Pharmacokinetics of enrofloxacin after oral, intramuscular and bath administration in crucian carp (Carassius auratus gibelio)
Abstract
The pharmacokinetics of enrofloxacin (ENR) was studied in crucian carp (Carassius auratus gibelio) after single administration by intramuscular (IM) injection and oral gavage (PO) at a dose of 10 mg/kg body weight and by 5 mg/L bath for 5 hr at 25°C. The plasma concentrations of ENR and ciprofloxacin (CIP) were determined by HPLC. Pharmacokinetic parameters were calculated based on mean ENR or CIP concentrations using WinNonlin 6.1 software. After IM, PO and bath administration, the maximum plasma concentration (Cmax) of 2.29, 3.24 and 0.36 μg/ml was obtained at 4.08, 0.68 and 0 hr, respectively; the elimination half-life (T1/2β) was 80.95, 62.17 and 61.15 hr, respectively; the area under the concentration–time curve (AUC) values were 223.46, 162.72 and 14.91 μg hr/ml, respectively. CIP, an active metabolite of enrofloxacin, was detected and measured after all methods of drug administration except bath. It is possible and practical to obtain therapeutic blood concentrations of enrofloxacin in the crucian carp using IM, PO and bath immersion administration.
Enrofloxacin (ENR) is an antibacterial drug of the fluoroquinolone group developed for use in veterinary medicine. It possesses a broad spectrum of activity against most gram-negative organisms and some gram-positive bacteria, as well as mycoplasmas. It has been demonstrated to be efficacious against common bacterial pathogens including Aeromonas salmonicida, Aeromonas hydrophila, Vibrio anguillarum, Yersinia spp and Renibacterium salmoninarum (Akhlaghi & Sharifi Yazdi, 2008; Hsu, Wooster, & Bowser, 1994; Maluping et al., 2005). China has given permission for ENR to be used in aquaculture, and it has become an essential strategy to confront and alleviate infectious disease and reduce animal mortality. The pharmacokinetics of ENR have been reported in a number of aquatic animals, including Korean catfish (Kim, Lim, Park, Hwang, & Yun, 2006), seabass (Intorre et al., 2000), snakehead fish (Fang, Zhou, & Liu, 2016), striped catfish (Phu et al., 2015) and turbot (Liang, Li, Zhao, Liu, & Chang, 2012). Nevertheless, information about the pharmacokinetics of ENR in crucian carp is deficient. This study was to compare and evaluate the pharmacokinetic properties of ENR and its active metabolite, ciprofloxacin (CIP) in crucian carp after oral, intramuscular and bath administration.
Six hundred healthy crucian carp (300 ± 30 g body weight), without previous treatment of pharmaceutical agents, were bought from a commercial farm and acclimatized for at least 2 weeks. They were divided randomly into three groups; each group was reared in an aquarium (20 m3), supplied continuously with circulating water and oxygen by an inflation pump. Heat rods were used to maintain the water temperature at 25 ± 0.5°C. They were fed a pelleted diet in fixed quantity twice daily, which was stopped 1 day before sampling. All animal treatment met the standards of the Institutional Animal Care and Use Committee guidelines.
Enrofloxacin solution (1%) for intramuscular and oral administrations was prepared by dissolving enrofloxacin hydrochloride in sterile 0.9% saline prior to use. The formulation of enrofloxacin for bath administration was prepared by dissolving enrofloxacin hydrochloride directly into the water to prepare 5 mg/L enrofloxacin solution. Previous to the drug administration, the fish were anaesthetized in well-aerated water containing 100 mg/L tricaine methane sulphonate (MS-222). Fish of group A were injected with enrofloxacin solution at the right epaxis of the third vertebrae (IM) at the dose of 10 mg/kg (b.w.), while those in group B were drenched with enrofloxacin solution with gavage needle (PO) at the same dose. The blood samples were taken from tail sinus of ten fish before and at 5, 15, 30 and 45 min and 1, 2, 4, 6, 8, 12, 24, 48, 72, 96, 120, 168, 216 and 264 hr after IM and PO treatment, respectively. Fish in group C were given enrofloxacin by immersing the fish in water containing 5 mg enrofloxacin/L of water for 5 hr. Samples of water in which the animals were bathed were collected prior to drug administration, at 1 min after drug administration and again at 5 hr. ENR concentrations in water were determined according to a previously reported HPLC method (Fang, Liu, Liu, & Lu, 2012). Approximately 1 ml of blood was taken from tail sinus of ten fish before treatment and at the following times post-treatment: 0, 0.5, 1, 2, 4, 6, 8, 12, 24, 48, 72, 96, 120, 168, 216 and 264 hr. Each fish was sampled only once and then removed to a separate recovery tank and not used again. Plasma was collected after centrifugation and stored at −70°C in heparinized microfuge tubes until assayed.
