In vitro anti-LPS dose determination of ketorolac tromethamine and in vivo safety of repeated dosing in healthy horses
Funding information
Support was provided by the Purdue University College of Veterinary Medicine Competitive Equine Research Funds.
Results were presented, in part, at the 2016 Forum of the American College of Veterinary Internal Medicine in Denver, CO.
Abstract
Flunixin meglumine (FM) is a commonly used Nonsteroidal anti-inflammatory drug (NSAID) in horses, but clinical efficacy is often unsatisfactory. Ketorolac tromethamine (KT) demonstrates superior efficacy compared to other NSAIDs in humans, but its anti-inflammatory effects have not been investigated in the horse. Safety of repeated dosing of KT has not been evaluated. The first objective was to conduct a dose determination study to verify that a previously described dosage of KT would inhibit Lipopolysaccharide (LPS)-induced eicosanoid production in vitro, and to compare KT effects of this inhibition to those of FM. Then, a randomized crossover study was performed using nine healthy horses to evaluate plasma concentrations of KT and FM following IV administration. Administered dosages of KT and FM were 0.5 mg/kg and 1.1 mg/kg, respectively. Safety following six repeated doses of KT was assessed. Ketorolac tromethamine and FM suppressed LPS-induced Thromboxane B2 (TXB2) and Prostaglandin E2 (PGE2) production in vitro for up to 12 hr. Intravenous administration produced plasma concentrations of KT and FM similar to previous reports. No adverse effects were observed. A KT dosage of 0.5 mg/kg IV inhibited LPS-induced eicosanoids in vitro, and repeated dosing for up to 3 days appears safe in healthy horses. Investigation of in vivo anti-inflammatory and analgesic effects of KT is warranted.
1 INTRODUCTION
Management of inflammation in horses represents an ongoing challenge in equine medicine. Systemic inflammatory response syndrome (SIRS) is defined as widespread and exaggerated inflammation that can be triggered by infectious or noninfectious stimuli (Lewis, Chan, Pinheiro, Armitage-Chan, & Garden, 2012; Moore & Vandenplas, 2014; Palmer, 2014; Roy, 2004). Possible complications of SIRS include coagulopathies and organ dysfunction, which can lead to death (Kelmer, 2009; Kilpatrick et al., 2016; Roy, 2004). Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly administered to horses with SIRS to reduce eicosanoid production through inhibition of cyclooxygenase (COX) enzymes. Several studies have documented the effectiveness of NSAIDs in reducing lipopolysaccharide (LPS)/endotoxin-induced eicosanoid production in horses, calves and rodents (Dogan, Ataoglu, & Akarsu, 2002; Jackman, Moore, Barton, & Morris, 1994; Moore, Hardee, & Hardee, 1986; Semrad & Dubielzig, 1993; Semrad, Hardee, Hardee, & Moore, 1987). Flunixin meglumine (FM) is currently considered the standard of care for LPS-induced inflammation in horses, based on efficacy, affordability and safety (Hardee, Moore, & Hardee, 1986; Moore et al., 1986). In comparison studies, FM has outperformed phenylbutazone in reducing production of LPS-induced mediators (Bryant, Farnfield, & Janicke, 2003; Hardee et al., 1986; Moore et al., 1986). The dosing schedule for FM of 1.1 mg/kg IV q12 hr is often used and is based on anecdotal perception of clinical efficacy (Jackman et al., 1994), but critically ill horses often display clinical signs associated with pain and SIRS despite standard-of-care FM therapy (Graubner, Gerber, Doherr, & Spadavecchia, 2011; Mair & Smith, 2005a,b). Identification of another NSAID with anti-inflammatory and analgesic properties superior to those of FM could potentially decrease illness and death associated with equine SIRS.
