Comparison of the pharmacokinetic profiles of two different amphotericin B formulations in healthy dogs
Abstract
This study was conducted to compare the pharmacokinetic profiles of conventional (Fungizone®) and liposomal amphotericin B (AmBisome®) formulations in order to predict their therapeutic properties, and evaluate their potential differences in veterinary treatment. For this purpose, twelve healthy mixed breed dogs received both drugs at a dose of 0.6 mg/kg by intravenous infusion over a 4-min period in a total volume of 40 ml. Blood samples were collected at 0, 0.5, 1, 1.5, 2, 3, 4, 8, 12, 24, 48, 72 and 96 hr after dosing, and concentrations of drug in plasma were determined by high-performance liquid chromatography (HPLC). Pharmacokinetics was described by a two-compartment model. Although both formulations were administered at the same doses (0.6 mg/kg), the plasma pharmacokinetics of liposomal amphotericin B differed significantly from those of amphotericin B deoxycholate in healthy dogs (p < .05). Liposomal amphotericin B showed markedly higher peak plasma concentrations (approximately ninefold greater) and higher area under the plasma concentration curve values (approximately 14-fold higher) compared to conventional formulation. It is concluded that AmBisome® reached higher plasma concentration and lower distribution volume and had a longer half-life compared to Fungizone®, and therefore, AmBisome® is reported to be an appropriate and effective choice for the treatment of systemic mycotic infections in dogs.
1 INTRODUCTION
Of almost the 70,000 species of fungi that have been recognized and described, about 50 species are reported to be pathogenic for animals (Rochette, Engelen, & Vanden Bossche, 2003). The commonest of the infections caused by these eukaryotic organisms are superficial and include diseases such as dermatophytosis in domestic animals (Bond, 2010; Nweze, 2011). However, fungi are also responsible for systemic fungal infections, causing significant morbidity and mortality, especially in dogs and cats (Atencia, Papakonstantinou, Leggett, McAllister, & Moonet, 2014; Davis, Papich, & Heit, 2009; Kerl, 2003). Systemic mycosis caused by pathogenic fungi (histoplasmosis, coccidioidomycosis, and blastomycosis) can contribute to an infection in a normal host and continues to be the most common infection identified in cats and dogs. On the other hand, opportunistic infections, including Aspergillus and Cryptococcus spp., require hosts that are incapacitated or immunocompromised, and such infections are increasing as the underlying causes of systemic infections (Atencia et al., 2014; Wiebe & Karriker, 2005). Over the last decade, it is believed that the incidence of invasive and severe fungal infections in small animals as well as humans has gradually been increasing, being associated with many factors including viral infections, cancer, endocrine, and metabolic disorders (Biegańska, Dardzińska, & Dworecka-Kaszak, 2014; Wiebe & Karriker, 2005).
Animal mycosis, particularly deep (systemic) mycosis, is one of the most challenging conditions encountered by veterinarians (Okabayashi et al., 2009). Treatment is complicated by limited availability of fungicidal antimicrobials and the necessity of long-term treatments with expensive drugs. The majority of antifungal drugs being only approved for human use further complicate this, as information from well-controlled veterinary trials or meta-analyses that could help guide the veterinarian's choice of drug and treatment regimen is largely unavailable (Hector, 2005; Kerl, 2003; Sykes & Papich, 2013). Among the restricted number of antifungal agents, amphotericin B is an important treatment option, due to its broad spectrum activity, being used primarily in the treatment of systemic fungal infections in veterinary medicine (Foy & Trepanier, 2010; Wiebe & Karriker, 2005). However, the use of this polyene antibiotic is limited due to serious adverse reactions (e.g., nephrotoxicity, phlebitis, and tachycardia); these toxicities frequently render the conventional formulation of amphotericin B deoxycholate (Fungizone) unsuitable for treatment (Ceylan, Akkan, Tütüncü, & Ağaoğlu, 2003; Hamill, 2013). To attenuate the toxicity and increase the therapeutic potential of amphotericin B, alternative liposomal (Ambisome) and lipid-based (Abelcet and Amphocil) formulations have been developed (Cifani, Costantino, Massi, & Berrino, 2012; Hector, 2005). Based on the pharmacokinetics and efficacy data, Ambisome was reported to be the most efficient formulation in terms of the spectrum of usage and is the least toxic among all these formulations (Cifani et al., 2012; Iman, Huang, Szoka, & Jaafari, 2011; Jain & Kumar, 2010). Neither conventional nor liposomal formulations of amphotericin B are approved for use in animals by the Food and Drug Administration. However, these preparations, used to treat serious fungal infections in dogs and cats, are prescribed by veterinarians as an extra-label use; most of the advice and studies addressing the recommended method of administration of amphotericin B are based on the nonlipid preparation (Boothe, 2012).
