Comparative pharmacokinetics of minocycline in foals and adult horses
Abstract
The objective of this study was to compare the pharmacokinetics of minocycline in foals vs. adult horses. Minocycline was administered to six healthy 6- to 9-week-old foals and six adult horses at a dose of 4 mg/kg intragastrically (IG) and 2 mg/kg intravenously (i.v.) in a cross-over design. Five additional oral doses were administered at 12-h intervals in foals. A microbiologic assay was used to measure minocycline concentration in plasma, urine, synovial fluid, and cerebrospinal fluid (CSF). Liquid chromatography–tandem mass spectrometry was used to measure minocycline concentrations in pulmonary epithelial lining fluid (PELF) and bronchoalveolar (BAL) cells. After i.v. administration to foals, minocycline had a mean (±SD) elimination half-life of 8.5 ± 2.1 h, a systemic clearance of 113.3 ± 26.1 mL/h/kg, and an apparent volume of distribution of 1.24 ± 0.19 L/kg. Pharmacokinetic variables determined after i.v. administration to adult horses were not significantly different from those determined in foals. Bioavailability was significantly higher in foals (57.8 ± 19.3%) than in adult horses (32.0 ± 18.0%). Minocycline concentrations in PELF were higher than in other body fluids. Oral minocycline dosed at 4 mg/kg every 12 h might be adequate for the treatment of susceptible bacterial infections in foals.
Introduction
Tetracyclines are bacteriostatic antimicrobial agents which bind primarily to the 30S ribosomal subunit, where they inhibit protein synthesis by blocking the binding of aminoacylated tRNA to the ribosomal acceptor (A) site (Chopra & Roberts, 2001). Tetracyclines have been used in human and veterinary medicine for years mainly because of their wide spectrum of activity against gram-positive and gram-negative bacteria, chlamydias, mycoplasmas, rickettsias, and some protozoa (Chopra & Roberts, 2001). Minocycline and doxycycline are semisynthetic analogs of tetracycline. They offer several pharmacological advantages over the parent compound including higher oral bioavailability, enhanced tissue penetration, and improved activity against gram-positive bacteria (Bryskier, 2005; Agwuh & MacGowan, 2006).
Pneumonia is a leading cause of morbidity and mortality in foals (Cohen, 1994). Gram-positive bacteria such as Streptococcus equi subspecies zooepidemicus and Rhodococcus equi are the most common causes of pneumonia in foals between 1 and 6 months of age (Hoffman et al., 1993; Giguère et al., 2002). Macrolide antimicrobial agents are commonly used in equine medicine for treatment of foal pneumonia, particularly when infection with R. equi is suspected or confirmed. However, administration of macrolide antimicrobial agents to foals results in a high incidence of adverse effects such as hyperthermia and diarrhea (Stratton-Phelps et al., 2000; Stieler et al., 2016). In addition, resistance to macrolides and rifampin in R. equi is an emerging problem with resistant isolates being cultured from up to 40% of foals at some farms (Giguère et al., 2010; Burton et al., 2013). Minocycline and doxycycline may be useful alternatives to currently used antimicrobial agents owing to their accumulation in lung tissue as well as their in vitro activity against many bacterial species commonly isolated from foals with pneumonia (Bryant et al., 2000; Jacks et al., 2003; Schnabel et al., 2012). In addition, tetracyclines are active against isolates of R. equi that are resistant to macrolides and rifampin (Giguère et al., 2010; Anastasi et al., 2015). Doxycycline has been investigated for the treatment of bacterial bronchopneumonia in foals (Venner et al., 2013) and proposed as an alternative treatment for foals infected with isolates of R. equi that are resistant to macrolides (Giguère et al., 2011). The recent increase in price and inconsistent availability of doxycycline in the United States has resulted in more frequent use of minocycline in foals with bronchopneumonia and other bacterial disorders. However, the rational use of minocycline in foals is precluded by the lack of pharmacokinetic studies. The objectives of this study were to investigate the pharmacokinetics of intravenous (i.v.) and intragastric (IG) minocycline in foals and to compare resulting variables to those determined in adult horses. An additional objective was to measure drug concentration in body fluids and bronchoalveolar (BAL) cells of foals after a multidose IG regimen.