Ketorolac tromethamine (KT) is a nonselective COX inhibitor that has been used in humans to provide potent anti-inflammatory and analgesic therapy since the 1980s (Litvak & McEvoy, 1990; Rooks, Tomolonis, Maloney, Wallach, & Schuler, 1982; Rooks et al., 1985). In several animal models, KT has been shown to have anti-inflammatory and analgesic properties that often exceed the efficacy of other NSAIDs (Dogrul, Yesilyurt, Deniz, & Isimer, 1997; Jett et al., 1999; Rooks et al., 1985; Waterbury et al., 2011; Yang et al., 2008). Ketorolac tromethamine is often administered as an IV bolus or as a constant rate infusion for morphine-sparing analgesia in postoperative patients (Beattie et al., 1997; Blackburn, Stevens, Wheatley, Madej, & Hunter, 1995; Etches et al., 1995; Ready et al., 1994). Although the pharmacokinetics of KT have been evaluated in a variety of veterinary species, including horses (Bianco, Constable, Cooper, & Taylor, 2016; Cagnardi et al., 2013; Nagilla, Deshmukh, Duran, & Ravis, 2007; Nagilla et al., 2009; Pasloske, Renaud, Burger, & Conlon, 1999; Planborg, Bondesson, Fredriksson, Larsson, & Kallings, 1994; Santos et al., 2001; Villa et al., 2015), there have been few studies evaluating its analgesic or anti-inflammatory efficacy in dogs, cats and horses (Cagnardi et al., 2013; Ferraresi et al., 2014; Mathews, Paley, Foster, Valliant, & Young, 1996; Villa et al., 2015). A single veterinary study found that KT was equal to FM in reducing SIRS parameters in an LPS-induced inflammatory model in calves (Semrad, 1993). In veterinary species, a dosage of 0.5 mg/kg, which was originally extrapolated from human literature, has been shown to provide analgesia (Bianco et al., 2016; Cagnardi et al., 2013; Ferraresi et al., 2014; Mathews et al., 1996; Villa et al., 2015). To date, this dosage has not been evaluated for anti-inflammatory efficacy in veterinary species, and few studies have evaluated plasma drug concentrations following administration of this dosage in horses (Bianco et al., 2016; Ferraresi et al., 2014).
Adverse effects of KT are similar to those caused by other nonselective NSAIDs, but overall incidence in postoperative human patients is low (Elia, Lysakowski, & Tramer, 2005; Forrest et al., 2002; Reinhart, 2000). No veterinary study has specifically evaluated KT for safety, but no adverse effects have been reported after single dosing in calves, sheep, goats, dogs, cats or horses (Bianco et al., 2016; Ferraresi et al., 2014; Nagilla et al., 2007, 2009; Pasloske et al., 1999; Santos et al., 2001; Semrad, 1993). Importantly, safety of repeated administration of KT in animals has not been evaluated.
Given that KT provides superior analgesia compared to other NSAIDs in human patients, and that this is highly correlated with its anti-inflammatory potency (Jett et al., 1999), our first objective was to verify that a previously described dosage of KT would inhibit LPS-induced eicosanoid production in vitro, and to evaluate KT effects of LPS-induced eicosanoid suppression compared to those of FM. Our second objective was to assess plasma concentrations of KT and FM following intravenous administration. A final objective was to evaluate the safety of KT following repeated dosing, as assessed by evaluating complete blood counts (CBC), serum biochemical analyses (SBA), urinalysis (UA) and fecal occult blood tests (FOBT) over a 3-day (6-dose) drug administration period.
2 MATERIALS AND METHODS
All procedures in this study were approved by the Institutional Animal Care and Use Committee at Purdue University. First, a dose determination study was performed to verify that KT at a dosage of 0.5 mg/kg could effectively suppress eicosanoid production from LPS-stimulated equine monocytes, and to evaluate KT effects of LPS-induced eicosanoid suppression relative to those of FM.