Enrofloxacin and CIP extraction and analysis were carried out according to the previously reported method (Fang et al., 2012). The limit of quantitation (LOQ) was 0.05 and 0.01 μg/ml for ENR and CIP, respectively. Concentrations showed good linearity within a range of 0.05–5 μg/ml and 0.01–1 μg/ml for ENR and CIP, respectively. In plasma, the mean recoveries were 94% and 91% for ENR and CIP, respectively. The coefficient of variations (intraday and interday) for ENR and CIP was less than 5.7% and 8.4%, respectively.
Pharmacokinetic analysis was performed using WinNonlin 6.1 software (Pharsight Corporation, Mountain View, CA, USA). Pharmacokinetic parameters of ENR after IM and PO dosings were calculated based on ENR mean concentrations by compartmental methods, and the compartmental analysis was evaluated based on coefficient of determination and Akaike's information criteria for the best fit model. The ENR concentration data after bath administration and the CIP concentration data after intramuscular and oral dosings were analysed by noncompartment modelling.
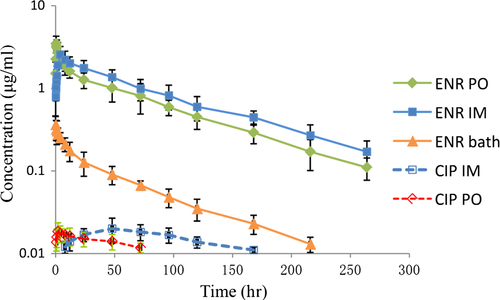
Plasma concentration of ENR and CIP vs. time curves and the pharmacokinetic parameters for ENR and CIP following IM, PO and bath administration of ENR are shown in Figure 1 and Table 1, respectively. The ENR concentration–time data of plasma after intramuscular and oral dosings were both best fit by a two-compartment open model with first-order absorption. Similar kinetic profiles of ENR have been observed in catfish (Kim et al., 2006), turbot scophthalmus maximus (Liang et al., 2012) and snakehead fish (Fang et al., 2016). Following PO administration, the T1/2ka (0.13 hr) of ENR was shorter compared with 0.82 hr in snakehead fish (Fang et al., 2016), 1.394 hr in Korean catfish (Kim et al., 2006), 1.99 hr in turbot scophthalmus maximus (Liang et al., 2012) and 6.03 hr in seabass (Intorre et al., 2000); this indicated that ENR was rapidly absorbed in crucian carp after gavage. T1/2β and AUC of ENR in crucian carp treated orally were 62.17 hr and 162.72 μg hr/ml, respectively; the values were considerably higher than those determined in Korean catfish (Kim et al., 2006), brown trout (Koc, Uney, Atamanalp, Tumer, & Kaban, 2009) and snakehead fish (Fang et al., 2016), while they were considerably lower than those of turbot scophthalmus maximus (Liang et al., 2012) and European eel (Fang, Yu, Cai, Zhou, & Huang, 2007). The reason for those differences is not clear.
| Parameter | Unit | Enrofloxacin | Ciprofloxacin | |||
|---|---|---|---|---|---|---|
| Intramuscular | Oral | Bath | Intramuscular | Oral | ||
| T1/2ka | hr | 0.65 | 0.13 | NA | NA | NA |
| T1/2α | hr | 24.07 | 2.22 | NA | NA | NA |
| T1/2β | hr | 80.95 | 62.17 | 61.15 | 137.71 | 129.99 |
| Tmax | hr | 4.08 | 0.68 | 0.00 | 48.00 | 2.00 |
| Cmax | μg/ml | 2.29 | 3.24 | 0.36 | 0.021 | 0.019 |
| AUC | μg hr/ml | 223.46 | 162.72 | 14.91 | 4.85 | 3.30 |
| Vd/F | L/kg | 5.23 | 5.51 | NA | NA | NA |
| CL/F | L/hr/kg | 0.04 | 0.06 | NA | NA | NA |
- T1/2ka, absorption half-life of the drug; T1/2α, distribution half-life of the drug; T1/2β, elimination half-life of the drug; Tmax, the time point of maximum plasma concentration of the drug; Cmax, the maximum plasma concentration; AUC, area under the concentration–time curve; CL/F, body clearance corrected for bioavailability; Vd/F, volume of distribution corrected for bioavailability.
After intramuscular injection of ENR in crucian carp, absorption was slow (Tmax = 4.08 hr), which was in agreement with that (Tmax = 4 hr) reported in red pacu (Lewbart et al., 1997), but longer than that in Atlantic salmon (Tmax = 0.29 hr) (Martinsen & Horsberg, 1995) and mitten-handed crab (Tmax = 0.08 hr) (Tang et al., 2006). However, an even longer Tmax (Tmax = 4.59 hr) has been reported in koi carp after IM administration (Udomkusonsri, Arthitvong, Klangkaew, & Kusucharit, 2007). ENR half-life after IM dose (10 mg/kg) was 80.95 hr, which was close to that (84.98 hr) determined in Atlantic salmon (Stoffregen, Wooster, Bustos, Bowser, & Babish, 1997), but considerably longer than that (18.96 hr) in koi carp (Udomkusonsri et al., 2007) and red pacu (28.9 hr) (Lewbart et al., 1997). AUC represents the extent of drug absorption and determines bioavailability. In this study, AUC was bigger when administered IM (223.46 μg hr/ml) than administered PO (162.72 μg hr/ml). However, the CL/F was smaller when administered IM (0.04 L hr−1 kg−1) than administered PO (0.06 L hr−1kg−1).