Rational antifungal therapy requires dosage regimes to be optimized, not only to guarantee clinical efficacy, but also to minimize the selection and spread of resistant fungal pathogens (Andes, 2006; Vandeputte, Ferrari, & Coste, 2012). Therefore, the disposition characteristics and pharmacokinetic parameters of drugs provide fundamental data for designing safe and effective dosage regimens (DeVane & Gill, 1997). In the current study, we conducted a comparative pharmacokinetic study to predict the therapeutic properties of liposomal amphotericin B (AmBisome), and evaluate its potential differences from the conventional amphotericin B (amphotericin B deoxycholate) (Fungizone) formulations in dogs.
2 MATERIALS AND METHODS
2.1 Animals
Twelve clinically healthy adult male dogs (golden retriever and German shepherd cross mix breed), weighing 23-28 kg and aged between 1.5 and 3 years, were used in this study. All dogs were determined to be healthy on the basis of physical examination and complete blood counts. Routine vaccinations and anthelmintic treatments were administered prior to arriving at the laboratory. The dogs were acclimatized for one week prior to initiation of the study. The dogs were housed in individual cages in temperature- and humidity-controlled rooms, fed ad libitum and allowed free access to water during the study period. The research protocol was approved by the Local Committee on Animal Research Ethics of University of Istanbul (Approval number: 2008/92).
2.2 Drug
Fungizone® (Bristol-Myers Squibb-ABD), a lyophilized preparation of amphotericin B in sodium deoxycholate, was reconstituted according to the manufacturer's instructions to give a 5 mg/ml solution amphotericin B. AmBisome® (Gilead Sciences Ltd.-Ireland), a lyophilized liposomal preparation of amphotericin B, was also reconstituted according to the manufacturer's instructions to give a 4 mg/ml solution amphotericin B. The solutions were diluted further with 5% dextrose solution to the concentrations required for injection.
2.3 Experimental protocol
The study was conducted in a single-dose, randomized, two-period and crossover design with a 21-day washout period between treatments. The 12 dogs were randomly allocated into two treatment groups: A single dose (0.6 mg/kg) of Fungizone® or AmBisome® was administrated by intravenous infusion over a 4-min period in a total volume of 40 ml before sampling started. The dose was selected based on maximal tolerated doses of amphotericin B in dogs (Bekersky et al., 1999). Blood samples were collected in heparinized tubes before and at 0.5, 1, 1.5, 2, 3, 4, 8, 12, 24, 48, 72 and 96 hr after dosing. Plasma was separated by centrifugation at 3,000 g for 10 min and stored frozen at −70°C until analyzed.
2.4 Measurement of amphotericin B concentrations
Plasma samples were transferred frozen to the Toxicology Laboratory of Pendik Veterinary Control Institute for analysis. Amphotericin B concentrations in plasma were determined using an HPLC method as previously described and validated according to the method of Egger, Bellmann, and Wiedermann (2001).
Retention times for amphotericin B were 3.36 min. The lower limit of quantification (LLOQ) of amphotericin B in plasma was 0.01 μg/ml. Control plasma sample collected from a dog, which had received no drug treatment, was spiked with pure amphotericin B (North China Pharmaceutical Huasheng CO., LTD, batch no: HAN07131301) to prepare standards, ranging from 0.05 to 5 μg/ml. Linearity of the standard curves was r2 = .9985. The percentage recovery of amphotericin B was 83.03 ± 2.73 (mean ± SD, n = 9). The intra- and interassay repeatability and reproducibility of the method were evaluated using spiked concentrations. Intra- and interassay precision (% coefficient of variation) was 2.9%-5.57%, and 1.43%-5.78%, respectively. Percentage accuracies were 96.3%-106.9%.
2.5 Pharmacokinetic analysis
The pharmacokinetic profiles of amphotericin B following Fungizone and AmBisome administration were analyzed using the “WinNonlin” 4.1 computer program (WinNonlin® Professional Version 4.1, Pharsight Corporation, Scientific Consulting Inc., North Carolina, USA). The appropriate pharmacokinetic model was determined by visual examination of individual plasma concentration versus time curves and by application of Akaike information criterion (Yamaoka, Nakagawa, & Uno, 1978). Based on these, the two-compartmental model was chosen for the analysis of the data. Pharmacokinetic parameters measured included alpha (α) and (β) rate constants, area under the concentration–time curve (AUC), maximum plasma concentration (Cmax, the concentration at time zero after a bolus dose), volume of distribution of central compartment (V1), volume of distribution of peripheral compartment (V2), apparent volume of distribution at steady-state (Vss), total clearance (Cl), and appropriate half-life (t1/2).