2.1 Dose determination study
Approximately 1 L of whole blood was collected aseptically from the jugular vein of a healthy horse from the Purdue University teaching herd and placed in a glass bottle containing 100 ml 40 mM EDTA. Monocyte isolation was performed using a sedimentation-gradient centrifugation protocol as previously described (Rooks et al., 1985). Total monocyte count was determined and cells were suspended in equine media. A cytospin slide was made to verify that the isolated cells were >75% monocytes. Serial drug dilutions were created from KT (Ketorolac tromethamine (30 mg/ml), Hospira, Inc., Lake Forest, Illinois, USA) and FM (Flunixin meglumine (50 mg/ml), VetOne Prevail, Boise, Idaho, USA) to create six concentrations of each drug: 80, 40, 20 (equivalent to 1.1 mg/kg FM), 10, 5, and 2.5 (equivalent to 0.5 mg/kg KT) μg/ml. Monocytes were then plated on 12-well tissue culture plates at a concentration of 1 × 106cells/well and incubated with serial dilutions of KT or FM. The plates were incubated at 37°C in a 5% CO2 atmosphere for 1 hr, followed by addition of LPS (E. coli 055:B5) at a final concentration of 1 μg LPS/ml. Positive and negative control wells contained monocytes alone without either drug. The final total volume in each well was 2.5 ml. In order to determine the duration of anti-inflammatory activity, the samples were allowed to incubate for 4, 8, 12, or 24 hr. Thus, there were four sets of duplicate wells, with one set for each time point (eight wells total for each of the four treatment groups). After each incubation period, the entire contents of each well were collected and frozen at −80°C until analysis. Concentrations of thromboxane B2 (TXB2) and prostaglandin E2 (PGE2) were measured from each sample using commercially available enzyme-linked immunosorbent assay (ELISA) kits validated for use in horses (Horse Elisa Kits, My BioSource, San Diego, CA, USA). Samples were analyzed per the manufacturer's instructions.
2.2 Crossover/pharmacokinetic and safety study
2.2.1 Animals and experimental design
Nine healthy adult horses from the Purdue University teaching herd were used in this randomized crossover study. The horses were determined to be systemically healthy based on history, physical examination, CBC (Abbott Cell-Dyn 3500 Hematology Analyzer, Abbott Park, IL, USA), SBA (Abbott Cell-Dyn 3500 Hematology Analyzer, Abbott Park), UA, and FOBT (Hemoccult, Beckman Coulter, Inc., Brea, CA, USA). All of the horses had been donated for chronic orthopedic diseases at least 2 months prior to use in this study, and their conditions were considered to be static. None of the horses had received any NSAID within 2 weeks prior to the onset of the study. The study was performed over a 5-week period. The horses were randomly assigned a number (1 through 9) and randomly divided into two groups. Phase 1 consisted of the even-numbered horses receiving KT and the odd-numbered horses receiving FM. Phase 2, the crossover, consisted of the odd-numbered horses receiving KT and the even-numbered horses receiving FM. A 2-week washout period separated the two phases.
2.2.2 Drug administration and sample collection
The day prior to drug administration (Day 0) for each of the two phases, the horses were weighed and a 14-gauge IV catheter was aseptically placed in each jugular vein. The right IV catheter in all horses and for both phases was used only for drug administration, and the left IV catheter was used only for blood collection. All blood samples were obtained by first withdrawing and discarding the first 10 ml of blood from the catheter before collecting 10–15 ml of blood for analysis. The catheters were flushed before and after each blood collection or drug administration with heparinized 0.9% saline and were removed following each of the two phases.
On Day 1 at Time zero (T = 0), horses received either KT at 0.5 mg/kg IV or FM at 1.1 mg/kg IV. All drug doses were rounded up to the nearest 0.1 ml. Heparinized blood was collected at T = 5 min (0.08 hr) to assess peak plasma concentration of drug (KT or FM) based on the rapid distribution of drugs after IV administration (Bianco et al., 2016; Semrad, Hardee, Hardee, & Moore, 1985). Heparinized blood was again collected at T = 4, 8, and 12 hr for assessment of plasma drug concentration.
