A comparative study between responses of isolated bovine and equine digital arteries to vasoactive mediators
Abstract
Hemodynamic perturbations, partly resulting from abnormal vasoconstriction of digital vessels, have been implicated in the pathogenesis of bovine and equine laminitis. This study compared the responsiveness of isolated bovine (BDA) and equine (EDA) digital arteries to pharmacological agents that stimulate receptor systems involved in the regulation of normal vessel tone. The role of the endothelium and the short- and longer-term effects of an experimentally induced endothelial damage were also evaluated. Species-related differences were found in the vessel reactivity to all of the receptor agonists tested. In intact BDA, as compared to intact EDA, norepinephrine was a more effective vasoconstrictor, 5-hydroxytryptamine a more effective but less potent vasoconstrictor, isoproterenol a less effective vasodilator and carbamylcholine a less potent vasodilator. In BDA, but not in EDA, the contractile responses to norepinephrine and 5-hydroxytryptamine were enhanced immediately after endothelium removal. However, the contractile reactivity of denuded BDA returned to basal values following overnight incubation. The differences suggest species specificity for the pathophysiology of digital vasomotor tone and function in horses and cattle.
1 INTRODUCTION
The neuro-humoral systems participating in the physiology and pathophysiology of digital vessel tone regulation have been extensively characterized in the horse (Bailey, Marr, & Elliott, 2004; Katz & Bailey, 2012). By contrast, the systems operating in the digital vessels of cattle have been investigated only in a limited number of studies (Belloli, Badino, Arioli, Odore, & Re, 2004; Comerma et al., 2008; Elmes & Eyre, 1977; Punzi, Belloli, Gogny, Desfontis, & Mallem, 2016; Risso et al., 2012).
Acute laminitis shows different clinical features in the two animal species. Indeed, although cases of subacute or chronic equine laminitis may have insidious onset, in horse most acute laminitis cases are painful and potentially life-threatening with clinical signs including severe lameness, elevated foot temperature and bounding digital pulse (Eustace, 2010; Hood, 1999a). In cattle, acute clinical signs are not usually perceived and the occurrence of the disease is first noticed when chronic condition has developed following repeated episodes of subclinical acute laminitis (Bergsten, 2003; Boosman, Németh, & Gruys, 1991; Hernandez, Garbarino, Shearer, Risco, & Thatcher, 2005). Despite the clinical differences, the pathogenesis of acute laminitis in horse and cattle, in which hemodynamic disturbances have been implicated (Bailey et al., 2004; Bergsten, 2003; Hood, 1999b), is generally considered to be comparable (Boosman et al., 1991). Indeed, dysfunction of digital circulation at the level of the digital vessels and in the laminar microvasculature has been well documented in horses during the prodromal phases of experimentally induced laminitis (Adair, Goble, Schmidhammer, & Shires, 2000; Allen, Clark, Moore, & Prasse, 1990; Eades, Stokes, & Moore, 2006; Eades et al., 2007; Schneider, Parks, Eades, & Tackett, 1999). Furthermore, evidence indicates that hemodynamic disturbances are also implicated in the development of laminitis in cattle (Bergsten, 2003).
Several potential mechanisms leading to the abnormal digital hemodynamic in horses during laminitis have been suggested, including increased systemic or local levels of endogenous vasoconstrictors, such as serotonin, thromboxane A2, endothelin-1 and catecholamines, as well as endothelial cell damage or dysfunction resulting in reduced endothelium-dependent vasodilation (Bailey et al., 2004, 2009; Baxter, Laskey, Tackett, Moore, & Allen, 1989; Schneider et al., 1999). Despite the lack of targeted investigation on the changes to vasomotor function in the bovine foot, the documented presence of thrombi and sole haemorrhages in affected claws after acute subclinical episodes of the naturally occurring disease (Bergsten, 2003; Boosman et al., 1991) provides indirect evidence that digital endothelial damage is also a characteristic feature of bovine laminitis.
It is known that there can be species-specific differences in the responses of vascular beds to vasoactive substances as well as in the role of the endothelium in the modulation of these responses (Karlsson, Bodelsson, Bodelsson, & Stjernquist, 1998; Mahajan & Tabrizchi, 2010; Nakagomi et al., 1998). Therefore, the possibility is here considered that the different clinical picture of acute laminitis in cattle and horse might in part due to differences in the vasomotor reactivity of their digital vessels.
