Pharmacokinetics of deflazacort in rabbits after intravenous and oral administration and its interaction with erythromycin
Abstract
The pharmacokinetic of deflazacort after intravenous and oral administration and the effect of erythromycin on the disposition of deflazacort in rabbits were investigated. A parallel study was carried out in twelve rabbits. The plasma concentration–time profiles of deflazacort were determined after intravenous and oral administration of single dosages of 5 mg/kg in the presence and absence (baseline) of multiple dose erythromycin regimens. Plasma concentrations of 21-desacetyldeflazacort were determined by HPLC. Plasma concentration–time curves were analysed by compartmental pharmacokinetic and noncompartmental methods. The t½λz values following intravenous and oral administration were 3.67 and 4.96 hr, respectively. The apparent volume of distribution at steady-state (Vss) was 4.08 ± 0.31 L/kg, this value indicates that deflazacort is widely distributed into the extravascular tissues. Moreover, bioavailability after oral administration of deflazacort (F = 87.48%) was high. Pharmacokinetic analysis after both routes of administration revealed a significant reduction in total body clearance, a significant increase in mean residence time, half-life and plasma concentrations of the steroid in the presence of multiple dose erythromycin. The results indicated the influence of the erythromycin on deflazacort disposition, which is consistent with a pharmacokinetic-type interaction in the elimination of the drug from the body. Moreover, this interaction should be considered to avoid adverse effects when using both drugs concomitantly.
1 INTRODUCTION
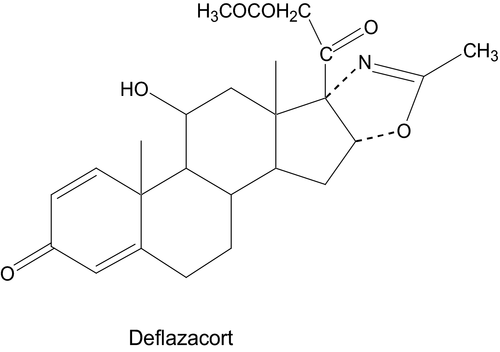
Deflazacort, (11β,16β)-21-(acetyloxi)-11-hydroxy-2′-methyl-5′H-pregna-1,4-dieno[17,16-d]oxazole-3,20-dione (Figure 1), is a synthetic heterocyclic glucocorticoid obtained from prednisolone and belonging to the class of oxazolino steroids. Deflazacort is an inactive prodrug which is rapidly converted in the body to its active alcohol metabolite, 21-desacetyldeflazacort (21HDFZ).
Studies performed using this drug in revealed a weaker effect on calcium and carbohydrate metabolism as well as a long-lasting immunosupressive action, when compared to prednisone or methylprednisolone (Marcolongo, Lalumera, Calabria, & Imbimbo, 1984; Schiatti, Selva, Barone, Restelli, & Glasser, 1980). Pharmacological studies in the rat showed that deflazacort was from 10 to 20 times as active as prednisolone in several experimental models which test anti-inflammatory activity (Nathansohn et al., 1969). Few pharmacokinetic data for deflazacort are available in animal species (Assandri, Buniva, Martinelli, Perazzi, & Zerilli, 1984; Assandri, Perazzi, Buniva, & Pagani, 1980; Assandri et al., 1983; Mollmann, Hochhaus, Rohatagi, Barth, & Derendorf, 1995). Deflazacort is rapidly absorbed and hydrolyzed after oral administration; average peak plasma concentrations of the active metabolite have been reported to be reached in 1.3 hr and the plasma half-life was 1.3 hr in human (Mollmann et al., 1995).
On the other hand, macrolide antibiotics, such as erythromycin or troleandomycin, are prescribed for many types of infections. As such they are often added to other drug therapy, thus, there are frequent opportunities for the interaction of these antibiotics with other drugs. Troleandomycin is known to delay the clearance of methylprednisolone by at least 60%, and it has been suggested that the delay in methylprednisolone clearance contributes to its efficacy (Szefler et al., 1980). Erythromycin reduces oral glucocorticoid metabolism, delaying methylprednisolone clearance by 46% (Laforce, Szefler, Miller, Ebling, & Brenner, 1983). However, there is no information available about any interaction between deflazacort and a macrolide antibiotic.