2.2.3 Drug concentrations
Within 1 hr of collection, heparinized blood from each time point (T = 0.08, 4, 8, and 12 hr) was centrifuged at 760 g at 4°C for 10 min. The plasma was harvested and frozen at −80°C until analysis. Quantitation was performed using high-performance liquid chromatography (HPLC) with a triple quadrupole mass spectrometer. Previous work details the sample preparation, instrumental settings, and method of validation for KT (Bianco et al., 2016). For this report, FM was added to the method and validated in a similar fashion. Reversed-phase HPLC was used, with retention times for KT, FM, and etodolac (the internal standard) being 1.8, 2.6, and 4.9 min, respectively. Quantitation was based on multiple reaction monitoring. For KT, electrospray ionization (ESI) positive mode was used with a transition of 256.1–104.9 and a collision energy (CE) of 18 V. For etodolac, ESI negative mode was used with a transition of 286.1–212.1 and a CE of 20 V. For FM, ESI positive mode was used with a transition of 296.8–278.8 and a CE of 15 V. For KT, quantitation was based on a six-point standard curve ranging from 0.0025 to 5 μg/ml. For FM, quantitation was based on an eight-point standard curve ranging from 0.15 to 100 μg/ml. All standard curves were prepared using unmedicated equine plasma. For KT, the limit of quantitation (LOQ) was 0.0019 μg/ml and the limit of detection (LOD) was 0.0006 μg/ml, defined as a peak-to-peak signal-to-noise ratio of 10:1 and 3:1, respectively. For FM, the LOQ was 0.0024 μg/ml and LOD was 0.0007 μg/ml.
2.2.4 Adverse effects
Following the initial dose, horses received KT or FM q 12 hr for 3 days (six doses total) to assess safety of repeated dosing. The horses were weighed each evening prior to each of the two drug administration phases and the drug dose was adjusted accordingly. For each phase, complete physical examinations were performed at T = 0, 4, 8, and 12 hr, and q 12 hr thereafter for up to 72 hr. A CBC and SBA were performed at T = 0, 24, 48, and 72 hr. Urinalysis and FOBT were performed on each horse on Days 1 and 4. Urine was collected via sterile catheterization without sedation in the mares; the geldings could not be catheterized without sedation; therefore, urine was collected via midstream free-catch as early in the day as possible.
2.2.5 Statistical analysis
Areas under the concentration–time curve (AUC) for KT and for FM were determined by the composite trapezoid rule, and compared by Welch test. Values of p < .05 were considered significant, and results were reported as mean ± SEM.
3 RESULTS
3.1 Dose determination study
Ketorolac tromethamine at a concentration equivalent to a 0.5 mg/kg dosage (2.5 μg/ml) and FM at a concentration equivalent to a 1.1 mg/kg dosage (20 μg/ml) suppressed LPS-induced TXB2 and PGE2 production from baseline for up to 12 hr. Specifically, KT suppressed TXB2 >35% from baseline and PGE2 production >30% from baseline for up to 12 hr at all concentrations (80, 40, 20, 10, 5, and 2.5 μg/ml). With drug concentrations ≥20 μg/ml, at 24 hr, KT suppressed PGE2 production >50% from baseline. Flunixin meglumine suppressed TXB2 >40% from baseline for up to 12 hr at all FM concentrations (≥ 2.5 μg/ml) and >40% from baseline for up to 24 hours at concentrations ≥20 μg/ml, while PGE2 suppression >40% from baseline required a FM drug concentration ≥5 μg/ml. Peak eicosanoid concentration in non-NSAID-treated samples occurred at 4 hr for PGE2 and 12 hr for TXB2.
3.2 Crossover/pharmacokinetic and safety study
Four mares and five geldings (median age 18 years; range 5–23 years) were included in the study. Six breeds were represented, including three Quarter Horses and one each of Saddlebred, Thoroughbred, Standardbred, Warmblood and Appaloosa. The median starting weight of the horses was 515 kg (range 416–616 kg); horses were weighed daily during each drug administration phase to ensure accurate dosing. One horse had been diagnosed with recurrent airway obstruction (RAO) and pars pituitary intermedia dysfunction (PPID), but had not received any medications during the 6 months prior to the start of the study. At the time of the study, the horse was not exhibiting signs of RAO. During the course of each phase, the horses were individually housed in box stalls. All horses had free access to fresh water. The horse with RAO continued on a complete pelleted feed and soaked alfalfa cubes, while all other horses had ad libitum access to grass and alfalfa hay. During the washout period, all horses were housed on pasture.