Materials and Methods
Animals
Three male and three female foals between 6 and 9 weeks of age and weighing between 102 and 156 kg were used in the study. In addition, six separate adult mares (i.e., not the foals’ dams) weighing between 527 and 611 kg were used for comparison. The animals were considered healthy on the basis of history, physical examination, complete blood count, and plasma biochemical profile. Animals were kept in individual stalls during the experiment with ad libitum access to grass hay and water. The foals were kept with their dams at all times and were allowed to nurse ad libitum. The study was approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Experimental design and sample collection
Minocycline was administered to each animal at a dose of 4 mg/kg IG and 2 mg/kg i.v. in accordance with a two-treatment, two-period randomized cross-over design. There was a washout period of at least 7 days between administrations for the two routes. For i.v. use, minocycline hydrochloride (PCCA USA, Houston, TX, USA) was dissolved in sterile water to a concentration of 7 mg/mL, sterilized through a 0.2-μm filter, and administered within 4 h of formulation as a bolus through a catheter placed in a jugular vein. For IG use, the contents of minocycline hydrochloride 100 mg capsules (Watson Pharma, Verna, Salcette, Goa, India) were dissolved in 50 mL of water and administered by use of a nasogastric tube. The tube was flushed with water to ensure complete delivery of the dose. Blood samples were collected from a catheter in the contralateral jugular vein before administration (time 0) and 5, 10, 20, 30, 45 min, as well as 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the drug was administered.
In foals only, additional doses of minocycline were administered orally (to minimize the frequency of nasogastric intubation) at 24, 36, 48, and 60 h and IG at 72 h. Blood samples were collected before each dose and 5, 10, 20, 30, 45 min, as well as 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the last dose. BAL lavage was performed, and samples of synovial, cerebrospinal fluid (CSF), and urine were collected aseptically 1.5 and 12 h after administration of the last IG dose. Foals were sedated by i.v. administration of xylazine hydrochloride (1.0 mg/kg) and butorphanol tartrate (0.07 mg/kg). A 10-mm in diameter, 2.4-m BAL catheter (Jorgenson laboratories, Loveland, CO, USA) was passed via nasal approach until wedged into a bronchus. The lavage solution consisted of four aliquots of 50 mL physiologic saline (0.9% NaCl) solution infused and aspirated immediately. Total nucleated cell count in BAL fluid was determined by use of an automated cell counter (Cellometer Auto T4; Nexcelom Bioscience, Lawrence, MA, USA). BAL fluid was centrifuged at 200 × g for 10 min to pellet the cells. The cells were washed, resuspended in 1 mL of phosphate-buffered solution, vortexed, and frozen at −80 °C until assayed. Supernatant BAL fluid was also frozen at −80 °C until assayed. Before assaying, the cell pellet samples were thawed, vortexed vigorously, and sonicated for 3 min to ensure complete cell lysis. The resulting suspension was centrifuged at 500× g for 10 min, and the supernatant fluid was used to determine intracellular concentration of minocycline.
Immediately after collection of BAL fluid, general anesthesia was induced by i.v. administration of diazepam (0.1 mg/kg) and ketamine (2.5 mg/kg). Samples of synovial fluid were collected from the intercarpal or radiocarpal joint by use of a 20-gauge needle. Samples of CSF were collected from the atlantooccipital space by use of a 3.5-inch, 20-gauge spinal needle. A flexible 8-F Foley catheter was used to collect urine directly from the bladder. Samples were centrifuged, and the supernatants were stored at −80 °C until assayed.
Measurement of minocycline concentration using a microbiologic assay
Minocycline concentration was determined in plasma, synovial fluid, CSF, and urine by use of an agar well diffusion microbiologic assay with Bacillus cereus (ATCC 11778; American Type Culture Collection, Rockville, MA, USA) as described previously (Womble et al., 2007). Known amount of purified doxycycline ranging in concentrations from 0.04 to 5.0 μg/mL was added to equine plasma, synovial fluid, CSF, and urine to produce standard curves for each type of matrix. BAL cells and BAL fluid were assayed with standards diluted in phosphate-buffered saline. Each sample or standard was assayed in triplicate, and mean values for three measurements of the zone diameters were determined. Negative control samples did not cause bacterial inhibition, which indicated no antibacterial activity of equine plasma, or body fluids, or BAL cell supernatants. Plots of zone diameters vs. standard doxycycline concentrations were linear between 0.08 and 5 μg/mL with r values ranging between 0.990 and 0.997. The lower limit of quantification of the assay was 0.156 μg/mL for plasma, synovial fluid, and urine, and 0.08 μg/mL for CSF and BAL fluid. The coefficients of variation for repeatedly assayed samples at concentrations >0.625 μg/mL and <0.625 μg/mL were <5% and <10%, respectively.