In recent years, rabbits have become increasingly popular as pet animals and are therefore often encountered in veterinary small animal practices. There are different diseases that required used of glucocorticoids alone or in concomitant use with antibiotics (Florin, Rusanen, Haessig, Richter, & Spiess, 2009; Jaberi, Nicfar, Tanideh, & Gramizadeh, 2003). Moreover, this animal may prove for further assessing mechanisms of drug- or disease-steroid interactions in veterinary medicine.
Because of this the aim of this study was to investigate the pharmacokinetics of deflazacort in rabbits after intravenous and oral administration alone and the effects of erythromycin on its disposition.
2 MATERIAL AND METHODS
2.1 Animals
Twelve healthy New Zealand white rabbits (4.1–4.8 kg) were obtained from the Laboratory Animal Farm of the University of Murcia (Spain). They were housed individually in cages under a 12-hr light/dark cycle and were fed pelleted feed concentrate with free access to food and water. Temperature was maintained at 20.1–22.3°C and relative humidity was 60–75%. They did not receive any drug treatment for at least 30 days preceding the experience.
2.2 Materials
Deflazacort pure substance, 21-desacetyldeflazacort and deflazacort hemissucinate were supplied by L. Zerilli from Research Laboratories of Gruppo Lepetit (Gerenzano, Italy). Erythromycin estearate suspension was a commercially available formulation (Pantomicina 250 suspensión®, Abbot Laboratories, S.A., Madrid, Spain). Deflazacort suspension for oral administration was a commercial formulation (Zamene®, Menarini Laboratories, Barcelona, Spain). Prednisolone reference standard was obtained from Sigma-Aldrich Química, S.A. (Madrid, Spain). All other chemicals and reagents used were commercially available and of guaranteed purity.
2.3 Study design
A parallel study was conducted in two phases, baseline and posterythromycin, with a washout period of 30 days. The study was sequential because a potential prolonged erythromycin inhibition of the hepatic metabolism might confound the interpretation of a complete cross-over study. The first phase was control in which only an aqueous solution of deflazacort hemisuccinate was intravenously (n = 6) or orally (n = 6) administered to each animal at a single dose of 5 mg/kg bodyweight. This permitted assessment in the stability of the disposition of deflazacort over time in the absence of other perturbing factors. After the washout period, the twelve rabbits received orally 50 mg/kg/12 hr of an erythromycin estearate suspension for 10 days. Finally, 12 hr after the last antibiotic intake the animals received another intravascular or oral single dose of 5 mg/kg of deflazacort hemisuccinate.
For the control studies, rabbits received oral water during 10 days previously to the corticoid administration. The oral dosing rate and oral dosing volumes in both the control studies and the interaction studies were identical. This controls for effects of stress exposure across treatments, an important consideration when investigating compounds which interact with the hypothalamic–pituitary–adrenal axis.
Following the final oral dose of erythromycin or water (control group), each rabbit was placed in a restraining device and deflazacort was administered by intravenous injection into the marginal vein of one ear or by oral administration. Blood samples (2 ml) were collected from the contralateral ear vein (in the case of intravenous administration) into heparinized tubes at 0 (pretreatment), 0.083, 0.167, 0.25, 0.5, 0.75, 1, 1.5, 2, 4, 6, 8, 10, 12, 18, 24 and 72 hr following drug administration. Samples were centrifuged at 1500 g over 10 min within 30 min after collection. Plasma was immediately removed and stored at −40°C until being assayed.
The protocol of this study adhered to the Principles of Laboratory Animal Care and was approved by the Bioethical Committee of the Faculty of Veterinary Medicine, University of Murcia (Spain).
