weeks after discontinuation of treatment (Burrows, MacAllister, Ewing, Stair and Burrows 1992). Currently, none of the published rifampin PK studies in adult horses and foals that employed a cross-over design included appropriate washout periods. Therefore, these studies do not sufficiently account for prolonged intestinal and hepatic enzyme induction (Reitman et al. 2011), rendering such studies clinically irrelevant.
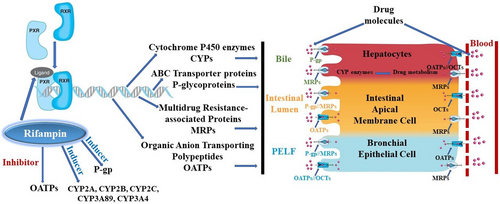
 ) and drug-uptake carriers (
) and drug-uptake carriers ( ) found primarily in drug-eliminating organs, including the kidney, liver, brain, intestinal lumen, testes, adrenal gland, pregnant uterus, tumour cells, and epithelial cells. CYPs, cytochrome P450 enzymes; MRPs, multidrug resistance-associated proteins; OATPs, organic anion-transporting polypeptides; OCTs, organic cation transporter; PELF, pulmonary epithelial lining fluid; P-gp, P-glycoproteins.
) found primarily in drug-eliminating organs, including the kidney, liver, brain, intestinal lumen, testes, adrenal gland, pregnant uterus, tumour cells, and epithelial cells. CYPs, cytochrome P450 enzymes; MRPs, multidrug resistance-associated proteins; OATPs, organic anion-transporting polypeptides; OCTs, organic cation transporter; PELF, pulmonary epithelial lining fluid; P-gp, P-glycoproteins.Hepatic enzyme auto-induction from multiple rifampin doses results in progressively lower peak serum rifampin concentrations and a decreased half-life (t½) (Frank 1990). In adult horses, a multidose, fasted study found the mean t½ of rifampin decreased significantly from 13.3 h (range: 10.5–26.0) after a single oral dose to 7.99 h (range: 6.98–9.32) after the seventh oral dose (Kohn et al. 1993). A repeated-dose oral study in foals found a t½ of 6.79 ± 0.70 h with a dose of 10 mg/kg and 7.61 ± 0.82 h with a dose of 20 mg/kg (Berlin et al. 2017). Also, Berlin et al. (2016) showed in foals that CYP3A4 induction increased more than 10-fold under the influence of rifampin. Furthermore, systemic exposure of rifampin was decreased by ~60% after chronic oral treatment (Berlin et al. 2017), due to upregulation of presystemic (hepatic first pass) and systemic elimination (Berlin et al. 2018).
Due to the maturation of metabolism and elimination processes, age-related changes in rifampin PK parameters have been identified in foals. In one study, six foals were followed over time, with single doses of oral rifampin (10 mg/kg), given at 1, 2, 4, 6, and 10 weeks. With increasing age, there were significant increases in area-under-the-curve (AUC) and decreases in the elimination rate in foals at 1, 2, 4, and 6 weeks of age as compared with 10 weeks, as well as adult horses (Burrows, MacAllister, Ewing, Stair, and Tripp 1992). These age-related changes make it difficult for cross-over studies to accurately determine PK parameters in growing foals. In people, polymorphisms in rifampin metabolism and elimination have been demonstrated, yet equine studies routinely use horses or foals all of one breed, so it is not known if breed-associated polymorphisms exist in horses.
Over the course of concomitant treatments (e.g., rifampin and clarithromycin) there are elevated concentrations of metabolites of rifampin (e.g., 25-O-desacetylrifampin) and macrolide metabolites (e.g., 14-hydroxyclarithromycin), which are bioactive. Desacetylrifampin has antibacterial properties, but fewer than the parent drug. For example, desacetylrifampin has approximately 20% of rifampicin's activity against M. tuberculosis (Donald et al. 2011). After short-term rifampin administration in foals, Peters et al. (2012) found that desacetylrifampin accumulated 1.4- to 6-fold in pulmonary epithelial lining fluid (PELF) and 8- to 60-fold in bronchoalveolar (BAL) cells, accounting for approximately 2% of the total drug exposure in PELF but 25% in BAL cells (Table 1) (Peters et al. 2012). The dilemma is that there is no information in terms of MICs for these metabolites against R. equi, and thus it is unclear if rifampin and macrolide metabolites contribute to clinical efficacy. Also, desacetylrifampin is more polar than rifampin and thus less likely to penetrate pulmonary abscesses and alveolar macrophages.