The aim of the study was to investigate whether there are differences in the vasomotor reactivity between bovine and equine digital vascular beds. As a first step in the present work, we evaluated and compared the responses of normal bovine and equine digital arteries (BDAs and EDAs, respectively) to vasoactive pharmacological agents that stimulate receptor populations involved in the normal regulation of vessel tone and that have been suggested to be involved in laminitis-associated vasomotor changes in horses (i.e., α-adrenergic, β-adrenergic, serotoninergic, cholinergic/muscarinic). Endothelium-intact and endothelium-denuded arteries were also evaluated to investigate the effect that endothelium had on these responses. Furthermore, because it has been suggested that when the vascular endothelium is chronically compromised, the underlying smooth muscle acquires the ability to change its reactivity to vasoactive stimuli (Binko, Meachem, & Majewski, 1999; Bishop-Bailey, Larkin, Warner, Chen, & Mitchell, 1997; Punzi et al., 2016), intact and denuded vessel preparations were assayed immediately after isolation or following overnight incubation in tissue culture medium. Direct comparability of the data obtained from the two species was guaranteed through preliminary work performed to help choose the correct experimental model and to establish uniform protocols for tissue handling and pharmacological stimulation.
2 MATERIALS AND METHODS
2.1 Isolated vessel preparation
BDAs and EDAs were collected from the forelimbs of healthy, adult, mixed-breed animals of both sexes immediately after slaughter at local abattoirs. The animals underwent a close clinical examination before slaughter, and the ones showing any signs of lameness or macroscopically evident foot lesion were discarded. Vessels were excised from the selected forelimbs, immediately flushed with saline to remove the remaining blood, placed in prechilled saline and transported to the laboratory within approximately 1 hr.
The arteries were cleared of surrounding tissues and flushed with Krebs–Henseleit solution at pH 7.4 (mM: NaCl 118.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.5 – Mallinckrodt Baker). Where appropriate, the endothelium was removed by endoluminal infusion of a 0.3% sodium deoxycholate solution (Sigma-Aldrich). A 3 to 4 mm wide ring was cut from the endothelium-intact (e+) or endothelium-denuded (e−) vessel segment and assayed immediately (fresh preparations—F) or following overnight incubation (approximately 16 hr) in tissue culture medium (DMEM supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin and 4 mM L-glutamine—Cambrex Bio Science) at 30°C (incubated preparations—I). The artery of each animal was randomly assigned to be prepared to receive just one treatment combinations (F/e+, F/e−, I/e+ or I/e−) for only one agonist.
2.2 Isometric tension recording
Artery rings were mounted in 10 ml organ baths (Ugo Basile and 2 Biological Instruments) containing oxygenated (95% O2–5% CO2) Krebs–Henseleit solution at 37°C. Artery rings were suspended from an isometric force transducer (FORT 10 and 25, WPI) connected via an amplifier to a computerized data acquisition system (PowerLab/400, ADInstruments; MP100, Biopac Systems). A 4 g passive tension was applied, which preliminary experiments showed gave optimal contractile response to depolarizing solution (80 mM KCl) in vessel rings of this size from both species. After washing and recovery of baseline tension, an equilibration period of 1 hr was allowed prior to the administration of the drugs. During this equilibration period, the Krebs solution was changed four times (i.e., every 15 min). Tissue viability was tested by addition of norepinephrine (NOR—Sigma-Aldrich), at a dose of 10−6 M, which induced, in preliminary concentrations–response curves (CRCs) assays, approximately 50% of maximal contraction. BDA and EDA rings responding with less than 20 and 10 g tension/g tissue, respectively, were discarded. Preliminary experiments identified these values as the minimum acceptable level of viability that ensures a reasonable chance generating complete and reliable concentration–response curves to selected agonists in the vessels of each species. At the plateau of NOR-induced contraction, the presence of functional endothelium was checked by addition of carbamylcholine (CARB—Sigma-Aldrich) at the maximal vasorelaxing dose of 10−5 M. For the assays on endothelium-intact vessels, rings were used if they showed at least 15%–20% relaxation in response to CARB. This cut-off was established by applying a statistical method (Jacobson & Truax, 1991) to the maximal relaxation values determined for acetylcholine in intact EDA rings under experimental conditions close to those of this study (Cogswell, Johnson, & Adams, 1995). The lack of any CARB-induced relaxations was prerequisite for the experimental use of endothelium-deprived rings.