2.6 Statistical analysis
All data, except t1/2α, t1/2β, and Mean residence time (MRT), were expressed as the mean ± standard deviation (SD). Harmonic means were calculated for t1/2α, t1/2β, and MRT. The Wilcoxon rank sum test was used to test for significant differences in t1/2α, t1/2β, and MRT. The other pharmacokinetic data were analyzed using the paired t test. Statistical significance was assigned at a p value of <.05. The SPSS (SPSS 15.0, Chicago, IL, USA) statistical software was used.
3 RESULTS
Mild-to-moderate gastrointestinal adverse effects were observed in several dogs during administration of Fungizone, and they returned to normal once the administration was completed. Following administration of Fungizone or AmBisome in dogs, no changes were detected in physical examination or complete blood counts or urinalysis data associated with the drugs.
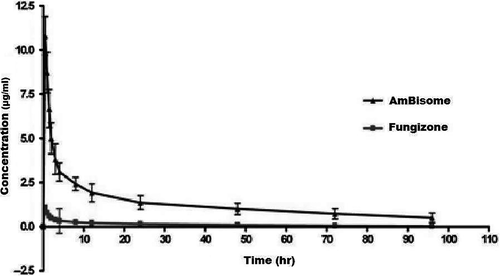
Dogs in this study mainly displayed similar diphasic plasma amphotericin B concentration profiles for both drugs (Figure 1). However, the pharmacokinetics of liposomal amphotericin B differed significantly from those of amphotericin B deoxycholate (Table 1). Although amphotericin B deoxycholate and liposomal amphotericin B were administered at the same dose, liposomal amphotericin B concentrations (Cmax: 14.8550 ± 1.845 μg/ml) were approximately ninefold higher than that of amphotericin B deoxycholate plasma concentrations (Cmax: 1.7103 ± 0.593 μg/ml) and liposomal amphotericin B (AUC: 164.9580 ± 54.491 μg·hr/ml) showed markedly higher area under the plasma concentration curve values (approximately 14-fold higher) compared to those determined for amphotericin B deoxycholate (AUC: 12.1572 ± 5.226 μg·hr/ml).
| Parameter (unit) | Conventional amphotericin B (Fungizone®) (Mean ± SD) | Liposomal amphotericin B (AmBisome®) (Mean ± SD) | p |
|---|---|---|---|
| α (Alpha) (1/hr) | 1.0584 ± 0.472 | 0.7516 ± 0.197 | .045 |
| β (Beta) (1/hr) | 0.0358 ± 0.016 | 0.0196 ± 0.012 | .031 |
| Alpha HL (t1/2α) (hr)a | 0.6549 ± 0.262 | 0.9221 ± 0.255 | .034 |
| Beta HL (t1/2β) (hr)a | 19.7064 ± 11.456 | 35.2114 ± 20.114 | .023 |
| k12 (1/hr) | 0.6999 ± 0.357 | 0.5241 ± 0.162 | .097 |
| k21 (1/hr) | 0.2218 ± 0.067 | 0.1502 ± 0.080 | .053 |
| V1 (ml/kg) | 380.1688 ± 97.184 | 40.9505 ± 4.952 | .000 |
| Cl (ml hr−1 kg−1) | 58.8164 ± 25.827 | 3.9769 ± 1.198 | .000 |
| MRT (hr)a | 24.2404 ± 16.347 | 45.1921 ± 27.412 | .028 |
| Vss (ml/kg) | 1480.0000 ± 243.376 | 201.8317 ± 49.153 | .000 |
| V2 (ml/kg) | 1099.8310 ± 220.770 | 160.8812 ± 52.423 | .000 |
| AUC (μg·hr−1 ml−1) | 12.1572 ± 5.226 | 164.9580 ± 54.491 | .000 |
| Cmax (μg/ml) | 1.7103 ± 0.593 | 14.8550 ± 1.845 | .000 |
- a Harmonic mean.
- Statistical significance was assigned at a p value of <.05.
- α, Distribution rate constant; β, Elimination rate constants; t1/2α, Distribution half-life; t1/2β, Elimination half-life; k12 and k21, Drug transfer rate constant from central to peripheral and from peripheral to central compartments, respectively; V1, Volume of distribution of central compartment; Cl, Total clearance; MRT, Mean residence time; Vss, Apparent volume of distribution at steady-state; V2, Volume of distribution of peripheral compartment, AUC, Area under the concentration–time curve; Cmax, Maximum plasma concentration.