| References | Peters et al. (2012) | Womble et al. (2006) | Berlin et al. (2017) | Berlin et al. (2017) | Peters et al. (2011) | Berlin et al. (2016) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
5 administrations (BID PO), either:
|
Monotherapy (clarithromycin: 7.5 mg/kg BID PO, 3 days) | Monotherapy (rifampin: 10 mg/kg SID PO, 10 days) | Monotherapy (rifampin: 20 mg/kg SID PO, 10 days) | Monotherapy (clarithromycin: 7.5 mg/kg BID PO, 7 days) followed by rifampin co-medication (10 mg/kg BID PO, 11 days) | Monotherapy (clarithromycin: 7.5 mg/kg BID PO, 5 days) followed by rifampin co-medication (10 mg/kg BID PO, 13 days) | |||||||
| PELF | BAL cells | PELF | BAL cells | PELF | BAL cells | PELF | BAL cells | PELF | BAL cells | PELF | BAL cells | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
μg/mL Mean ± S.D. |
μg/mL Mean ± S.D. |
μg/mL Mean ± S.D. |
μg/mL Mean ± S.D. |
μg/mL Mean ± S.D. |
μg/mL Mean ± S.D. |
|||||||
| Clarithromycin | ||||||||||||
| Without Rifampin | 9.95 ± 19.9 | 116 ± 137 | 21.36 ± 20.53 | 117.27 ± 107.84 | 9.49 ± 6.12 | 264 ± 375 | 2.77 ± 2.50 | 47.0 ± 26.7 | ||||
| With Rifampin | 3.34 ± 5.54 | 24.8 ± 29.0 | 0.69 ± 0.66 | 10.2 ± 10.2 | 0.07 ± 0.05 | 0.75 ± 0.85 | ||||||
| 14-Hydroxyclarithromycin | ||||||||||||
| Without Rifampin | 0.16 ± 0.23 | 1.90 ± 1.17 | 0.41 ± 0.20 | 4.73 ± 5.49 | 16.9 ± 7.68 | 301 ± 137 | ||||||
| With Rifampin | 0.08 ± 0.10 | 1.15 ± 0.78 | 0.13 ± 0.08 | 1.71 ± 1.22 | 9.27 ± 4.97 | 39.3 ± 21.5 | ||||||
| Rifampin | ||||||||||||
| Without Clarithromycin | 8.49 ± 4.10 | 4.91 ± 1.04 | 1.01 ± 0.20 | 1.25 ± 0.29 | 2.71 ± 1.25 | 3.09 ± 1.63 | ||||||
| With Clarithromycin | 8.12 ± 5.86 | 6.65 ± 3.66 | 3.12 ± 1.47 | 2.10 ± 0.85 | ||||||||
| 25-O-Desacetyl-Rifampin | ||||||||||||
| Without Clarithromycin | 0.19 ± 0.10 | 1.44 ± 0.62 | 0.03 ± 0.01 | 0.22 ± 0.06 | 0.06 ± 0.03 | 0.53 ± 0.16 | ||||||
| With Clarithromycin | 0.18 ± 0.07 | 1.73 ± 1.42 | 0.09 ± 0.04 | 0.38 ± 0.20 | ||||||||
- Abbreviations: BAL cells, bronchoalveolar cells (e.g., epithelial cells, alveolar macrophages); BID, twice daily administration; PELF, pulmonary epithelial lining fluid; PO, oral route of administration; S.D., standard deviation; SID, once daily administration.
Deacetylated and parent rifampin are excreted primarily in bile with only a small amount eliminated in urine. Parent rifampin undergoes enterohepatic circulation, but desacetylrifampin is poorly absorbed (Street and Korman 2010). Rifampin is passively filtered through the kidneys. In adult horses, Kohn et al. (1993) found the mean urinary recoveries of rifampin and desacetylrifampin were 6.25% ± 1.96% and 0.57% ± 0.22% of the total IV dose of rifampin, respectively. The rate of elimination increases with repeated doses and metabolizing enzyme induction.
4 Pharmacodynamics (PD) (The Paradox of Rifampin co-Treatments)
Although in vitro evidence suggests rifampin is synergistic with other antimicrobials, in vivo evidence reveals the opposite, where rifampin exhibits complex interactions with other drugs. The confusing aspect of the complex interactions between rifampin and macrolides is the paradox that within the gastrointestinal tract, the actions of rifampin are strongly inhibitory to macrolide oral absorption (bioavailability), but in the case of pulmonary penetration, rifampin may be complimentary for macrolide uptake.
The efficacy of antimicrobials for R. equi treatment is dependent on active unbound concentrations at the target site (lungs), and exceeding the MICs for R. equi within the environment of the respective bacteria (i.e., pulmonary abscesses, bronchial, and alveolar epithelial cells, PELF, and BAL cells). The cell membranes of lung tissue also possess uptake carriers of the organic anion-transporting polypeptide (OATP), organic cation transporter (OCT), and peptide transporter families and with efflux carriers of the ATP-binding cassette (ABC) family (Bosquillon 2010). Both P-glycoproteins (ABCB1 and ABCC2) are expressed in the apical membrane of the epithelial and BAL cells, and both may mediate active efflux of their substrates into the PELF and the environment of macrophages, respectively (Bosquillon 2010). Both OATP1A2 and OATP2B1 are known in horses, whereby OATP1A2 is expressed on epithelial cells and OATP2B1 is expressed on both epithelial and BAL cells, and regulated by rifampin (Venner et al. 2010; Peters et al. 2011).
Specifically, rifampin is a potent inducer of intestinal P-glycoproteins as well as an inhibitor of intestinal and hepatic OATPs (Tamai and Nakanishi 2013). In foals, rifampin induces mRNA expression of the ABC intestinal uptake transporter ABCB1 coding for P-glycoprotein (membrane-bound drug efflux pump), as well as CYP3A89 and CYP3A4 in primary equine hepatocytes (Berlin et al. 2016). Inducing intestinal P-glycoproteins results in certain drug substrates (e.g., digoxin, macrolides) being pumped from apical duodenal cells back into the lumen of the intestine and thus not being absorbed into the body (Greiner et al. 1999; Togami et al. 2012).