Measurement of minocycline concentrations in BAL fluid and cells
Bronchoalveolar fluid supernatant (50 μL) was mixed with 70 μL of methanol and spiked with 1 μL of 13.8 μg/mL internal standard demeclocycline, followed by vortexing. The liquid was loaded into the sample vial before liquid chromatography–tandem mass spectrometry (LC/MS/MS) injection. Cell lysates were vacuum-dried and resuspended into 150 μL of 80% methanol. Subsequently, a 50-μL aliquot was mixed with 50 μL blank supernatant and 20 μL methanol and spiked with 1 μL 13.8 μg/mL internal standard demeclocycline, followed by vortexing. The liquid was loaded into the sample vial before LC/MS/MS injection.
Samples were analyzed with the 5500 QTRAP LC/MS/MS system (Sciex, Framingham, MA, USA), and software Analyst 1.6.2 was used for data acquisition and analysis. The 1200 series HPLC system (Agilent Technologies, Santa Clara, CA, USA) used included a degasser, an autosampler, and a binary pump. The LC separation was performed on an Agilent SB-Aq column (4.6 × 50 mm, 5 μm) with mobile phase A (0.1% formic acid in water) and mobile phase B (0.1% formic acid in acetonitrile). The flow rate was 0.35 mL/min. The linear gradient was as follows: 0–2 min, 100%A; 7–10.5 min, 0%A; 11–15.5 min, 100%A. The autosampler was set at 15 °C. The injection volume was 15 μL. Mass spectra were acquired under positive electrospray ionization (ESI) with the ion spray voltage at +5500 V. The source temperature was 450 °C. The curtain gas, ion source gas 1, and ion source gas 2 were 32, 50, and 65 psi, respectively. Multiple reaction monitoring was used for quantitation of minocycline (m/z 458.2 –> m/z 441.1) with demeclocycline as the internal standard (m/z 465.2 –> m/z 448.2). The lower limit of quantification was 0.005 μg/mL.
Estimation of PELF and BAL cell volumes and determination of minocycline concentrations in PELF and BAL cells
Estimation of the volume of PELF was determined by urea dilution method (Rennard et al., 1986). Urea nitrogen concentrations in BAL fluid (UreaBAL) and concurrent plasma samples (UreaPLASMA) were determined by use of a commercial quantitative colorimetric kit (Biochain Urea Assay Kit, Hayward, CA, USA). The volume of PELF (VPELF) in BAL fluid was derived from the following equation: 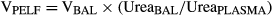 , where VBAL is the volume of recovered BAL fluid. The concentration of minocycline PELF (MINOPELF) was derived from the following relationship: MINOPELF = MINOBAL × (VBALC/VPELF), where MINOBAL is the measured concentration of minocycline in BAL fluid and VBALC is the volume of a BAL cell. A VBALC of 1.20 μL/106 cells was used for calculations based on a previous study in foals (Jacks et al., 2001).
, where VBAL is the volume of recovered BAL fluid. The concentration of minocycline PELF (MINOPELF) was derived from the following relationship: MINOPELF = MINOBAL × (VBALC/VPELF), where MINOBAL is the measured concentration of minocycline in BAL fluid and VBALC is the volume of a BAL cell. A VBALC of 1.20 μL/106 cells was used for calculations based on a previous study in foals (Jacks et al., 2001).
Pharmacokinetic analysis
For each animal, plasma minocycline concentration vs. time data were analyzed based on noncompartmental pharmacokinetics using commercially available software (PK Solutions 2.0; Summit Research Services, Montrose, CO, USA). Maximum plasma concentration (Cmax) and time to achieve maximum plasma concentration (Tmax) were determined directly from the plasma concentration data. The rate constant of the terminal phase (λz) was determined by linear regression of the logarithmic plasma concentration vs. time curve using a minimum of three data points. Half-life of the terminal phase (t½λz) was calculated as ln 2 divided by λz. The area under the concentration–time curve (AUC) and the area under the first moment of the concentration–time curve (AUMC) were calculated using the trapezoidal rule, with extrapolation to infinity using C24 h/λz, where C24 h is the plasma concentration at the 24-h sampling time. Mean residence time (MRT) was calculated as AUMC/AUC. Bioavailability was calculated as (AUCIG/AUCi.v.) × (dose i.v./doseIG). Apparent volume of distribution based on the AUC (Vdarea) was calculated as i.v. dose/AUC·λz, apparent volume of distribution at steady-state (Vdss) was calculated as (i.v. dose/AUC)/(AUMC/AUC), and systemic clearance (CL) was calculated from i.v. dose/AUC.