Bath immersion treatment caused lower plasma drug concentration in plasma compared with the others. Following bath administration, the T1/2β, Cmax and AUC were 61.15 hr, 0.36 μg/ml and 14.91 μg hr/ml, respectively (Table 1). No detectable levels of ENR were present at 264 hr postbath administration. CIP was not detected in any samples. Different results are found in koi carp (Udomkusonsri et al., 2007), for which the T1/2β, Cmax and AUC were 42.1 hr, 0.86 μg/ml and 29.1 μg hr/ml after bath with the same dosage, respectively. In addition, after bath seabass with 5 mg/L enrofloxacin for 4, 8 and 24 hr, the Cmax was 0.17, 0.31 and 0.72 μg/ml, respectively (Intorre et al., 2000). The mean ± SD of water concentrations of ENR at 1 min and 5 hr were 4.42 ± 0.56 μg/ml and 3.74 ± 0.38 μg/ml, respectively. This represents 88% and 75% of the total ENR concentration expected in the bath water. Our results indicate that it was possible to obtain therapeutic blood concentration of enrofloxacin in crucian carp using bath administration and enrofloxacin is absorbed into the bloodstream from the water. As freshwater fish do not drink, the most likely point of absorption during a bath treatment is across the high-surface area gill epithelium (Lewbart et al., 1997). This method is not practical to add fluoroquinolone drugs into hard water or water with high levels of divalent cations, for example, magnesium, in which enrofloxacin will bind to divalent cations and causes noneffective therapy. For addition, biologic filtration in the system may be compromised by this delivery method. Then, fish should be bathed in other container temporarily. Nevertheless, this study showed a potential for this method to treat a large population suffering from disease when drugs cannot be administered orally.
In this study, after IM treatment with ENR, the mean Cmax of CIP was 0.021 μg/ml at 48 hr and declined to 0.011 μg/ml by 168 hr, while after PO treatment, the mean Cmax of CIP was 0.019 μg/ml at 2 hr and declined to 0.012 μg/ml by 72 hr. After IM and PO treatment, the AUC ratios of CIP and ENR were 2.17% and 2.03%, respectively. In catfish, CIP could be detected in plasma with an AUC ratio of 3.33% after PO treatment (Danyi et al., 2011). In turbot, the AUC ratios were 2.04% and 1.84% after PO treatment at 16°C and 10°C, respectively (Liang et al., 2012). However, CIP was not detected in serum samples of the sea bream after oral administration (Della Rocca, Di Salvo, Malvisi, & Sello, 2004). These results indicate a low extent of deethylation of ENR in aquatic animals.
The MIC values of ENR against the most common fish pathogens were 0.01–0.06 μg/ml for V. anguillarum, 0.01–0.03 μg/ml for Yersinia ruckeri, 0.01–0.06 μg/ml for Escherichia coli, 0.0125–0.025 μg/ml for A. hydrophila and 0.006 μg/ml for A. salmonicida (Martinsen, Oppegaard, Wichstrom, & Myhr, 1992; Prescott & Yielding, 1990; Roque, Molina-Aja, Bolan-Mejia, & Gomez-Gil, 2001; Wang & Zhu, 2005). However, it should be pointed out that the MIC values described above are very old and now, perhaps, no longer effective. It was widely accepted that treatment with dosage regimens, which lead to high PK-PD indices as AUC/MIC > 125 and Cmax/MIC > 8, resulted in less frequent selection of resistant mutants (McKellar, Sanchez Bruni & Jones, 2004). Using this guideline, the IM dose of 10 mg/kg produced AUC and Cmax values that would be sufficient to treat organisms with an MIC ≤ 0.28 μg/ml, and the PO dose of 10 mg/kg produced AUC and Cmax values that would be sufficient to treat organisms with an MIC ≤ 0.40 μg/ml. However, because of our reluctance to use high concentrations in the water, the bath produced low Cmax and AUC values compared with the others. These values would only be sufficient to treat bacteria with MIC values of 0.045 μg/ml. For bacteria with higher MIC values, a larger dose would need to be investigated to determine whether higher plasma levels are possible.
ACKNOWLEDGMENTS
This work was supported financially by the National Natural Science Foundation of China (31502125) and the Natural Science Foundation of Guangdong Province (2015A030310086).