In foals, the most studied in vivo interactions between rifampin and macrolide co-treatments involve clarithromycin. It has been shown that concomitant treatment with rifampin (short and long-term) leads to a lowering of the average steady-state plasma concentrations of clarithromycin by up to > 90% with subsequent lower and variable concentrations within pulmonary epithelial lining fluid (PELF) and bronchoalveolar (BAL) cells (Table 1) (Peters et al. 2011, 2012; Berlin et al. 2016). The very low clarithromycin plasma and tissue concentrations with concomitant rifampin treatment were the consequence of significant reductions in clarithromycin oral bioavailability, since the plasma elimination half-lives of each drug remained unchanged (Peters et al. 2011). Regardless of whether the two drugs were given together (concomitant—at the same time) or separated in time (consecutive), clarithromycin plasma and tissue concentrations fell below the MIC90 of 0.5 μg/mL for R. equi (Peters et al. 2012; Berlin et al. 2016). Decreases in macrolide oral bioavailability with rifampin co-administration have also been shown in rats (azithromycin or clarithromycin bioavailability were reduced 65% and 45%, respectively, when co-administered with rifampin), with a reduction of 45% (Garver et al. 2008) and 30%–40% in humans (Taki et al. 2007).
The drug interactions between rifampin and clarithromycin are further complicated by the fact that macrolides have some inhibitory effects on rifampin absorption and metabolism (Berlin et al. 2016). Macrolide antimicrobials inhibit uptake transporters (e.g., OATP1A2, OATP1B1, OATP1B3, OAT2) (Seithel et al. 2007), efflux carriers (e.g., P-glycoprotein), and drug-metabolizing enzymes (e.g., CYP3A) (Quinney et al. 2013) and, therefore, can influence the pharmacokinetics of rifampin (Berlin et al. 2017). Of note, rifampin is an OATP1B1 substrate whereas clarithromycin is an inhibitor (Berlin et al. 2016). Furthermore, all macrolides exert pro-kinetic effects on gastric emptying, which can influence the rate of absorption and apparent half-life of rifampin. Concomitant use with clarithromycin leads to reduced rifampin exposure (AUC) and the metabolic ratio (AUCDAc-RIF/(AUCRIF + AUCDAc-RIF)), versus consecutive clarithromycin (4 h apart from rifampin) leading to prolongation of half-life, increased systemic exposure, and better pulmonary penetration of rifampin (Berlin et al. 2016, 2017).
Other macrolide/azalide formulations are available in veterinary medicine for intramuscular and subcutaneous administration (tulathromycin, gamithromycin), thus avoiding gastrointestinal interactions with rifampin, and have been investigated in foals in combination with rifampin. Combination of gamithromycin with rifampin resulted in significantly increased plasma exposure of gamithromycin (AUC of 16.2 ± 4.77 vs. 8.57 ± 3.10 μg × h/mL), due to rifampin inhibition of hepatic uptake and biliary excretion leading to a reduction in the total body clearance of gamithromycin (Berlin et al. 2018). However, gamithromycin significantly lowered rifampin plasma exposure (AUC0–168 h) of single (~ 50% reduction) and multiple-dose (~ 30% reduction) rifampin (83.8 ± 35.3 and 112 ± 43.1 vs. 164 ± 96.7 μg × h/mL), without a change in metabolic ratio and half-life (Berlin et al. 2018). Multiple doses of gamithromycin reduced plasma concentrations of rifampin by lowering intestinal absorption of rifampin by inhibition of an as yet unknown uptake mechanism.
Combination of rifampin with tulathromycin in horses resulted in significantly decreased AUC of tulathromycin (18.5 ± 4.0 vs. 24.4 ± 3.7 μg × h/mL), including both lowered plasma concentrations (24 h, 0.17 ± 0.05 vs. 0.24 ± 0.05 μg/mL; 192 h, 0.05 ± 0.01 vs. 0.06 ± 0.01 μg/mL) as well as BAL cell concentrations (24 h, 0.84 ± 0.36 vs. 1.56 ± 1.02 μg/mL; 192 h, 0.60 ± 0.23 vs. 1.23 ± 0.90 μg/mL), compared with the respective tulathromycin concentrations from monotherapy (Venner et al. 2010). Rifampin concentrations were not measured to investigate whether tulathromycin concomitantly affected rifampin plasma and lung concentrations, but such an interaction is possible given the above-mentioned negative drug interactions between rifampin–clarithromycin and rifampin–gamithromycin.
While specific equine clinical studies have not examined drug interactions between rifampin and erythromycin or azithromycin, erythromycin has high affinity for CPY3A4, is a substrate of p-glycoprotein, and thus likely a similar risk of negative drug interactions when administered concurrently with rifampin. Azithromycin has a weak affinity for CYP34A, but is both a substrate of p-glycoproteins and MRP2.
Paradoxically, a higher distribution of clarithromycin into PELF has been found, despite markedly lower plasma concentrations, after rifampin concomitant therapy, with no change in total body clearance (Peters et al. 2011; Berlin et al. 2016). For example, with rifampin co-administration, clarithromycin, and 14-hydroxyclarithromycin accumulated approximately 20- to 40-fold and 1.5- to 4.5-fold in PELF and 300- to 1800-fold and 25- to 90-fold in BAL cells, respectively (Peters et al. 2012). However, this still represents an overall lower penetration of clarithromycin in PELF and BAL cells, by approximately 65% and 80%, respectively (Peters et al. 2012), compared to monotherapy. Pulmonary penetration of drugs is complex, poorly understood, and not only mediated by efflux transporters (Peters et al. 2011). Macrolides move against a steep concentration gradient from plasma via the PELF into BAL cells to reach concentrations above plasma concentrations. The delivery of a macrolide to its site of action in PELF and BAL cells may be influenced by CYP3A4 and the drug transporters, P-glycoprotein (ABCB1), ABCC2, and OATPs, which all can be modulated and/or upregulated via the nuclear PXR by rifampin (Figure 1). Conversely, Peters et al. (2012) showed, after short-term concomitant administration, that the penetration of rifampin and its metabolite into pulmonary compartments of foals was not influenced by clarithromycin into PELF, but exhibited approximately 40%–80% lower concentrations in BAL cells.