Volumes of distribution for liposomal amphotericin B (V1:40.9505; V2:160.8812; Vss:201.8317 ml/kg) were smaller than those for amphotericin B deoxycholate (V1:380.1688; V2:1099.8310; Vss:1480.0000 ml/kg). The central compartment volumes (V1) of both drugs were smaller than the V2 and Vss values. The t1/2α and t1/2β values of Ambisome (t1/2α: 0.9221 hr; t1/2β: 35.2114 hr) were significantly longer compared to Fungizone values (t1/2α: 0.6549 hr; t1/2β: 19.7064 hr) (p < .05). The total clearance of liposomal amphotericin B (3.9769 ± 1.198 [ml hr−1 kg−1]) was less than the clearance of amphotericin B deoxycholate (58.8164 ± 25.827 [ml hr−1kg−1]).
4 DISCUSSION
The use of conventional amphotericin B in systemic mycoses of canine is limited in clinical practice for several reasons: (i) the recommended doses, based on the type of disease caused by a particular fungal organism (such as blastomycosis, histoplasmosis) range between 0.25 and 0.5 mg/kg daily (Ford, 2004; Kerl, 2003). In previous pharmacokinetic studies performed in beagle dogs, the plasma peak concentrations after application of 0.6 mg/kg and 1 mg/kg doses of amphotericin B were detected as 0.232 μg/ml and 1.10 μg/ml, respectively (Fielding, Singer, Wang, Babbar, & Guo, 1992; Fukui et al., 2003). These peak concentrations may be insufficient for some resistant candida and aspergillus species; therefore, greater amphotericin B concentrations may be required for adequate fungicidal activity. (ii) Also, eradication of several fungal infections (e.g., cryptococcosis) in animals necessitates long-term therapy with high-dose amphotericin B (O'Brien et al., 2006; Boothe, 2012). (iii) The tolerability of this drug is limited by considerable renal toxicity due to total cumulative dose and duration of therapy (Boothe, 2012). (iv) Despite its long history of usage, much is still unknown concerning the pharmacokinetics of amphotericin B, especially in the veterinary treatment (Davis, Papich, & Heit, 2009). Moreover, data on the pharmacokinetics of amphotericin B-containing liposomes are scarce in the literature and are mostly obtained from beagle dogs (Fukui et al., 2003; Serrano et al., 2013), which have generally different pharmacokinetics from the other breeds (Lemetayer, Dowling, Taylor, & Papich, 2015). The present study was therefore conducted with mixed breed dogs instead of beagle dogs, to represent the pharmacokinetics of general dog population more precisely.
In the present study, the pharmacokinetic properties of liposomal amphotericin B were compared with conventional amphotericin B deoxycholate in healthy dogs. Although both formulations were administered at the same doses (0.6 mg/kg), the plasma pharmacokinetics of liposomal amphotericin B differed significantly from those of amphotericin B deoxycholate in healthy dogs (p < .05). Liposomal amphotericin B showed markedly higher peak plasma concentrations (approximately ninefold greater) and area under the plasma concentration curves (approximately 14-fold higher) compared to those determined for other amphotericin B formulation. These results are consistent with previous reports. A study by Fukui et al. (2003) showed that AmBisome, administered to beagle dogs at a dose of 1.0 mg/kg, caused a higher (5.74-fold) plasma concentration in 5 min and AUC was 1.9-fold greater than Fungizone (in 0.6 mg/kg). In another study performed in beagle dogs which applied AmBisome at a dose of 5 mg/kg, Serrano et al. (2013) observed that the median Cmax value for AmBisome was 52-fold greater than for Free-Dimeric amphotericin formulation (in 5 mg/kg), prepared as a colloidal dispersion similar to the marked reference formulation Fungizone. The enhanced drug levels in plasma with liposomal amphotericin B presumably occur by a decrease in volume of distribution, as well as a different rate of clearance occurring during the circulation compared to amphotericin B deoxycholate. Accordingly, the steady-state volume of distribution and the clearance with liposomal amphotericin B were, respectively, 7.3-fold and 14.8-fold less than conventional formulation of the drug in dogs in the present study. Liposomes are cleared from the circulation to a substantial extent by the phagocytic cells of the mononuclear phagocyte system (MPS) (Van Etten, Ten Kate, Snijders, & Bakker-Woudenberg, 1998). Liposomal amphotericin B is small in size and has a negative charge, which prevents significant uptake with MPS. Its properties lead to high plasma levels and a larger area under the concentration–time curve (Kaufman & Manzoni, 2015). Moreover, the reduced volume of distribution of the liposomal formulation of amphotericin B may be related to a decrease in nonspecific binding with proteins and/or membrane cholesterol of amphotericin B and allows for significantly greater