2.4 Analytical method
Plasma concentrations of 21HDFZ were analysed by high-performance liquid chromatography according to the method of Bernareggi, Poletti, Zanolo, and Zerilli (1987). The HPLC system was equipped with a model 305 and 306 pumps, 811C dynamic mixer, 805 manometric module, 115 UV detector (Gilson, Middleton, USA), and a model SIL-10ADVP autoinjector (Shimadzu, Kyoto, Japan) connected to a computer with a Shimadzu Class-VP™ Chromatography Data System programme (Shimadzu, Columbia, USA). A 1 ml plasma sample, 200 mg NaCl and 2.5 μl of the internal standard working solution (500 ng of prednisolone) were added to a 10 ml glass tube and mixed by shaking. After that the specimen was extracted in two serial steps, with 10 and 5 ml of methylene chloride also by shaking for 10 min, at 300 inversions per min. Each sample was then centrifuged at 3000 g for 10 min (ambient temperature) and aqueous layer as well as creamy interface was aspirated and discarded. The remaining organic phase was twice washed using 1 ml of an aqueous solution of 0.1 M sodium hydroxide saturated with sodium chloride and subsequently using 1 ml of a saturated aqueous solution of sodium chloride. After shaking and centrifuging once again, the aqueous phase was aspirated and discarded; then 1 g of anhydrous sodium sulphate was added to dry the organic phase. Finally, after shaking and centrifuging, the organic phase was transferred to a conical tube and dried down under a gentle stream of nitrogen at 37°C.
This residue was redissolved in 100 μl of the mobile phase and 20 μl injected onto the HPLC column (Zorbax Sil 250 × 4.6 mm, 5 μm, Hichrom Ltd., Bekshire, UK). An isocratic elution at ambient temperature was carried out using methylene chloride-methanol (94:6) as the mobile phase. Flow rate was 1 ml/min. Measurements were made by ultraviolet detection at 254 nm with a 0.01 absorbance units full scale (A.U.F.S.).
The retention time was 8 min for 21HDFZ and 6 min for the internal standard (prednisolone). The assay was validated by measuring concentration of known amounts of deflazacort (DFZ) and 21HDFZ in a range from 10 to 5000 μg/L. The average (±SE) recoveries between and within-batch were for DFZ 96.7 ± 0.7, 98.5 ± 0.4 per cent and for 21HDFZ 97.6 ± 0.5, 98.9 ± 1.3 per cent, respectively. The limit of detection was 5 μg/L for DFZ and 21HDFZ.
2.5 Pharmacokinetics
The concentration–time data obtained after each treatment in each individual rabbit were initially fitted to one-, two- and three-exponential equations by the retroprojection method (Gibaldi & Perrier, 1982), and the PKCALC computer program (Shumaker, 1986) was used to obtain the best estimates of the parameters of these equations. The final curve fitting was carried out using nonlinear regression with the MULTI computer program and Gauss-Newton damping algorithm (Yamaoka, Tanaka, Okumura, Yasuhara, & Hori, 1986). The data were analysed on an individual animal basis using a weighting of 1/concentration. The minimum Akaike's information criterion (Yamaoka, Nakagawa, & Uno, 1978) and coefficient of determination were used to select the best equation that defined plasma concentration–time data for each animal.
Pharmacokinetic parameters were obtained from the individual fitted equations (Gibaldi & Perrier, 1982). The pharmacokinetic parameters were calculated for each rabbit and reported as the mean ± SD.
A noncompartmental analysis was used to determine the area under the concentration–time curve (AUC), using the linear trapezoidal rule with extrapolation to infinity time, mean residence time (MRT), mean absorption time (MAT = MRTSC, IM − MRTIV), systemic clearance (Cl), apparent volume of distribution at steady-state (Vss) and bioavailability (F = AUCSC,IM × 100/AUCIV).
2.6 Statistical analysis
Statistical parameters were calculated and the Kolmogorov–Smirnov test and the Mann–Whitney test were employed to verify the homogeneity of the data and to test for between-rabbit differences in the parameters. The Wilcoxon Rank Sum test and the Student's t-test were used to test parameters for significant differences between baseline and posterythromycin situation in each rabbit. The Mann–Whitney test was also used to compare experimental values with theoretical concentrations and was completed with a multivariate correlation analysis (Powers, 1990). In all cases the minimum probability level tested was 95% (p < 0.05).