Rifampin and macrolide concomitant therapies are not without risk to horses. Severe and fatal enterocolitis has been reported in mares whose foals are treated with concomitant therapies, and associated with infection/proliferation of Clostridioides difficile. Experimentally reproduced enterocolitis in mares has implicated sub-therapeutic doses of erythromycin (Gustafsson et al. 1997), suggesting the macrolide portion is responsible for this adverse side-effect. Mares could be exposed to low concentrations of macrolides, in foals treated for R. equi, possibly due to ingesting small amounts of the active drug during coprophagia or contamination of feeders/water buckets shared by the mare and foal. Since macrolide oral bioavailability is inhibited by rifampin, then higher macrolide concentrations would be present in the foal's feces compared to macrolide monotherapy. Thus, perhaps a risk factor for macrolide-induced fatal enterocolitis in mares, secondary to foal R. equi treatments, is the concurrent rifampin usage. Also, in human medicine, rifampin usage has been associated with occasional cases of C. difficile (Street and Korman 2010).
5 In Vitro Rifampin Microbiological Studies and Bacterial Resistance
In vitro microbiology studies were part of the original basis for the tradition of rifampin concomitant therapies for R. equi in foals. They established the susceptibility of R. equi to rifampin and other antimicrobials, as well as claiming evidence for synergy of rifampin and macrolide combinations against R. equi. Furthermore, a common in vitro method to compare antimicrobials for selection of resistant mutants is based on measurement of the mutant prevention concentration (MPC), defined as the lowest antibiotic concentration that can prevent all single-step resistant mutants within a large population size (e.g., 109–1010 colony forming units). Traditional MIC testing is not a reliable measure of the relative tendency of antimicrobial agents to enrich resistant mutant subpopulations (Berghaus et al. 2013). The range of antimicrobial concentrations between the bacteria's MIC and the MPC is known as the mutant selection window (MSW), representing the danger zone for emergence of resistant mutants.
Berghaus et al. (2013) found that of 10 antimicrobial agents studied against R. equi, rifampin had the highest MPC, indicating that rifampin monotherapy is very likely to select for resistance. However, combining rifampin with erythromycin, clarithromycin, or azithromycin resulted in a significant decrease in MPC for R. equi (Berghaus et al. 2013). Lower R. equi MICs and MPCs for rifampin–macrolide combinations are largely the basis for the belief that rifampin–macrolide combinations are synergistic.
Previous PK/PD studies have investigated clarithromycin/rifampin concentrations, either as monotherapy or combination, at the target site of R. equi infection (pulmonary epithelium lining fluid, alveolar macrophages) in foals (Table 1). Berghaus et al. (2013) compared previously published mean rifampin/macrolide target site concentrations to their MPCs for R. equi and stated concentrations were above the MPC. However, Berghaus et al. (2013) only considered the results from Peters et al. (2012), which represented short-term administration of clarithromycin/rifampin and also did not consider the variability (standard deviations) around the estimate of antimicrobial concentrations. The PK/PD of rifampin concomitant therapies is dynamic, changing over time with repeated administrations due to PXR induction of drug metabolizing enzymes and p-glycoproteins. Also, these PK/PD studies were performed in healthy foals and thus without important barriers for antimicrobial penetration (e.g., pyogranulomatous abscess/s); therefore, the true antimicrobial (rifampin, macrolides) target site concentrations of naturally infected foals are unknown. Considering all PK studies measuring target site antimicrobial concentrations, representing short-to-longer term administrations, reveals high variability as well as decreasing parent antimicrobial target site concentrations, with increasing clarithromycin metabolite concentrations for which there is no information about MICs and MPCs (Table 1). For lower MPCs represented by rifampin concomitant therapies, it is assumed that both antimicrobials must reach, or increase above, MPC concentrations at target site/s to prevent selection of first-step resistant mutants. After PXR induction, any surviving R. equi will be exposed to clarithromycin concentrations that will not predictably reach these concomitant antimicrobial MPC concentrations (Table 1); also acknowledging that PK studies could not account for all barriers of antimicrobial penetration (e.g., pyogranulomatous abscess/s).
Although the MIC for each bacterial antibiotic-strain pair is typically considered a single value with high repeatability, it is unclear if this is true for the MPC. For example, the MPC is dependent on the probability and timing of mutations that confer resistance, which imposes a greater variance than MICs. Gianvecchio et al. (2019) investigated variations in MPC and found that even in highly controlled laboratory environments, MPCs vary widely, not only from differences in strain and antibiotic, but from replicates with the same strain and same antibiotic. Although Berghaus et al. (2013) and Erol et al. (2022) performed MPC testing in triplicates for each R. equi isolate, the study by Gianvecchio et al. (2019) found this to be insufficient to examine effects of stochasticity on the appearance of mutants, suggesting that p value estimates would be unreliable. Erol et al. (2022) also investigated MPC's for R. equi and found a greater variance in MPC for all antimicrobials examined.