drug peak concentrations, which again relate to greater AUC values. To what extent greater concentrations of liposome associated amphotericin B are correlated with a greater antifungal activity in vivo is not known yet. Amphotericin B has a concentration-dependent antifungal activity. It is primarily related to the concentration which it reaches in the fungal cell. For a Cmax/MIC ratio of four, amphotericin B is already clearly effective, and its efficacy becomes optimal at a ratio of 10 (Cifani et al., 2012). In practice, maximization of the concentration-dependent activity of amphotericin deoxycholate is difficult due to its dose-related nephrotoxicity. Therefore, new studies evaluating dose-related pharmacodynamic properties of lipid amphotericin formulations such as liposomal amphotericin B are needed. This study was not designed to measure prophylactic efficacy. However, the plasma liposomal amphotericin B concentrations were within the range of MICs for susceptible strains of Candida (0.02 to 3 mg/L) and Aspergillus (0.012 to 3 mg/L) (Boothe, 2012; Mallie et al., 2005). Therefore, the data of the present study suggest that on the basis of the total plasma drug concentrations, such a dose may be practical in the prophylactic setting.
In the present study, it is also determined that both drugs have greater distribution volume of peripheral compartment in proportion to central compartment. The smaller peripheral compartment distribution volume of liposomal drug compared to conventional form may be considered to result from the slow transfer of active substance from the liposomes in the bloodstream or the slow uptake of liposomes into deep tissue sites. It is known that drug distribution throughout the body depends largely on organ blood flow and physicochemical properties of the drug (Struys, Kalmar, & De Paepe, 2008). Relatedly, Vogelsinger et al. (2006) have reported that colloidal amphotericin B displays a larger volume of distribution in comparison with liposomal amphotericin B, because the larger disk-like particles formed by colloidal amphotericin B are rapidly taken up by the peripheral tissues, particularly by cells of the reticuloendothelial system. The clinical impact of these pharmacokinetic differences between different amphotericin B formulations is unclear.
The concentration of a drug in systemic circulation is reduced by its metabolism and extraction as well as its distribution into body tissue and other fluids (Hillery, 1997). In this study, it is determined that liposomal amphotericin B has a relatively long elimination half-life (35.21 hr) compared to that of conventional amphotericin B, which is approximately two times shorter. The elimination process of the liposomal drug not only depends on the drug itself but also on the clearance of the liposomal carrier from the circulation and the release rate of the entrapped drug from the liposomal carrier (Drummond, Noble, Hayes, Park, & Kirpotin, 2008). It is stated that inclusion of cholesterol into the liposomal composition leads to a decrease in liposomal clearance and to an increase in liposomal elimination half-life (Semple, Chonn, & Cullis, 1996). Moreover, several reports have demonstrated that particle sizes of liposomes are critical factors in the elimination process of the liposomal drugs and liposomes with small particle size (100 nm) provide longer drug elimination half-life (Ait-Oudhia, Mager, & Straubinger, 2014; Azanzo, Sadada, & Reis, 2015; Sercombe et al., 2015). While the exact metabolic pathways and elimination of amphotericin B have been insufficiently elucidated (Gregio, Veiga, Rinaldi, & Bettega, 2015), the long elimination half-life of liposomal amphotericin B suggests that its elimination mechanism differs from that amphotericin B deoxycholate. In particular, the chemical properties and physical form of liposomal amphotericin B (Adler-Moore, Gangneux, & Pappas, 2016) may provide advantages based on the extended elimination half-life of the drug. Therefore, AmBisome may lead to a decrease in the frequency and length of drug administration.
In conclusion, this study that compares the pharmacokinetic profile of liposomal amphotericin B and conventional amphotericin B in dogs showed that both formulations fit to the two-compartmental model, AmBisome® reached higher plasma concentration and lower distribution volume and had a longer half-life compared to Fungizone®. Based on these results, it is concluded that AmBisome® would be an appropriate and effective choice for the treatment of systemic mycotic infections in dogs by maintaining higher plasma concentrations compared to Fungizone®.
ACKNOWLEDGMENTS
This work was supported by a grant from the Scientific Research Projects Coordination Unit of Istanbul University (Project No.3202 and UDP-45767).