2.3 Pharmacological stimulation protocols
BDA and EDA rings were assayed for their responses to NOR (α-adrenergic agonist), 5-hydroxytryptamine (serotoninergic agonist, 5-HT—Sigma-Aldrich), (-)isoproterenol (β-adrenergic agonist, ISOP—Sigma-Aldrich) and CARB (cholinergic agonist). CRCs to the agonists were constructed under all the above experimental conditions (fresh or incubated endothelium-intact/deprived preparations), except for CARB which was tested only in endothelium-intact preparations. Each vessel ring was exposed to only one of the selected pharmacological agents. CRCs to each agonist were constructed in the same cumulative (5-HT and ISOP: 10−10–10−4 M) or non-cumulative (NOR: 10−8–10−4 M, CARB: 10−9–10−4 M) fashion and attention was paid to ensure equally effective preconstriction levels to test the relaxant responses: the effects of ISOP were studied in rings preconstricted to 80%–90% of their maximum contraction with the thromboxane mimetic U44069 (Sigma-Aldrich) at a dose of 10−6 M and 10−5 M in EDA and BDA rings, respectively. CARB-dependent relaxation was studied after induction of 50% maximal tension with NOR at a dose of 10−6 M in both EDA and BDA rings.
2.4 Data analysis and statistics
Contractile responses were expressed as g tension/g tissue wet weight and relaxant responses were expressed as percentage reduction in precontracting agent-induced tone. The effects of each agonist were plotted against Log molar drug concentration and efficacy (Emax) and potency (pD2 = −LogEC50) best fit values were derived for each assayed vessel ring by a computerized nonlinear regression procedure (GraphPad Prism, v. 3.02; GraphPad Software). The significance of species (grouping variable), endothelium removal and incubation (experimental variables) as putative factors affecting, either alone or in combination, the efficacy and potency (dependent variables) of NOR, 5-HT and ISOP was evaluated first by a univariate three-way general linear model analysis of variance (ANOVA), with 2 × 2 × 2 design (species: horse/bovine; endothelium removal: not performed/performed; incubation: not performed/performed). Separate two-way ANOVAs and/or unpaired Student's t tests were conducted in a top-down fashion, in order to identify the location more precisely and to confirm the differences found. As for CARB, differences in efficacy and potency were analysed in dependence of the factors species and incubation by two-way ANOVA, followed by unpaired Student's t tests in the event of any significant results. Statistical analyses were performed using SPSS statistical software (v. 18.0) and GraphPad Prism (v. 3.02; GraphPad Software). The level of significance was set at P < .05.