Other modern in vitro methods to explore synergy between antimicrobial combinations include checkerboard assays (determination of MICs for each antibiotic in combination) as well as bacterial time-kill assays. Checkerboard assays rely on inhibition of visible bacterial growth at a single time point, whereas time-kill assays assess the dynamic activity of the tested drug combination at multiple time points. Both Erol et al. (2022) and Huguet et al. (2024) found that checkerboard analysis of R. equi against the rifampin–macrolide combinations were indifferent (i.e., no synergy, antagonism or additive effect). Also, time-kill assays demonstrated only minor differences in the extent of bacterial kill, regardless of whether antimicrobials were tested individually or in combination or against clarithromycin/rifampin resistant R. equi strains (Erol et al. 2022; Huguet et al. 2024). These other in vitro experiments provide evidence contrary to previously held beliefs that rifampin/macrolide combinations are synergistic against R. equi. Although checkerboard analysis and time-kill assays are preferred in vitro methods to explore antimicrobial synergy, there is no true gold standard. While it is interesting that several in vitro synergy testing methods incorporate physiological antimicrobial concentrations, these data do not inform as to the clinical outcomes that correlate with these methods (Doern 2014).
Antimicrobial resistance in R. equi is an important issue in equine medicine due to limited therapeutic options. For example, a study found that the odds of nonsurvival for foals infected with isolates of R. equi resistant to macrolides and rifampin was approximately seven times that for foals infected with susceptible isolates (Giguère et al. 2010). The most common type of bacterial resistance to rifamycins involves mutations in a limited number of highly conserved amino acids of the RNA polymerase β-subunit (RNAP) encoded by the chromosomal rpoB gene, thereby reducing rifamycin binding affinity and dictating the level of antimicrobial resistance (Fines et al. 2001). The rpoB gene is clustered into three regions: Clusters I, II, and III of which Type I is the most reported in R. equi as well as other bacterial genera (Fines et al. 2001). Rifampin resistance in R. equi are typically single-point substitutions, where the most common mutation involves Ser531Leu/Trp of the RNAP amino acid sequence and occasionally several different amino acids substitutions at the codon site 526 (Fines et al. 2001). Resistant mutants may be concentration-sensitive and contain RNA polymerases with one of a variety of sensitivities to rifampin. Spontaneous resistance occurs at a high rate (1 in 107 or 108 bacteria), even within the treatment period.
Other reported mechanisms of resistance include duplication of the rpoB target, possession of an RNAP-binding protein rbpA, bacterial modification of rifampin by glucosylation, ribosylation, phosphorylation, and decolorization, and modification of cell permeability (Tupin et al. 2010). For example, rifampin resistance has been identified in two nocardioform species, Rhodococcus erythropolis and Mycobacterium smegmatis, in which rifampin is inactivated. In addition, a putative efflux system was reported in Rhodococcus, responsible for persistence and multidrug resistances and similar to that found in other microorganisms, such as mycobacteria. One study reported a protein encoded by the req_39680 gene in R. equi isolated from clinical (66.7%), soil (66.7%), and feces (46.7%) samples responsible for phenotypic expression of this efflux mechanism (Gressler et al. 2014).
Several years ago, a global trend changed the way foals are screened/assessed for the presence of R. equi as well as the decision for antimicrobial treatments. What changed is a move away from clinical examinations with transtracheal wash for R. equi culture/susceptibility to the routine screening of foals with thoracic ultrasound, allowing for early detection of subclinical or mild-to-moderate R. equi pulmonary abscesses, without culture/susceptibility samples (Venner et al. 2007; Giguère et al. 2017). Ultrasonographic screening has revealed the most frequently recognized type of R. equi infection is a subclinical form (> 50% of cases) for which foals develop sonographic evidence of peripheral pulmonary consolidation or small abscessation without manifesting clinical signs (Giguère et al. 2017). It is presumed foals with this subclinical form of R. equi infection could either self-cure or further develop to either mild-to-moderate or severe R. equi pneumonia. Consequently, the popularization of thoracic ultrasonographic screening has substantially increased antimicrobial prescriptions of foals with subclinical, or mild-to-moderate R. equi pneumonia resulting in overuse of critically important antimicrobials (Venner et al. 2009; Álvarez-Narváez et al. 2020). Prior to ultrasound screening these subclinical and mild-to-moderate cases would largely have been undetected and not treated. After the implementation of ultrasound screening in the early 2000's, the prevalence of antimicrobial resistance in R. equi in clinical isolates from foals increased significantly (Burton et al. 2013; Huber et al. 2019).
Burton et al. (2013) conducted a retrospective study on clinical R. equi isolates from a Kentucky breeding farm and revealed that 7 years after the initiation of the ultrasound screening program in foals, rates of macrolide and rifampin resistance rose significantly. They also found that 24% of pretreatment isolates showed resistance compared to a 62% of post-treatment isolates, whereby MICs for erythromycin were consistently higher than rifampin. Buckley et al. (2007) investigating the MIC values toward rifampin and erythromycin among 94 clinical R. equi isolates collected over a 10-year period, found a steady increase in MIC concentrations for both drugs: to rifampin from 0.081 μg/mL pre-2000 to 0.187 μg/mL in 2006 and to erythromycin from 0.258 μg/mL pre- 2000 to 0.583 μg/mL in 2006. Huber et al. (2019) performed a retrospective observational study of all clinical samples from foals submitted to veterinary diagnostic laboratories in Kentucky between January 1995 and December 2017. Between 1995 and 2006, the proportion of resistant R. equi isolates was 0.7% for erythromycin and 2.3% for rifampin. Between 2007 and 2017, after the popularization of thoracic ultrasound, 13.6% and 16.1% of R. equi isolates were resistant to erythromycin and rifampin, respectively.