Data analysis
Normality of the data was assessed based on histograms of differences in means, normal quantile plots of the residuals, and the Shapiro–Wilk test. Constant variance of the data was assessed by plotting residuals against predicted values and with Levene's test. In preliminary analyses, the Student's t-test was used to ensure that there was not a significant effect of order of route of administration on pharmacokinetic variables. Comparison of each pharmacokinetic variable between foals and adults was made using the paired t-test, except for Tmax for which the Wilcoxon signed rank test was used. Differences were considered significant at P < 0.05.
Results
Adverse effects were not noted after i.v. or IG administration of minocycline. Plasma concentration vs. time profiles of minocycline after i.v. administration to foals and adult horses are presented in Fig. 1. After i.v. administration to foals, minocycline had a mean (±SD) t½λz of 8.5 ± 2.1 h, a systemic clearance of 113.3 ± 26.1 mL/h/kg, and a Vdss of 1.24 ± 0.19 L/kg (Table 1). Pharmacokinetic variables determined after i.v. administration to adult horses were not significantly different from those determined in foals (Table 1).
| Variable | Age | P value | |
|---|---|---|---|
| Adults | Foals | ||
| i.v. | |||
| λz (/h) | 0.0839 ± 0.0169 | 0.0857 ± 0.00851 | 0.873 |
| t½λz (h) | 8.6 ± 2.2 | 8.5 ± 2.1 | 0.923 |
| Cinitial (μg/mL) | 4.25 ± 1.17 | 3.88 ± 0.78 | 0.538 |
| Vdarea (L/kg) | 1.31 ± 0.17 | 1.34 ± 0.21 | 0.816 |
| Vdss (L/kg) | 1.16 ± 0.18 | 1.24 ± 0.19 | 0.446 |
| CL (mL/h/kg) | 108.9 ± 22.9 | 113.3 ± 26.1 | 0.761 |
| AUC0–24 h (μg·h/mL) | 16.4 ± 2.6 | 15.9 ± 3.4 | 0.755 |
| AUC0–∞ (μg·h/mL) | 19.1 ± 3.9 | 18.6 ± 5.0 | 0.864 |
| AUMC0–∞ (μg·h2/mL) | 219.2 ± 111.5 | 219.5 ± 106.3 | 0.995 |
| MRT (h) | 11.0 ± 3.3 | 11.3 ± 2.5 | 0.866 |
| C24 h (μg/mL) | 0.20 ± 0.08 | 0.21 ± 0.09 | 0.827 |
| IG | |||
| Cmax (μg/mL) | 1.57 ± 0.94 | 2.29 ± 0.99 | 0.221 |
| Tmax (h)† | 1.0 (0.5–4.0) | 1.0 (0.3–2.0) | 0.589 |
| AUC0–24 h (μg·h/mL) | 11.7 ± 6.3 | 22.2 ± 9.2 | 0.045 |
| AUC0–∞ (μg·h/mL) | 14.4 ± 6.7 | 26.6 ± 11.2 | 0.045 |
| C24 h (μg/mL) | 0.19 ± 0.06 | 0.27 ± 0.12 | 0.224 |
| C ss(max) | NA | 4.15 ± 0.93 | NA |
| C ss(min) | NA | 1.32 ± 0.41 | NA |
| F (%) | 32.0 ± 18.0 | 57.8 ± 19.3 | 0.038 |
- †Median and range; NA, not applicable; λz, rate constant of the terminal phase; t½λz, half-life of the terminal phase; Cinitial, initial measured plasma concentration (at 5 min); Vdarea, apparent volume of distribution based on AUC; Vdss, apparent volume of distribution at steady-state; CL, systemic clearance; AUC0–24 h, area under the plasma concentration vs. time curve from time 0 to 24 h; AUC0–∞, area under the plasma concentration vs. time curve extrapolated to infinity; AUMC0–∞, area under the first moment of the concentration vs. time curve extrapolated to infinity; MRT, mean residence time; Cmax, maximum plasma concentration (observed) after the first dose; Tmax, time to maximum plasma concentration (observed) after the first dose; C24 h, plasma concentration 24 h after administration of the first dose; Css(max), maximum plasma concentration at steady-state when administered q 12 h (observed); Css(min), minimum plasma concentration at steady-state when administered q 12 h (observed); F, bioavailability.