3 RESULTS
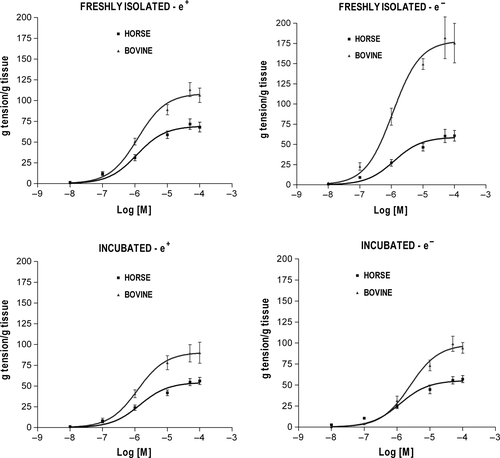
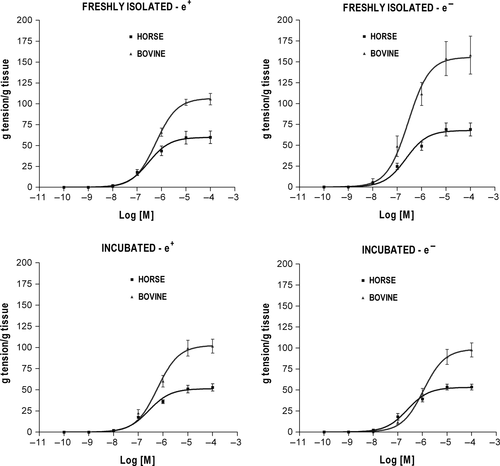
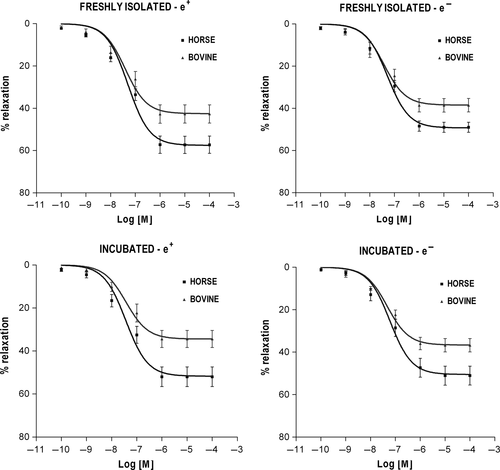
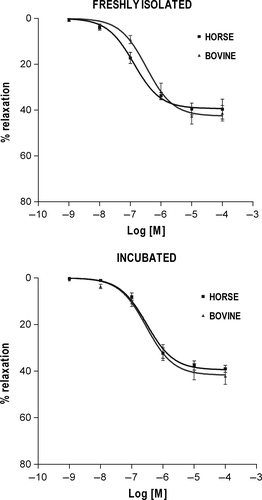
In both BDAs and EDAs, the functional responses evoked by each agonist were dose-related and showed qualitatively similar contractile (NOR, 5-HT) or relaxant (ISOP, CARB) patterns regardless of the experimental condition (Figures 1-4). The curve fitting parameters (Emax, pD2) calculated from the comparative experiments are presented in Tables 1-4.
| Horse | Bovine | |||
|---|---|---|---|---|
| F | I | F | I | |
| Emax | ||||
| e+ |
69.51 ± 5.26A (9) |
54.23 ± 3.71C (7) |
(6) |
90.45 ± 10.00C (6) |
| e− |
58.90 ± 5.67B (6) |
55.84 ± 4.40D (9) |
(6) |
(8) |
| pD2 | ||||
| e+ |
5.95 ± 0.10 (9) |
5.84 ± 0.09 (7) |
5.90 ± 0.06 (6) |
5.92 ± 0.09 (6) |
| e− |
5.91 ± 0.10 (6) |
5.90 ± 0.10 (9) |
5.97 ± 0.08 (6) |
5.60 ± 0.08 (8) |
- A,a,DP < .001.
- B,b,CP < .01.
| Horse | Bovine | |||
|---|---|---|---|---|
| F | I | F | I | |
| Emax | ||||
| e+ |
59.96 ± 7.55A (9) |
51.09 ± 4.07C (12) |
(6) |
102.89 ± 8.81C (6) |
| e− |
67.46 ± 7.58B (10) |
53.02 ± 3.62D (9) |
(9) |
(7) |
| pD2 | ||||
| e+ |
6.54 ± 0.10E (9) |
6.57 ± 0.05F (12) |
(6) |
6.23 ± 0.08F (6) |
| e− |
6.64 ± 0.11 (10) |
6.61 ± 0.13G (9) |
(9) |
(7) |
- A,B,C,D,dP < .001.
- F,GP < .01.
- E,a,b,cP < .05.
| Horse | Bovine | |||
|---|---|---|---|---|
| F | I | F | I | |
| Emax | ||||
| e+ |
57.59 ± 4.07A (13) |
51.94 ± 4.58C (12) |
42.30 ± 4.03A (9) |
34.66 ± 3.98C (9) |
| e− |
49.12 ± 2.17B (9) |
50.98 ± 4.09D (12) |
38.81 ± 3.16B (7) |
36.61 ± 3.09D (7) |
| pD2 | ||||
| e+ |
7.33 ± 0.08 (13) |
7.47 ± 0.15 (12) |
7.45 ± 0.14 (9) |
7.41 ± 0.13 (9) |
| e− |
7.27 ± 0.13 (9) |
7.24 ± 0.13 (12) |
7.42 ± 0.15 (7) |
7.35 ± 0.05 (7) |
- A,B,C,DP < .05.
| Horse | Bovine | |||
|---|---|---|---|---|
| F | I | F | I | |
| Emax | ||||
| e+ |
39.36 ± 2.88 (10) |
39.30 ± 1.96 (10) |
42.28 ± 3.02 (12) |
41.18 ± 3.60 (14) |
| pD2 | ||||
| e+ |
(10) |
6.54 ± 0.08b (10) |
6.47 ± 0.11a (12) |
6.53 ± 0.11 (14) |
- a,bP < .05.