3.2.1 Plasma drug concentrations
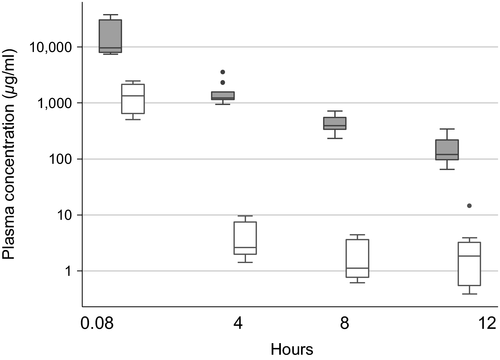
Mean ± SEM plasma drug concentrations of KT and FM over time are shown in Figure 1. Both drugs followed a similar pattern, with the highest mean plasma concentrations detected at T = 5 min (0.08 hr) after drug administration, and then gradually decreasing over time. Drug concentrations of KT were lower at each time point compared to FM, and their AUC significantly differed (p = .007). At T = 5 min (0.08 hr), the mean plasma concentration of KT was 1.52 ± 0.30 μg/ml and decreased to 0.0031 ± 0.0015 μg/ml at T = 12 hr. In contrast, the mean plasma concentration of FM at T = 5 min (0.08 hr) was 25.39 ± 8.82 μg/ml and decreased to 0.16 ± 0.03 μg/ml at 12 hr.
3.2.2 Adverse effects
No significant change was noted in the physical or hematological variables of any horse during the course of the study. There were no significant changes in UA values; only one horse had a positive FOBT, which occurred on Day 1 of Phase 2. The subsequent sample (Day 4) from the same horse was negative. None of the horses demonstrated any notable change in attitude, nor displayed colic behavior or diarrhea during the period of drug administration. There was no evidence of thrombophlebitis or a catheter site reaction in any horse. One horse had an episode of colic two days after the completion of Phase 1 (Day 6, during the washout period) when the horse was on pasture. The horse was hospitalized, and the cause of colic was determined to be a mild impaction of the pelvic flexure. One gallon of mineral oil was administered via nasogastric tube, and the horse was hospitalized for observation without feed for 24 hr. The horse did not receive any medications and showed no further signs of colic. The horse was returned to pasture before returning for Phase 2.
4 DISCUSSION
Based on the results of the in vitro study, a concentration equivalent to a 0.5 mg/kg dosage of KT suppressed LPS-induced TXB2 and PGE2 production for up to 12 hr. However, it remains to be determined whether this effect would be significant in vivo or in the face of inflammatory stimuli. Other similar studies have evaluated the efficacy of NSAIDs, including FM, in horses without the induction of inflammation and demonstrated significant inhibition of PGE2 and/or TXB2 (Beretta, Garavaglia, & Cavalli, 2005; Brideau, Van Staden, & Chan, 2001; Galbraith & McKellar, 1996; Jackman et al., 1994; Kim et al., 2015; Knych, Arthur, McKemie, & Chapman, 2015; Lees, Ewins, Taylor, & Sedgwick, 1987; Soma, Uboh, Rudy, & Fegely, 1992). Given that FM is currently considered the standard of care for LPS-induced inflammation in horses, it was important to compare the ability of KT and FM in suppressing LPS-induced eicosanoids with the same experimental conditions applied. This not only allowed us to compare endogenous eicosanoid suppression between drugs but also prepares us to perform subsequent experiments evaluating the in vivo anti-inflammatory and analgesic properties of NSAIDs in horses.
The eicosanoids evaluated in this study, TXB2 and PGE2, were chosen due to their correlation to COX-1 and COX-2 activity, respectively. Thromboxane B2 is a stable and inactive metabolite of thromboxane A2 (TXA2) and is used as a marker of whole body expression of COX-1, while PGE2 concentrations are a reflection of COX-2 expression. Given the role of COX-1 expression in maintaining the integrity of the gastrointestinal tract mucosa and protecting blood flow to the stomach and kidney, inhibition of COX-1 activity is typically viewed as an undesirable effect. However, targeted inhibition of COX-1 is utilized in cases where the potential for hypercoagulopathy exists as COX-1 expression by activated platelets leads to production of TXA2 and promotion of platelet aggregation. Critically ill human and animal patients often exhibit signs of coagulopathy, with a hypercoagulable state preceding deterioration into disseminated intravascular coagulopathy (DIC). Several studies have documented that horses with ischemic or inflammatory gastrointestinal disease are at increased risk for coagulopathy that may progress to DIC and death (Cesarini, Cotovio, Rios, Armengou, & Jose-Cunilleras, 2016; Dolente, Wilkins, & Boston, 2002; Epstein, 2014; Epstein, Brainard, Giguere, Vrono, & Moore, 2013). Therefore, in patients with potential hypercoagulability, treatment with COX-1-specific inhibitors (e.g., aspirin) might be indicated (Epstein, 2014). While their method of inhibition differs, nonselective NSAIDs have been shown to be as effective at reducing TXB2 as aspirin when compared directly (Lees et al., 1987).