Rifampin–macrolide concomitant therapies assessed in PK/PD studies, summarized in this review, demonstrate consistent negative drug interactions between rifampin and macrolides in foals. This both compromises macrolide target site concentrations against R. equi infection, as well as oral bioavailability, leading to greater antimicrobial residue pollution into the environment. Rhodococcus equi is a well-known soil saprophyte commonly found in the environment of horse facilities and thus is also affected by environmental antimicrobial residues (Higgins et al. 2024). Thus, rifampin concomitant treatments are also risk factors for macrolide-resistant R. equi. Macrolide resistance in R. equi is typically the result of acquiring the erm(46) gene, which confers resistance to all macrolides, lincosamides, and streptogramin B (Anastasi et al. 2016). Furthermore, this erm(46) gene in R. equi was part of the extrachromosomal, conjugative plasmid, pRErm46, also carrying the sul1 gene and the tetRA gene conferring resistance to macrolides and streptogramins B (MLSB), sulfonamides, and tetracyclines, respectively. This plasmid, pRErm46, is a highly transferable mobile genetic element, between R. equi strains at frequencies up to 10−3, as well as to other bacterial species (Alvarez-Narvaez et al. 2019; Anastasi et al. 2016). Genetic sequencing of 144 isolates collected from the soil of 100 horse-breeding farms identified multidrug-resistant (MDR) R. equi carrying both the known pRErm46 plasmid and a novel MLSB resistance gene, erm(51) (Huber et al. 2020). In addition, the majority of MDR-R. equi isolated from foals or their environment have a rpoB mutation conferring resistance to rifampin. The environment is the main source of R. equi infections in foals; therefore, higher rates of MDR R. equi in the environment contribute to clinical infections with MDR R. equi (Higgins et al. 2024). Moreover, environmental and clinical isolates carrying erm(46) were found to be part of the same clonal population, indicating that a complex environmental–animal cycle allows the reinfection of foals with MDR-R. equi from the soil. These resistance traits may circulate (lost or acquired via conjugal transfer) between isolates and be maintained as different variants within the environment and farm animals (e.g., pigs, ruminants, and equines) through the oral-fecal route.
In conclusion, macrolide–rifampin concomitant therapies do not appear to prevent either the selection of single-step resistant mutants or MDR R. equi as well as resistant gastrointestinal commensals that are shed into their environment where they persist and potentially colonize horses and infect other foals (Álvarez-Narváez et al. 2020). While Berghaus et al. (2013) did demonstrate in vitro that rifampin concomitant use does significantly lower the MPC than that of each individual respective drug for R. equi, other in vitro experiments (checkerboard analysis, time-to-kill assays) do not identify additive or synergistic effects of rifampin/macrolide combinations on R. equi. The popularization of thoracic ultrasound has led to global increases in macrolide–rifampin concomitant therapies with subsequent increases in MDR R. equi. Furthermore, Álvarez-Narváez et al. (2020) demonstrated horizontal transfer of the Rhodococcus equi conjugative plasmid pRErm46 between different environmental organisms among Rhodococcus, Nocardia and Arthrobacter spp. at frequencies ranging from ∼10−2 to 10−6. These environmental actinobacteria may act as reservoirs of antimicrobial resistance determinants and contribute to the maintenance and spread of erm(46)-mediated macrolide resistance within equine farms (Álvarez-Narváez et al. 2020). Similarly, MDR R. equi isolates have been reported in human medicine. Given that R. equi is not infrequently associated with opportunistic infections in humans, the emergence of macrolide and rifampin resistance might also adversely impact human health (Huber et al. 2019).
6 Randomized Clinical Trials
It is challenging to predict whether an antimicrobial/s administered to foals will reach therapeutic concentrations at the target site of R. equi infection because of the complex PK/PD interactions and pathophysiological processes involved. For example, antimicrobials chosen must penetrate three target sites (pulmonary epithelium lining fluid, pyogranulomatous abscess, alveolar macrophages) to concentrations above the MIC for R. equi. A variety of confounders must be considered, including age-dependent differences in bioavailability, volume of distribution, clearance, and elimination (e.g., the function of drug-metabolizing enzymes and drug transporters), drug–drug interactions, and disease-related factors (Berlin et al. 2017). Therefore, randomized clinical trials involving naturally infected foals are an important source of information for understanding the clinical efficacy of rifampin. Since 2012, randomized blinded and double-blinded controlled clinical trials (RCTs) have been published comparing various antimicrobial treatments in groups of foals with confirmed pulmonary abscesses, from R. equi endemic farms. Criteria for study inclusion have been modified over the years, based on ultrasound measurements of pulmonary abscesses, to develop a lesion score (defined as the sum of the largest diameter of all pulmonary lesions ≥ 1 cm), from 1.0 to 10.0 cm (Venner et al. 2012), 5.0–10.0 cm (Venner, Astheimer, et al. 2013), 8.0–15 cm (Venner, Credner, et al. 2013), 8.0–20.0 cm (Hildebrand et al. 2015), and 10.0–15.0 cm (Rutenberg et al. 2017; Wetzig et al. 2020).
