Effect of feeding on the pharmacokinetics of oral minocycline in healthy adult horses
Funding information
Supported by the American Quarter Horse Association.
Abstract
Minocycline is commonly used to treat bacterial and rickettsial infections in adult horses but limited information exists regarding the impact of feeding on its oral bioavailability. This study's objective was to compare the pharmacokinetics of minocycline after administration of a single oral dose in horses with feed withheld and with feed provided at the time of drug administration. Six healthy adult horses were administered intravenous (2.2 mg/kg) and oral minocycline (4 mg/kg) with access to hay at the time of oral drug administration (fed) and with access to hay delayed for 2 hr after oral drug administration (fasted), with a 7-day washout between treatments. Plasma concentration versus time data was analyzed based on noncompartmental pharmacokinetics. Mean ± SD bioavailability (fasted: 38.6% ± 4.6; fed: 15.7% ± 2.3) and Cmax (fasted: 1.343 ± 0.418 μg/ml; fed: 0.281 ± 0.157 μg/ml) were greater in fasted horses compared to fed horses (p < .05 both). Median (range) Tmax (hr) in fasted horses was 2.0 (1.5–3.5) and in fed horses was 5.0 (1.0–8.0) and was not significantly different between groups. Overnight fasting and delaying feeding hay 2 hr after oral minocycline administration improve drug bioavailability and thus plasma concentrations.
Minocycline, a semisynthetic tetracycline derivative with bacteriostatic activity, represents an attractive addition to available oral antimicrobials for adult horses due to its wide spectrum of activity, excellent tissue penetration, relatively low cost, and potential anti-inflammatory properties (Agwuh & MacGown, 2006; Bishburg & Bishburg, 2009; Sapadin & Fleischmajer, 2006). Minocycline use in equine veterinary practice has increased due to price increases and decreased availability of doxycycline. While recent pharmacokinetic studies of oral minocycline in adult horses indicate that adequate drug concentrations are achieved in several tissues, there is considerable variability in reported bioavailability of minocycline (23%–48%) across studies (Echeverria et al., 2016; Giguère et al., 2016; Schnabel et al., 2012). It is not known whether differences in study feeding protocols contributed to this finding.
Food exerts complicated effects on the pharmacokinetics of a drug (Gu et al., 2007) Decreased oral bioavailability in fed versus fasted horses has been reported for several drugs (Bouckaert et al., 1994; Davis, Salmon, & Papich, 2006; Sykes et al., 2015; Van Duijkeren et al., 1995) and may result from delayed gastric emptying or from a physical barrier to absorption with high-roughage diets (Toothaker & Welling, 1980). In humans, food is not reported to effect tetracycline bioavailability (Agwuh & MacGown, 2006; Bishburg & Bishburg, 2009). In an adult horse, feeding resulted in decreased Cmax when doxycycline was administered via nasogastric tube (Davis et al., 2006). Similarly, feeding in dogs decreased Cmax for minocycline when administered orally (Hnot, Cole, Lorch, Rajala-Shcultz, & Papich, 2015). In horses provided ad libitum hay, the addition of grain did not result in decreased plasma minocycline concentrations (Schnabel et al., 2012). To date, the impact of feeding hay on the pharmacokinetics of oral minocycline has not been investigated in horses. The objective of this study was to compare the pharmacokinetics of oral minocycline in adult horses fasted or allowed access to hay at the time of drug administration.
Three nonpregnant mares, two geldings, and one stallion (7–16 years of age; weight of 48–650 kg) were housed in individual stalls for 24 hr prior to and throughout each experimental period and were maintained on pasture between experimental periods. This study was approved by the University of Illinois Institutional Animal Care and Use Committee (protocol No. #16055).
In a three-treatment randomized cross-over design with a 7-day washout between treatments, minocycline hydrochloride was administered IV once at a dose of 2.2 mg/kg (Nagata et al., 2010) and orally once at a dose of 4 mg/kg without and with access to alfalfa/grass mixed hay (oral-fasted and oral-fed). Feed was withheld from all horses 12 hr prior to oral drug administration. Fasted horses were provided two flakes of hay 2 hr after drug administration. Fed horses were fed two flakes (mean weight 4.35 ± 0.42 lbs/flake) of hay 20 min prior to drug administration and were allowed to continue eating. Concentrates were not fed at any time. Water was provided ad libitum.
For oral administration, the contents of minocycline hydrochloride 75 mg capsules (Aurobindo Pharam USA, Inc, Dayton, New Jersey, USA) were dissolved in 50 ml of water and administered within 2 hr of preparation using an oral syringe. The syringe was flushed with water to ensure complete delivery of the drug.
Intravenous minocycline was prepared with minocycline hydrochloride powder (Fagron, Inc, St. Paul Minnesota, USA) dissolved in sterile water (Hospira, Inc., Lake Forest, Illinois, USA) to a 5 mg/ml concentration, filtered through a 25-mm syringe filter with a 0.2 micron Supor® membrane (Pall Animal Health, Port Washington, New York, USA) and administered within 2 hr of preparation as a 5-min infusion via dedicated right jugular catheter (Mila International, Inc., Erlanger, Kentucky, USA).