3.1 Vasoconstrictor response to NOR
The three-way ANOVA of NOR efficacy revealed a significant main effect of species (P < .001) and a significant three-way interaction among species, endothelium removal and incubation (P < .05). The efficacy was overall greater in bovine than in horse (Figure 1) and separate t tests run between corresponding subgroups of the two species confirmed the significance for each of the four experimental conditions examined (P < .01) (Table 1). The separate two-way ANOVAs run for endothelium removal and incubation within each species revealed significant interaction between the two factors in BDA (P < .05) and no main or interaction effects in EDA. Direct comparisons between specific bovine subgroups (t tests) showed that endothelium removal was associated with significant increase in NOR Emax in fresh vessels (P < .001) and that performing incubation resulted in a significant decrease in NOR Emax in endothelium-denuded vessels as compared to vessels freshly deprived of the endothelium (P < .01) (Table 1). As concerns NOR potency, three-way ANOVA did not reveal any significant differences between bovine and horse.
3.2 Vasoconstrictor response to 5-HT
The three-way ANOVA of 5-HT efficacy gave similar results to those obtained for NOR, namely a significant main effect of species (P < .001) with an overall greater Emax in BDA than in EDA (Figure 2) that was confirmed at each experimental condition (t test between corresponding subgroups of the two species; P < .001) (Table 2), as well as a significant three-way interaction among species, endothelium removal and incubation (P < .05). Interaction between endothelium removal and incubation proved to be significant only in BDA (two-way ANOVAs; P < .05), where endothelium removal was associated with a significant increase in 5-HT Emax in fresh vessels (t test: P < .05) and performing incubation resulted in a significant decrease in 5-HT Emax in denuded vessels as compared to vessels freshly deprived of the endothelium (t test: P < .05) (Table 2).
The three-way ANOVA of 5-HT potency also disclosed a highly significant main effect of species (P < .001), with overall lower pD2 in bovine than in horse, which was confirmed at all experimental conditions (t test; P < .05) except for fresh-denuded vessels (Table 2). The three-way interaction was close to significance (P = .06), but the endothelium removal by incubation and species by incubation two-way interactions were significant (P < .05). When two-way ANOVAs were run for bovine and horse separately, a significant interaction between endothelium removal and incubation was present only in BDA (P < .01). Further comparisons (t tests) revealed that removing the endothelium was associated with significant 5-HT pD2 increase in fresh vessels (P < .05) and that performing incubation in endothelium-denuded vessels was associated with significant 5-HT pD2 decrease (P < .001).
3.3 Vasodilator response to ISOP
The three-way ANOVA of ISOP efficacy only revealed a highly significant main effect of species (P < .001) and direct comparisons between corresponding subgroups of the two species (t tests) confirmed that mean Emax was overall lower in BDA than in EDA at each experimental condition (P < .05) (Figure 3, Table 3). The same analysis for ISOP potency did not show any significant result.
3.4 Vasodilator response to CARB
The two-way ANOVA of CARB efficacy did not reveal significant differences between bovine and horse (Figure 4). As for CARB potency, the same analysis showed a significant main effect of species (P < .05) with pD2 value being overall lower in BDA than in EDA. Separate t tests confirmed CARB potency to be lower in BDA than in EDA only when fresh vessels were compared (P < .05) and a significant decrease in potency was associated with incubation in EDA (P < .05), but not in BDA.
4 DISCUSSION
The results of this work revealed that there are clear-cut differences between BDA and EDA in reactivity to a panel of vasoactive receptor agonists under various experimental conditions.