An important outcome of all these controlled RCTs is that the majority of foals with small pulmonary lesions recover without antimicrobial therapy and that antimicrobial treatment of foals with small lesions (median abscess score ≤ 6 cm) does not significantly accelerate lesion resolution relative to the placebo group. Self-recovery rates of up to 88% in placebo groups have been noted in subclinical cases (Venner, Astheimer, et al. 2013). These RCTs have focused primarily on subclinical and mild-to-moderate pneumonia cases in foals, and without culture and susceptibility data or confirmation of bacteria-free foals upon recovery. Unfortunately, R. equi culture and susceptibility is not routinely performed in practice, in lieu of ultrasonic screening (McCracken and Slovis 2009). While the popularity of diagnostic ultrasound screening for R. equi pneumonia has been regarded as an advancement, this has paradoxically been counterproductive to antimicrobial stewardship. These RCTs reflect field diagnostics but are inconsistent with prudent-use principles of critically important antimicrobials, as ultrasound screening does not represent a gold standard for R. equi diagnostics. It remains to be described the ultrasound characteristics of the normal developing foal lung to better understand the accuracy of ultrasound for foal pneumonia, including the presence of small pulmonary lesions.
Results of these RCTs can be split into two broad categories, including monotherapy groups (e.g., macrolide or doxycycline) as well as concomitant therapy groups (e.g., rifampin plus a macrolide or doxycycline), and compared to respective placebo groups (Figure 2). Re-analyzing RCTs for treatments of foals with pulmonary abscesses can be done using different outcome measures: relative risk, odds ratio, or absolute risk difference along with the corresponding calculated 95% confidence intervals (95% CI) (Figures 2 and 3). For this review paper, a further analysis was performed with either random-effects or mixed-effects models. Mixed-effects modeling of these R. equi RCTs revealed no statistically significant difference in outcomes (p = 0.28) between monotherapy versus rifampin concomitant therapy from foals with subclinical or mild-to-moderate pulmonary abscesses that recovered without the need for a change in antimicrobial therapy. This analysis is without one treatment arm from the trial by Wetzig et al. 2020 (azithromycin + doxycycline).
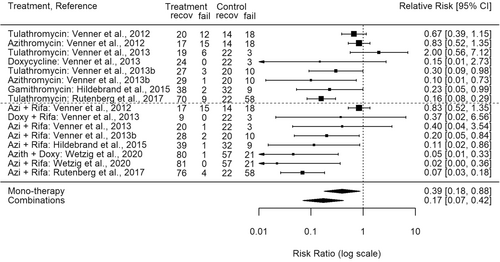
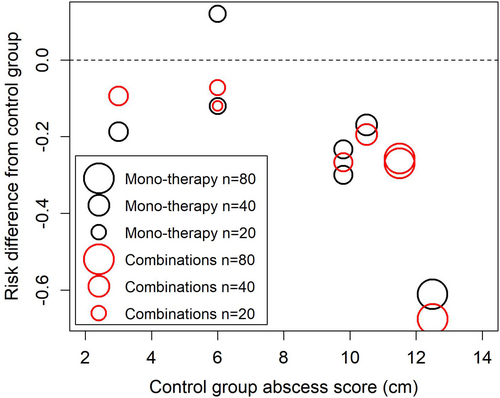
Furthermore, analysis of the same RCTs revealed a statistical trend of greater treatment efficacy compared to placebo, identified as an absolute risk difference, with increasing initial ultrasonic measured mean abscess score (cm); whereby, monotherapy groups follow the same increasing efficacy trend as do concomitant therapy groups (Figure 3). A mixed-effects model confirmed that the initial ultrasonic measured mean abscess score (cm) had a significant effect on therapy outcomes (p < 0.0001); however, with mean abscess scores included in the mixed-effects model there was still no significant difference between monotherapy versus concomitant therapy groups (p = 0.36). This model also investigated whether there might be an additional benefit of concomitant therapy at higher abscess scores, using an interaction term (Control group Abscess Score X Concomitant therapy). This interaction was not significant (p = 0.1114). Thus, based on the analysis of results from these RCTs, rifampin concomitant therapies do not offer any significant added clinical benefit over monotherapy for foals with subclinical or mild-to-moderate pulmonary R. equi infections, regardless of initial abscess score.
The positive results of monotherapy for treatment of R. equi infections are supported by pharmacokinetic studies in foals that show clinical doses result in drug concentrations in serum, PELF, and BAL cells higher than the MIC90 for R. equi for the entire dosing interval; this includes clarithromycin (Womble et al. 2006; Suarez-Mier et al. 2007), azithromycin (Suarez-Mier et al. 2007), tulathromycin (Venner et al. 2010), and erythromycin (Suarez-Mier et al. 2007).
7 Rifampin Nonantibacterial Clinical Properties
Rifampin has been found to possesses potent clinical immunosuppressive effects in humans and animal models. Rifampin inhibits innate immune function through a direct molecular target for binding to myeloid differentiation protein-2, the key co-receptor for innate immune toll-like receptor-4 (TLR4) that plays a fundamental role in the activation of innate immunity (Wang et al. 2013). Higher rifampin doses also reduce phagocytic activity of immune cells.
Rifampicin has also been shown to have neuroprotective roles for various central nervous system diseases (e.g., stroke, meningitis, Parkinson disease, multiple sclerosis, and Alzheimer disease) (Ma et al. 2016). Rifampin also exerts anti-inflammatory effects through inhibiting the production of interleukin-6 and prevents excessive activation of nuclear factor kappa B (NF-кB) (Ma et al. 2016).