3 RESULTS
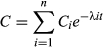
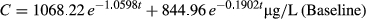
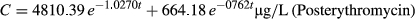
For oral administration, a one-compartment model with first order absorption always provided the best description of the concentration–time data of 21HDFZ in both experimental conditions.
The mean plasma concentrations of 21HDFZ at the times of sample collection after intravenous and oral administration for both experimental conditions are plotted in Figures 2 and 3, respectively. Erythromycin pretreatment resulted in a significantly higher mean plasma 21HDFZ concentrations compared with baseline conditions.
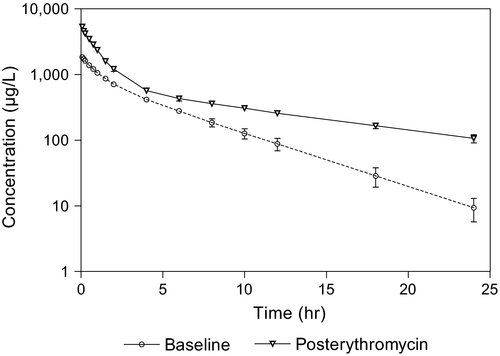
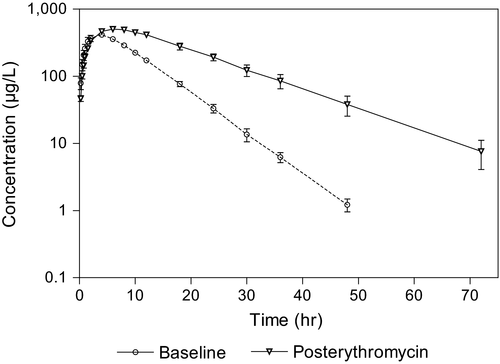
The mean (±SD) pharmacokinetic parameters based on compartmental pharmacokinetic analysis and noncompartmental methods are presented in Table 1.
| Parameters | Baseline | Posterythromycin | Level of significance (p) |
|---|---|---|---|
| Intravenous administration | |||
| λ1 (hr−1) | 1.06 ± 0.17 | 1.03 ± 0.05 | n.s. |
| λz (hr−1) | 0.19 ± 0.02 | 0.08 ± 0.01 | <.01 |
| t½λ1 (hr) | 0.67 ± 0.13 | 0.68 ± 0.03 | n.s. |
| t½λz (hr) | 3.67 ± 0.36 | 9.14 ± 0.69 | <.01 |
| Vss (L/kg) | 4.08 ± 0.31 | 3.32 ± 0.22 | <.01 |
| AUC (μg hr/L) | 5541.73 ± 309.49 | 13538.80 ± 357.43 | <.01 |
| AUMC (μg hr2/L) | 24922.6 ± 4022.3 | 119626.0 ± 12039.5 | <.01 |
| MRT (hr) | 4.48 ± 0.51 | 8.83 ± 0,74 | <.01 |
| Cl (L/kg hr) | 0.90 ± 0.05 | 0.37 ± 0.01 | <.01 |
| Vz (L/kg) | 4.78 ± 0.33 | 4.89 ± 0,34 | n.s. |
| Oral administration | |||
| ka (hr−1) | 0.53 ± 0.08 | 0.31 ± 0.02 | <.01 |
| λz (hr−1) | 0.14 ± 0.01 | 0.07 ± 0.01 | <.01 |
| t½a (hr) | 1.34 ± 0.23 | 2.23 ± 0.16 | <.01 |
| t½λz (hr) | 4.96 ± 0.18 | 10.14 ± 1.06 | <.01 |
| AUC (μg hr/L) | 4837.13 ± 192.12 | 11292.64 ± 1078.63 | <.01 |
| MRT (hr) | 9.15 ± 0.49 | 17.81 ± 1.61 | <.01 |
| MAT (hr) | 4.67 ± 0.62 | 8.98 ± 1.46 | <.01 |
| F (%) | 87.48 ± 5.42 | 83.39 ± 7.43 | n.s. |
| tmax (hr) | 3.46 ± 0.38 | 6.26 ± 0.41 | <.01 |
| Cmax (μg/L) | 420.14 ± 31.66 | 501.66 ± 11.92 | <.01 |
- Ka, the absorption disposition rate constant; t½a, the absorption half-life associated with the initial slope (ka) of a semilogarithmic extravascular concentration–time curve; t½λ1, the disposition half-life associated with the initial slope (λ1) of a semi-logarithmic concentration–time curve; t½λz, the elimination half-life associated with the terminal slope (λz) of a semilogarithmic concentration–time curve, Vss, the apparent volume of distribution at steady-state; Vz, the apparent volume of distribution calculated by the area method; Cl, the total body clearance of drug from the plasma; AUC, the area under the plasma concentration–time curve from zero to infinity; AUMC, area under the moment curve; MRT, mean residence time; F, the fraction of the administered dose systemically available (bioavailability); Tmax, the time to reach peak or maximum plasma concentration following intramuscular and subcutaneous administration; MAT, mean absorption time; Cmax, the peak or maximum plasma concentration following intramuscular and subcutaneous administration.