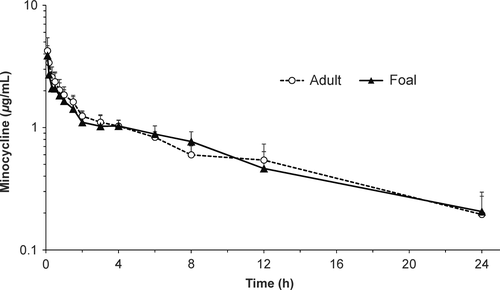
After IG administration, quantifiable minocycline concentration was found in two of six foals at 5 min and in all six foals at 10 min. Median time to peak plasma minocycline concentration (Tmax) after administration of the first IG dose to foals was 1 h (range 0.5–4.0 h), and Cmax was 2.29 ± 0.99 μg/mL (Table 1). Bioavailability was significantly higher in foals (57.8 ± 19.3%) than in adult horses (32.0 ± 18.0%). After multiple IG doses in foals, peak plasma concentration was 4.15 ± 0.93 μg/mL (Fig. 2). Minocycline concentrations in BAL cells and CSF were lower than concurrent plasma concentration (Table 2). Minocycline concentration in PELF at 12 h was higher than that found at other sites.
| Sample | Time after minocycline administration (h) | |
|---|---|---|
| 1.5 | 12 | |
| Plasma† | 3.59 ± 0.28 | 1.32 ± 0.41 |
| Synovial fluid† | 1.54 ± 0.16 | 0.93 ± 0.23 |
| Urine† | 2.71 ± 2.01 | 2.01 ± 1.75 |
| PELF‡ | 27.50 ± 28.54 | 19.24 ± 16.05 |
| BAL cells‡ | 0.08 ± 0.06 | 0.13 ± 0.12 |
| CSF† | 0.30 ± 0.16 | 0.21 ± 0.10 |
- †Minocycline concentration measured using a microbiologic assay; ‡Minocycline concentration measured using LC/MS/MS.
Discussion
A safe antimicrobial agent providing therapeutic drug concentrations in the lungs after oral administration would be a useful addition to currently available antimicrobial agents for the treatment of pneumonia and other bacterial disorders in foals. This study demonstrates that minocycline has good oral bioavailability in foals and achieves drug concentrations above the minimum inhibitory concentration (MIC) of many common bacterial pathogens of horses when administered at a dose of 4 mg/kg q 12 h to foals.
The present study used a microbiologic assay to measure minocycline concentration in all sample types apart from PELF and BAL cells, for which the assay was not sensitive enough. Microbiologic assays cannot differentiate between a drug and its active metabolite or metabolites. In people, minocycline has six metabolites that have little to no antimicrobial activity and are mainly found in urine (Nelis & De Leenheer, 1982; Bocker et al., 1991). As a result, both HPLC and microbiologic assays are still considered acceptable for the measurement of minocycline concentrations in people (Agwuh & MacGowan, 2006) and there is a good correlation between both types of assays in pigs (Pijpers et al., 1991). The plasma minocycline concentration vs. time profile after administration of i.v. minocycline to adult horses in this study is almost identical to that determined in a prior study using HPLC to measure minocycline concentrations (Nagata et al., 2010). Elimination t1/2, apparent Vdss, and systemic CL measured after i.v. administration to adult horses in this study (8.6 ± 2.2 h, 1.16 ± 0.18 L/kg, and 109 ± 23 mL/h/kg, respectively) were similar to those measured after administration of the same i.v. dose in the aforementioned study (7.7 ± 1.9 h, 1.53 ± 0.09 L/kg, and 160 ± 40 mL/h/kg, respectively) (Nagata et al., 2010). In contrast, Cmax was considerably higher after administration of a single IG 4 mg/kg dose in this study (1.57 ± 0.94 μg/mL) than that reported after IG administration in a prior study (0.30 ± 0.11 μg/mL) (Schnabel et al., 2012). The reason for this discrepancy is unknown; in both studies, horses had ad libitum access to hay and the drug was given via nasogastric intubation.