The most striking between-species differences were found in vessel reactivity to the vasoconstrictors and involved, in particular, the contractile responses of intact vessels. NOR showed greater contractile efficacy in intact BDA than in intact EDA, whereas potency values did not differ between the two species and were in line with previous studies (Baxter, 1995; Baxter, Laskey et al., 1989; Belloli, Carcano, Arioli, & Beretta, 2000; Belloli et al., 2004; Cogswell et al., 1995). This suggests that the species-related differences in the whole vessel reactivity to NOR probably involve intracellular pathways mediating downstream the effects of receptor activation. 5-HT, too, showed greater contractile efficacy in intact BDA than in intact EDA, but potency was higher in the latter, suggesting that species-related differences also may involve the density and/or type of the receptors. The high sensitivity to 5-HT is a well-known property of isolated EDA (Bailey & Elliott, 1998a; Bailey & Elliott, 1998b Baxter, 1995; Baxter, Laskey et al., 1989), and several studies suggest that 5-HT could play an important role in maintaining normal vasoconstrictor tone of the equine digital vessels as well as in mediating digital vasospasm in developing laminitis (Bailey et al., 2004). The 5-HT EC50 values estimated here for EDA (~45 ng/ml) were about three times lower than those recorded for BDA (~127 ng/ml). Although caution must be taken when data from in vivo and in vitro findings are related, the greater 5-HT potency recorded in equine may be consistent with the lower free plasma levels of 5-HT measured (by HPLC) in healthy horses (~3.5 ng/ml) than in cows (~170 ng/ml) (Bailey & Elliott, 1998a; Bruschetta, Di Pietro, Sanzarello, Giacoppo, & Ferlazzo, 2010; Menzies-Gow, Bailey, Katz, Marr, & Elliott, 2004).
Further between-species differences in vessel contractile reactivity to NOR and 5-HT were revealed with respect to the influence of the endothelium and to the short- and longer-term changes induced in vascular reactivity by endothelium removal. In EDA, the contractile responses of denuded vessels to both agonists were similar to those of intact vessels, irrespective of whether vessels were fresh or incubated. Earlier studies have already reported that the endothelium has no influence on the responses of the EDA to NOR and 5-HT (Bailey & Elliott, 1998a; Cogswell et al., 1995) and, consistently, digital vessels collected from laminitic horses showed evidence of impaired endothelial function without any associated changes in contractile reactivity to adrenergic agonists (Schneider et al., 1999). By contrast, the endothelium of the BDA was found to exert an inhibitory modulation of the responses to both NOR and 5-HT, as indicated by the increased reactivity of freshly denuded vessels. As this condition may mimic the endothelial damage occurring in laminitis, it can be speculated that these two vasoconstrictors may play an important role in bovine as mediators of the digital arterial vasospasm associated with laminitis. Interestingly, the BDA responses to NOR and 5-HT were differentially modulated by endothelium, as endothelium removal in freshly denuded vessels increased both the efficacy and potency of the latter, while it increased only the efficacy of the former. The enhancing effects acutely produced by endothelium removal on the reactivity of the BDA to NOR and 5-HT could no longer be observed after incubation. The present observations parallel those reported for the rat aorta, in which endothelium removal increased the contractile response to NOR (Vinet, Brieva, Pinardi, & Penna, 1991) and a 24 hr organ culture decreased the vasoconstriction evoked by phenylephrine in denuded but not in intact vessels (Binko et al., 1999). The incubation-associated decrease in the contractile response of denuded rat aorta was found to partly depend on the induction of NO synthase and consequent autocrine release of NO in the smooth muscle layer (Binko et al., 1999). These studies can support the hypothesis that as a reaction to endothelial removal, compensatory pathways are activated in the smooth muscle layer of BDA during incubation leading to the re-establishment of inhibitory control over the action of vasoconstrictors.
In both BDA and EDA, the endothelium showed no modulating role in the vasorelaxant effects of ISOP in freshly deprived vessels, which is consistent with previous observations (Belloli et al., 2004; Zizzadoro et al., 2011), as well as after incubation. The latter finding suggests that the functionality of β-adrenoceptors is not affected by pathways responsible for the incubation-associated effects revealed for the α-adrenergic and serotoninergic receptor agonists.
ISOP showed a lesser degree of vasorelaxant efficacy in the BDA than in the EDA but not different potency between the two species, suggesting species-related differences at postreceptor level. The vessel reactivity to ISOP, however, was evaluated here in the absence of any α-adrenergic blockers. As reported in previous studies, α-adrenoceptors are dominant over β-adrenoceptors in both EDA and BDA (Belloli et al., 1999, 2004) and ISOP can display α-adrenergic agonistic properties even at low concentrations (Elmes & Eyre, 1977; Pourageaud, Leblais, Bellance, Marthan, & Muller, 2005). Therefore, in the face of equi-effective levels of U44069-induced tone, the smaller ISOP-induced vasorelaxation in the BDA might result from a greater counteractive vasoconstriction evoked by the simultaneous activation of α-adrenoceptors.