Blood samples were collected via direct left jugular venipuncture into Na-heparin tubes (Covidien Ltd., Dublin Ireland) before drug administration (time 0) and at 5, 10, 15, 20, 30 min and 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 36, and 48 hr after drug administration. Samples were centrifuged (400 g × 10 min at 4°C), plasma aliquoted and then stored at −80°C until analysis.
Plasma concentrations were determined via liquid chromatography–mass spectrometry (LC/MS/MS) analysis using a hybrid triple Quadrupole-Linear Accelerator trap mass spectrometer (5500 QTrap LC/MS/MS system, Sciex, Framingham Massachusetts, USA) (1200 series HPLC system, Agilent Technologies, Santa Clara, California, USA) and specialized software for data acquisition and analysis (Analyst 1.6.2 software. AB Sciex LP, Concord, Ontario, Canada). Plasma samples were thawed and 50 μl of plasma mixed with 70 μl of methanol, spiked with 2 μl of internal standard, demeclocycline 13.8 μg/ml (Sigma-Aldrich Corp, Atlanta, Georgia, USA), vortexed, and centrifuged.
The LC separation specifications were as follows: column 4.6 × 50 mm, 5 μm; mobile phase A 0.1% formic acid in water; mobile phase B 0.1% formic acid in acetonitrile; flow rate 0.35 ml/min; linear gradient at 0–2 min 100%A, 2–7 min transition 100%A to 100%B, 7–10.5 min 0%A and 100%B, 10.5–11 min transition to 100%A, 11–15.5 min 100%A, autosampler at 15°C, injection volume 15 μl. Mass spectra were acquired under positive electrospray ionization with the ion spray voltage at +5500 V. Source temperature was 450°C. Curtain gas, ion source gas 1, and ion source gas 2 were 32, 50, and 65 psi, respectively. Multiple reaction monitoring was used for quantitation of minocycline (m/z 458.2 to >m/z 441.1) with demeclocycline as the internal standard (m/z 465.2 to >m/z 448.2).
Prior to sample analysis, standard calibration curves were constructed using aliquots of drug-free equine plasma. Values (ng/ml) used for calibration solutions were 10, 20, 40, 80, 200, 1000, 2,000, and 5,000 and the standard curves revealed an R value of ≥0.98 in all cases. At plasma concentrations of 20, 200, and 2000 ng of minocycline/ml, extraction efficiency was 70.0 ± 1.7%, 70.7 ± 3.7%, and 89.4 ± 1.1%, respectively. Extraction efficiency of the internal standard was 83.4 ± 2.2%. Within- and between-run accuracy (CV) and precision for minocycline detection in plasma were determined (Table 1).
| Minocycline concentration (ng/ml) | Within-run accuracy (%) | Within-run CV (%) | Between-run accuracy (%) | Between-run CV (%) |
|---|---|---|---|---|
| 20 | 102.5 ± 1.8 | 5.0 | 102.5 ± 4.4 | 1.1 |
| 200 | 100.1 ± 2.8 | 3.7 | 100.1 ± 3.2 | 2.3 |
| 2000 | 100.1 ± 4.0 | 2.6 | 102.8 ± 2.3 | 3.8 |
- Within-run precision (CV) was calculated by use of three concentrations of control samples (20, 200, and 2000 ng of minocycline/ml) repeated six times in a single run. Between-run precision (CV) was determined by comparing results for three concentrations of control samples (20, 200, and 200 ng of minocycline/ml) over three consecutive daily runs (total six runs). Within and between-run accuracy were established by determining the ratio of the calculated response to the expected response for the three concentrations of control samples over six runs.
Plasma minocycline concentration versus time data was analyzed using noncompartmental methods and commercially available software (PK Solutions 2.0, Summit Research Services, Montrose, Colorado, USA). Maximum plasma concentration (Cmax) and time to maximum plasma concentration (Tmax) were collected directly from the data for oral dosing. The rate constant of the terminal phase (λz) was determined by linear regression of the logarithmic plasma concentration versus time curve using a minimum of four data points. Half-life of the terminal phase (t½λz) was calculated as ln 2 divided by λz. Area under concentration–time curve (AUC) and area under the first moment of the concentration–time curve (AUMC) were calculated using the trapezoidal rule, with extrapolation to infinity using Cmin/λz, where Cmin is plasma concentration at the last measurable time point. Mean residence time (MRT) was calculated as follows: AUMC/AUC. Bioavailability (F) was calculated as (AUCoral/AUCIV) × (doseIV/doseoral). Apparent volume of distribution based on the AUC (Vdarea) was calculated as follows: IV dose/AUC λz, and systemic clearance (CL) was calculated from IV dose/AUC.