The Wilcoxon Rank Sum Test and the Student's t-test performed on pharmacokinetic parameters after intravenous administration of deflazacort revealed significant differences for all pharmacokinetic parameters between baseline and postantibiotic situations except for λ1, t½λ1 and Vz (Table 1).
The value obtained for t½λz was 9.14 hr after the antibiotic intake (3.67 in the baseline situation) and for MRT 8.83 hr (4.48 hr in baseline conditions) both parameters were significantly different between the two situations. Finally, plasma clearance was reduced 2.45 times after erythromycin pretreatment.
Both statistical tests revelead significant differences in all of the pharmacokinetic parameters between baseline and postantibiotic situations obtained after oral administration, except in systemic availability of the drug.
Absorption of deflazacort from the digestive tract was relatively fast although was delayed in the case of pretreatment with erythromycin, so that peak levels were attained at 3.46 and 6.26 hr, respectively. As occurred for intravenous administration, higher mean plasma 21HDFZ concentrations and longer half-life were obtained after pretreatment with erythromycin. The extent of oral availability was high and without significant differences between the two situations.
All of the findings are consistent with a pharmacokinetic interaction between erythromycin and deflazacort when administered together.
4 DISCUSSION
The t½λz values following intravenous (i.v.) and oral administration were 3.67 and 4.96 hr, respectively, these values are very shorter to that determined for Assandri et al. (1980) in rat, man and dog. Consequently, the systemic clearance in our study (Cl = 0.90 ± 0.05 L/hr kg) was faster than those described in other species for deflazacort (Assandri et al., 1980).
The apparent volume of distribution at steady-state (Vss) was 4.08 ± 0.31 L/kg, this value indicates that deflazacort is widely distributed into the extravascular tissues. In other studies, larger volumes of distribution (Vd) were described for glucocorticoids in pigs (2.78 L/kg; Wyns et al., 2013) and dogs (5.2–7.7 L/kg; Ryrfeldt, Tonnesson, Nilsson, & Wikby, 1979).
In the present study, bioavailability after oral administration of deflazacort (F = 87.48%) was high, but this value was lower than to values have been obtained for other glucocorticoids in mammalian species (Wyns et al., 2013). These results indicate that glucocorticoids show good absorption after extravascular routes of administration.
Previous studies on interactions between macrolide antibiotics and steroids have been carried out using methylprednisolone. In this way, Ebling, Rich, Szefler, and Jusko (1987) analysed the effect of troleandomycin related to this drug in rabbits, finding a significant decrease in total plasma clearance of methylprednisolone in the presence of multiple dose of troleandomycin.