Unlike COX-1, which is constitutively expressed throughout the body, COX-2 is primarily an inducible enzyme which increases in response to growth factors and inflammatory stimuli such as LPS. In horses, constitutive expression of COX-2 has been demonstrated in the glandular mucosa of the stomach (Morrissey, Bellenger, Ryan, & Baird, 2010; Nieto, Aleman, Anderson, Fiack, & Snyder, 2012), mucosa of the urinary bladder (Nieto et al., 2012), jejunum (Cook et al., 2009; Hilton et al., 2011), and left dorsal colon (Morton et al., 2009). Higher COX-2 expression in these tissues is likely beneficial as PGE2 promotes local inflammation and cytotoxic immune responses to prevent pathogen entry (Kalinski, 2012).
In the current study, mean plasma drug concentrations of KT at all time points were nearly identical to those reported in a pharmacokinetic analysis of KT in healthy adult horses administered the same dosage of 0.5 mg/kg IV (Bianco et al., 2016). A similar study investigating the pharmacokinetics of KT at 0.5 mg/kg IV administered prior to castration in horses reported a relatively high LOQ (0.01 μg/ml) that did not allow comparison to the low (< 0.01 μg/ml) plasma drug concentrations we observed at 4, 8, and 12 hr (Ferraresi et al., 2014). Mean plasma drug concentrations of FM were similar to previous pharmacokinetic studies investigating the same dosage used here (1.1 mg/kg IV) in healthy adult horses (Foreman et al., 2012; Lee & Maxwell, 2014; Semrad et al., 1985; Toutain, Autefage, Legrand, & Alvinerie, 1994). As the therapeutic concentration of KT has not been elucidated in horses, the significance of the observed difference in plasma drug concentrations between FM and KT is unknown.
No adverse effects were associated with either KT or FM in this study. Several studies in human medicine have reported adverse effects following KT administration, which has led to establishment of labeling guidelines that include a limit of five consecutive treatment days (Reinhart, 2000). However, it is important to consider patient population. The primary indication for KT in human medicine is as a postoperative NSAID for abdominal, gynecological, or orthopedic surgery, and these are patients who might inherently be at higher risk of adverse effects (Gillies, Kenny, Bullingham, & McArdle, 1987; Horlocker, Hebl, Kinney, & Cabanela, 2002; Kim et al., 2005). Furthermore, KT is not available without a prescription, limiting its use in treating minor conditions in healthy patients. Lastly, human analgesics are typically administered at a prescribed dose rather than adjusted according to body weight, the latter of which is standard in veterinary medicine. In the veterinary literature, the safety of KT has only been evaluated in healthy dogs, cats, and horses undergoing elective surgery (Cagnardi et al., 2013; Ferraresi et al., 2014; Mathews et al., 1996; Villa et al., 2015). Here, we demonstrated that 3 days of q12 hr dosing of KT was safe in healthy horses. Additional studies designed to evaluate the clinical efficacy of the dosage used in this study (0.5 mg/kg IV q12 hr) as well as the safety of longer courses of KT administration in horses are warranted. In conclusion, given these data, it is reasonable to investigate the anti-inflammatory and analgesic effects of KT compared to FM in horses in a future study. A KT dosing schedule of 0.5 mg/kg IV q12 hr for 3 days appeared safe in healthy horses.
ACKNOWLEDGMENTS
This work was performed at Purdue University in West Lafayette, IN. The authors thank Dr. Vanessa Cook, Anna Smith, and Anisa Dunham for technical assistance.