The endothelium-dependent vasodilator CARB showed similar efficacy in relaxing intact BDA and EDA, with the maximum CARB relaxation in the EDA being close to the maximum relaxation reported for ACh in the same vessel (Baxter, Tackett, & Moore, 1989; Cogswell et al., 1995). The precontraction levels induced in BDA and EDA, however, were equivalent in terms of percentage of the maximal tension (50%), while their absolute magnitude was greater in BDAs than in EDAs (~50 and 30 g tension/g tissue, respectively) owing to the aforementioned different efficacy of α-adrenergic stimulation in the two species. Therefore, the ability of CARB to reduce these two different absolute precontraction levels by the same percentage turns into vasorelaxant effects of greater absolute magnitude in the BDA than in the EDA. This may reflect species specificity in the endothelial production of relaxing factors (e.g., NO) in response to muscarinic receptor stimulation and/or of the vascular smooth muscle responsiveness to the endothelial-derived mediators.
CARB proved more potent in the EDA than in the BDA, which is in line with the observation by other authors of high EDA sensitivity to cholinergic vasodilator agonists (Baxter, 1995; Baxter, Tackett et al., 1989; Cogswell et al., 1995; Schneider et al., 1999; Zerpa et al., 2005). This might involve a higher muscarinic receptor density and/or coupling efficiency in the EDA as compared to BDA. The species-related difference disappears when vessel preparations were incubated, but the mechanisms through which incubation modifies muscarinic receptor sensitivity in EDA endothelial cells remain to be established.
A limitation of the current study was the evaluation of larger digital arteries (conductance vessels) rather than the smaller lamellar arteries (resistance vessels). It is well known that equine digital veins are more sensitive to vasoconstrictors than arteries, while differences in vascular responses are apparent between large conductance arteries and veins and small resistance arterioles and venules (Peroni et al., 2006; Robertson, Bailey, & Peroni, 2009). Thus, the results of the current study should not be extrapolated to other vessels within the equine and bovine digit and do not supply a complete species-specific overview of the differences in horse and cow digital haemodynamic regulation.
A further limit of the work concerns the organ bath temperature (37°C) selected for the experimental assays which is not physiologically relevant, at least for horse. Temperature is a relevant experimental design issue to consider when evaluating the vascular response to different mediators or when comparing literature data on vascular studies (Zerpa, Berhane, Elliott, & Bailey, 2010). Therefore, as the aim of this work did not envisage investigating the pathophysiology of acute bovine and equine laminitis but was planned to compare normal bovine and equine digital vascular responses to various vasoactive mediators, the standard temperature historically used in our laboratory was chosen to take advantage of a well-tested experimental procedure and to allow for a correct comparison of these results with those from our previous experimental trials.
5 CONCLUSIONS
The results from the present study identified differences in responses of BDA and EDA to vasoactive mediators. Taken together, they suggest that under physiologic condition (intact vessels), BDA is probably under the control of a greater vasoconstrictor tone than EDA due to the greater vasoconstrictor influence of NOR and 5-HT and the smaller vasorelaxant influence of ISOP. Moreover, it can be suggested that, following acute endothelial damage, BDA develops more intense vasoconstriction than EDA, due to the increase in the endothelium-modulated vasoconstrictor component. However, the BDA seems to be able to partly compensate for endothelial damage, probably through the vicarious function of the smooth muscle layer, and this would lead to spontaneous attenuation of acute vasospasm.
Finally, the results obtained broaden our knowledge of the vascular physiology of the BDA. Future work needs to be performed investigating the neuro-humoral systems operating in the regulation of other bovine digital vessels and how changes in digital vascular flow may contribute to the onset of laminitis in this animal species.
ACKNOWLEDGMENTS
The authors thank Dr. Maddalena Curci (Department of Soil, Plant and Food) and Dr. Valeriana Colao (Department of Veterinary Medicine) of the University of Bari for help and advice with statistical analysis and Mr. Giuseppe Sasso for skilful technical assistance. Some preliminary results were published in an abstract in Proceedings of the LXIV National SISVet Congress, 2010. This study was supported by a Grant from University of Bari (ORBA07XSJH).
CONFLICT OF INTEREST
The authors have no conflicts of interest.