As previously described, plasma concentrations after intravenous administration of corticosteroid in the present trial were higher after antibiotic pretreatment (Figure 2), and for both experimental conditions the two-compartment open model always provided the best description of the concentration–time data. The range of concentrations for 21HDFZ in the baseline situation was similar to those previously reported in pharmacokinetic studies performed with animals and human beings (Assandri et al., 1984). Therefore the differences observed after erythromycin pretreatment are due to an interaction between the macrolide and the corticosteroid. Erythromycin therapy resulted in a 59% reduction of 21HDFZ clearance, significantly higher mean plasma concentrations and an increased MRT and half-life compared with pre-erythromycin concentrations. Similarly troleandomycin (Szefler et al., 1980), erythromycin (Laforce et al., 1983) and clarithromycin (Fost et al., 1999) therapies have shown to significantly reduce the clearance of methylprednisolone in human clinical studies by at least 60%, 46% and 65%, respectively, and it has been suggested that the delay in methylprednisolone clearance contributes to its efficacy. In addition to clearance, erythromycin appears to cause a significant decrease in steady-state volume of distribution of 21HDFZ. The mechanism for this effect has not been investigated but it has been suggested that may reflect a decrease in tissue binding or an increase in plasma protein binding of the corticosteroid (Ludden, 1985).
After oral administration, a one-compartment model with first order absorption always provided the best description of the data in rabbits. This result is similar to that obtained previously by Assandri et al. (1984) in rats, dogs, monkeys and humans. This is a common phenomenon for drugs whose disposition after intravenous administration fits a two-compartment model, because if the value of the absorption rate constant is the same or lower than the rate constant for the distribution phase (λ1), this phase will not appear in the curves and the drug's disposition is best interpreted according to an open one-compartment model (Gibaldi & Perrier, 1982).
In rabbits, the control oral administration of deflazacort appears to be absorbed as rapidly as in monkeys, but slower than in rats and humans (Assandri et al., 1984). The oral half-lives longer than after intravenous administration and values of MAT higher than the intravenous MRT values indicated that the 21HDFZ follows a flip–flop model in which the initial phase of the plasma concentration curve determined the apparent elimination rate constant (λz) and the last phase defined the absorption rate constant (which is the limiting factor for the elimination of the drug). This effect is also observed after the treatment with the macrolide antibiotic.
On the other hand, although the analysis of other metabolites of deflazacort was beyond the scope of the present study, metabolite III (6-β-hydroxy-21-desacetyldeflazacort) was detectable in the chromatograms after the erythromycin administration. This finding could be due to the delay of the elimination phase.
The results of our study indicated a influence of the macrolide antibiotic on deflazacort disposition, which is consistent with a pharmacokinetic-type interaction in the elimination of the drug from the body. Then, the long-term use of erythromycin in animals receiving chronic deflazacort therapy may place those animals at increased risk for steroid-induced adverse effects, but clinical studies are needed to verify this fact. Nevertheless, deflazacort has revealed a weaker effect on calcium and carbohydrate metabolism as well as a long-lasting immunosupressive action, when compared to prednisone or methylprednisolone (Markham & Bryson, 1995). Moreover, using long-term therapy of deflazacort instead prednisolone has been associated with decreased loss of total skeleton bone mineral density, helped to prevent redistribution of total body fat and worsening of the lipid profile in kidney transplant patients (Lippuner, Casez, Horber, & Jaeger, 1998).
Finally, we can conclude that erythromycin affects deflazacort disposition in rabbits, these animals may serve as a useful animal model for further studies of the erythromycin/deflazacort interaction previously to evaluate its clinical efficacy and applications.
ACKNOWLEDGMENTS
Thanks are due to Dr L. Zerilli from Lepetit Research Center (Gerenzano, Italy) and to Marion Merrell Dow Laboratories for supplying deflazacort pure substance and its metabolites. This study was supported by the University of Murcia (SPAIN). The authors wish to thank Dr. M. Alvinerie for his invaluable help and comments on this research. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.