Normality was assessed based on histograms of differences in means, normal quantiles plots of the residuals, and the Shapiro–Wilk test. Constant variance was assessed by plotting residuals against predicted values and with Levene's test. One-way ANOVA for repeated measures was used to compare λz, t½λz, and MRT between the three methods of administration. When indicated, multiple pairwise comparisons were carried out using Holm–Sidak method. Comparison of AUC, Cmax, Tmax, and bioavailability between fed and fasted treatments was done using paired t test, except for Tmax for which Wilcoxon signed-rank test was used. Differences were considered significant at p < .05.
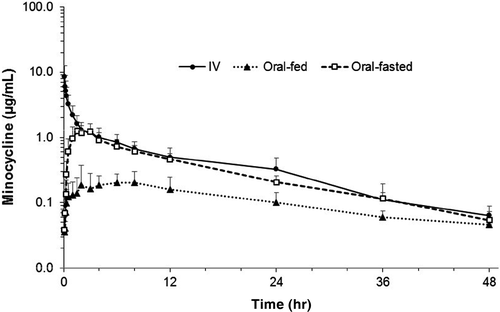
Plasma minocycline concentration versus time curves for IV and oral (fasted and fed) were constructed (Figure 1). Pharmacokinetic parameters following IV and oral (fasted and fed) minocycline administration are listed in Table 2. Significant differences in Tmax (median; range) were not identified between fasted (2.0 hr; 1.5–3.5) and fed (5.0 hr; 1.0–8.0) horses. Significant differences in all other pharmacokinetic parameters were identified between fasted and fed horses, but not between fasted horses and horses administered IV minocycline. Bioavailability was significantly greater for fasted (38.6% ± 4.6) versus fed (15.7% ± 2.3) horses (p < .05). Cmax was also significantly greater for fasted (1.343 ± 0.418 μg/ml) versus fed (0.281 ± 0.157 μg/ml) horses (p < .05).
| Variable | Intravenous | Oral | |
|---|---|---|---|
| Fasted | Fed | ||
| λz (hr−1) | 0.060 ± 0.009 | 0.062 ± 0.008 | 0.038 ± 0.012ab |
| t½λz (hr) | 11.8 ± 1.9 | 11.3 ± 1.2 | 20.0 ± 7.0ab |
| AUC0–24 hr (μg hr/ml) | 22.2 ± 7.5 | 16.0 ± 4.5 | 5.3 ± 2.0b |
| AUC0–∞ (μg hr/ml) | 23.3 ± 7.7 | 17.0 ± 4.8 | 6.8 ± 2.7b |
| MRT (hr) | 13.9 ± 1.6 | 15.4 ± 4.5 | 30.7 ± 11.0ab |
| Cmax (μg/ml) | NA | 1.343 ± 0.418 | 0.281 ± 0.157b |
| Tmax (h)c | NA | 2.0 (1.5–3.5) | 5.0 (1.0–8.0) |
| F (%) | NA | 38.6 ± 4.6 | 15.7 ± 2.3b |
| Vdarea (L/kg) | 1.78 ± 0.75 | NA | NA |
| CL (ml/hr/kg) | 104.4 ± 37.3 | NA | NA |
- a Indicates a statistically significant difference between fed and intravenous (p < .05).
- b Indicates a statistically significant difference between fed and fasted (p < .05).
- c Median and range; NA, not applicable.
- λz, rate constant of the terminal phase; t½λz, half-life of the terminal phase; AUC0–24 hr, Area under the plasma concentration versus time curve from time 0 to 24 hr; AUC0–∞, Area under the plasma concentration versus time curve extrapolated to infinity; MRT, Mean residence time; Cmax, Maximum plasma concentration (observed) after the first dose; Tmax, Time to maximum plasma concentration (observed) after the first dose; F, Oral bioavailability; Vdarea, Apparent volume of distribution based on AUC; CL, Systemic clearance.
In this study, bioavailability and Cmax of minocycline in adult horses were superior when hay was withheld. While significant differences in Tmax were not identified between oral-fasted and oral-fed groups, the Tmax of 5 hr in oral-fed versus 2 hr in oral-fasted horses suggests that hay might delay drug absorption. In contrast to fasted bioavailability (38.6%), oral-fed bioavailability (15.7%) was much lower than previously reported for minocycline in adult horses (Echeverria et al., 2016; Giguère et al., 2016; Schnabel et al., 2012). Previous minocycline studies reported intragastric administration with various feeding protocols: ad libitum grass hay with pelleted feed twice daily (Schnabel et al., 2012); ad libitum grass hay (Giguère et al., 2016); or two flakes of grass hay twice daily (Echeverria et al., 2016). In this study, minocycline was administered via an oral dosing syringe thought to more accurately reflect drug administration in practice. It is possible that this route of administration and access to feed contributed to reduced bioavailability.
In conclusion, these results suggest that overnight fasting and delaying feeding of hay for 2 hr after minocycline administration improve drug absorption. We chose this feeding protocol as boarded horses are typically fed twice daily and are often administered medications at feeding times.
CONFLICTS OF INTEREST
The authors have no conflicts of interest.