8 Conclusions on Rifampin Concomitant Therapies for Rhodococcus equi
Rifampin concomitant therapies have remained the traditional treatment or prevention of R. equi infections in foals for nearly 40 years. This was based on previously held beliefs that rifampin concomitant therapies are synergistic, improving efficacy, and reducing the emergence of resistance to either single agent. However, evidence has accumulated that greatly improves our understanding of rifampin pharmacokinetics and pharmacodynamics that challenges these beliefs as well as the need for rifampin use in foals. Based on the accumulated evidence, rifampin's negative interactions within the body and other drugs are complex and dynamically change over the course of treatment. During the first week of R. equi treatment, it appears the main antimicrobial effect is from bacteriostatic decreasing concentrations of rifampin and its 25-desacetylrifampin metabolite to a lesser extent since there is strong inhibition of macrolide oral bioavailability and subsequent plasma concentrations with rifampin concomitant therapy. Rifampin may assist the penetration of macrolides into the lungs but not to recognized MIC90 concentrations in all cases. After the first week of treatment, rifampin induces accelerated and long-lasting activity of hepatic cytochrome P450 enzymes leading to increased hepatic biotransformation of both rifampin and other drugs and decreasing plasma/lung concentrations of the parent drugs. While these drug metabolites (e.g., 25-desacetylrifampin, 14-hydroxyclarithromycin) are known to retain antibacterial effects, there is no information about their MICs, MPCs of these drug metabolites against R. equi, and sparse information about pharmacokinetics/pharmacodynamics within equine lung compartments. These antagonistic drug interactions are further reflected in the results of randomized blinded and double-blinded controlled clinical trials that show rifampin concomitant therapies do not demonstrate a significant advantage over monotherapy for subclinical and mild-to-moderate bronchopneumonia in foals on R. equi endemic facilities, regardless of the initial pulmonary abscess score. Rifampin concomitant therapies are themselves risk factors for selecting multi-resistant clonal Rhodococcus equi associated with several independent genetic events, including transferable erm(46) gene macrolide resistance along with chromosomal rpoB mutations with no linking cross-resistant mechanism (Alvarez-Narvaez et al. 2019; Burton et al. 2013). These clonal MDR strains may persist in the environment, representing future concerns for foal health and public health.
Given increasing evidence of negative drug interactions in vivo, and the rapid spread of MDR R. equi, as well as results of RCTs of natural R. equi infections in foals, then the use of rifampin (WHO critically important antimicrobial) is seriously questioned for subclinical and mild-to-moderate cases of R. equi pneumonia in foals, representing the majority of R. equi cases. Despite the accumulated evidence against the use of concomitant rifampin and a macrolide, such use continues to be recommended (Higgins and Huber 2023; Bordin et al. 2022; Sanz 2023). As highlighted by Morris et al. (2011) in The Journal of the Royal Society of Medicine, the translation of research into clinical practice can take approximately 20 years. This delay is particularly evident in antimicrobial stewardship, where entrenched habits and the comfort of traditional protocols—like rifampicin-macrolide combinations for Rhodococcus equi—resist change, even when evidence, such as that presented in our review, demonstrates limited efficacy and significant risks (e.g., resistance development). Thus, revision of clinical practice guidelines for horses is needed to be in-line with evidence-based principles omitting rifampin as an option for subclinical and mild-to-moderate R. equi pneumonia. In human medicine, rifampin is not recommended in combination with clarithromycin, erythromycin or doxycycline since their concentrations are substantially decreased by rifamycins (Street and Korman 2010).
9 Revised Recommendations Can Be Suggested Regarding Antimicrobial Treatments for R. equi in Foals
- –
Ultrasonographic screening of foals for early R. equi detection reveals the subclinical form as the most common (peripheral pulmonary consolidation or abscessation without manifesting clinical signs). RCTs have revealed a strong performance in the placebo group (self-cure) for foals with subclinical cases, especially with a median ultrasound abscess score ≤ 6 cm, whereby antimicrobial therapy does not significantly accelerate lesion resolution relative to the placebo group. Clinical and ultrasound monitoring only is warranted for these cases. Since ultrasonography only allows scanning of the lung periphery for lesions as well as individual variation in disease susceptibility, then there is no lesion score cut-point that will ideally differentiate foals requiring therapy from foals that will spontaneously recover.
- –
Mild-to-moderate R. equi bronchopneumonia cases appear to warrant antimicrobial therapy. Differences between placebo and antimicrobial treatment have only been reported among foals with a total maximum diameter of thoracic ultrasound lesions > 10 cm, but a study by Wetzig et al. (2020) found 73.1% of the untreated foals from the control group with an abscess score from 10 to 15 cm recovered. An analysis of RCTs has confirmed that monotherapy with macrolides performs equally well as rifampin concomitant therapies, regardless of initial pulmonary abscess score. Clinical and ultrasound monitoring are also warranted, with antimicrobial therapy best guided by lower airway sampling for culture and susceptibility.
- –
Foals with severe R. equi bronchopneumonia require antimicrobial therapy but have not been fully evaluated in clinical studies, and evidence of the need for concomitant antimicrobial therapy is lacking. The best antimicrobial concomitant therapy with the least antagonistic drug interactions, resulting in appropriate MIC concentrations for both parent drugs at the target site/s of infection, has not been identified.
Author Contributions
Keith Edward Baptiste: substantial contributions to conception and manuscript design, acquisition of data, and interpretation of data, drafting the manuscript and revising it critically for important intellectual content. Niels Christian Kyvsgaard: formal analysis and interpretation of data, drafting the manuscript and revising it critically for important intellectual content. Mohamed Omar Ahmed: drafting the manuscript and revising it critically for important intellectual content. Peter Damborg: drafting the manuscript and revising it critically for important intellectual content. Patricia M. Dowling: substantial contributions to manuscript design, drafting the manuscript and revising it critically for important intellectual content.
Ethics Statement
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as no animals were used.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
This manuscript does not report original results of a clinical trial or secondary results of clinical trial data. All references can be provided upon request to the corresponding author.