Elimination t1/2, apparent Vd, and systemic CL after i.v. administration of minocycline to foals were similar to those of adult horses. However, oral bioavailability was significantly higher in foals (57.8 ± 19.3%) than in adult horses (32.0 ± 18.0%). This was not unexpected as the oral bioavailability of several antimicrobial agents such as amoxicillin, cefadroxil, and rifampin is higher in foals than in adult horses (Baggot et al., 1988; Burrows et al., 1992; Duffee et al., 1997). The oral bioavailability of minocycline in adult horses in this study is considerably higher than the oral bioavailability of doxycycline, which is estimated to be only 3% (Davis et al., 2006). Plasma Cmax of minocycline after IG administration of a single (2.29 ± 0.99 μg/mL) and multiple (4.15 ± 0.93 μg/mL) 4 mg/kg doses of minocycline to foals in this study is similar to that achieved after IG administration of single and multiple (q 12 h) 10 mg/kg doses of doxycycline to foals of similar ages (2.54 ± 0.27 and 4.05 ± 0.83 μg/mL, respectively) (Womble et al., 2007).
The optimal dosing of antimicrobial agents is dependent on the pharmacokinetics as well as the pharmacodynamics of the drug. Pharmacodynamic properties of a drug address the relationship between drug concentration and antimicrobial activity. The most important pharmacodynamic variables in predicting the efficacy of tetracyclines are the AUC/MIC ratio and length of time that plasma concentrations exceed the MIC (T > MIC) (van Ogtrop et al., 2000; Agwuh & MacGowan, 2006; Bowker et al., 2008). However, the optimal AUC/MIC ratio or target T > MIC that would predict the efficacy of minocycline in vivo has not been established. Bacterial isolates with a MIC ≤ 4 μg/mL are reported as susceptible to minocycline as established by the Clinical and Laboratory Standards Institute guidelines. After administration of multiple 4 mg/kg doses q 12 h to adult horses, Cmax is only 0.67 ± 0.26 μg/mL (Schnabel et al., 2012). As a result, it has been recommended that minocycline be used only for the treatment of highly susceptible pathogens (MIC ≤ 0.25 μg/mL) in adult horses (Schnabel et al., 2012). Based on the results of the present study, a dose of 4 mg/kg, orally, every 12 h results in a Cmax of 4.15 ± 0.93 μg/mL and trough concentrations of 1.32 ± 0.41 μg/mL. These concentrations might be adequate for the treatment of foals infected with bacteria with a MIC ≤ 1 μg/mL (Ensink et al., 1993; Schnabel et al., 2012). These would include most Actinobacillus spp., Staphylococcus spp., R. equi, and β-hemolytic streptococci. These would also include approximately 50% of Escherichia coli (Ensink et al., 1993; Schnabel et al., 2012).
The rate and extent of penetration of a drug into most sites outside the vascular space are determined by the drug's concentration in plasma, molecular charge and size, extent of plasma protein binding, and blood flow (Nix et al., 1991). The percentage of binding of minocycline to plasma proteins in adult horses is approximately 68% (Nagata et al., 2010). Concentration of minocycline in synovial fluid and urine in this study was similar to or slightly lower than concurrent plasma concentration. Minocycline concentration in the urine in the present study was approximately 23- to 69-fold lower than that of doxycycline in foals of similar ages receiving doses of 10 mg/kg q 12 h, whereas drug concentration in the CSF was similar to that achieved with doxycycline (Womble et al., 2007). In people, minocycline accumulates in lung tissue with a lung-to-serum concentration ratio of 3.7 (Watanabe et al., 2001). In the present study, minocycline concentration in PELF was higher than that of plasma at 12 h and similar to PELF concentrations achieved with doxycycline (Womble et al., 2007). However, mean intracellular minocycline concentration in BAL cells in the present study (0.08 ± 0.06–0.13 ± 0.12 μg/mL) was considerably lower than that determined with doxycycline in foals (Womble et al., 2007) and well below the MIC of minocycline against R. equi, a facultative intracellular pathogen. Despite achieving higher intracellular concentrations, doxycycline was recently shown to be poorly active against intracellular R. equi in equine monocyte-derived macrophages (Giguère et al., 2015). To the authors' knowledge, the activity of minocycline against intracellular R. equi has not been studied.
On the basis of the pharmacokinetic variables, MIC of common bacterial pathogens of horses, and drug concentrations in PELF and BAL cells, a dose of 4 mg/kg orally every 12 h might be adequate for the treatment of susceptible bacterial infections in most foals. Additional studies will be required to determine the safety and efficacy of this dosage in a clinical setting.
Acknowledgments
This project was funded by the Hodgson Equine Research Endowment of the University of Georgia. We thank Melanie Fratto, Emily Hart, Shune Hiroto, Kasey Williams, and Dr. Lisa Fultz for assistance with sample collection.




