Pharmacological evaluation of a selective bradykinin B1 antagonist in a canine model of arthritis
Abstract
The effects of a selective bradykinin 1 receptor antagonist, compound A, were evaluated in a canine model of acute inflammatory model of arthritis. Despite detection of the B1 receptor in canine type B synoviocytes using a fluorescent ligand, oral administration of compound A (9 and 27 mg/kg) did not improve weight bearing of dogs injected intra-articularly with IL-1β in a force plate analysis. Analysis of the synovial fluid of IL-1β-treated dogs indicated high levels of bradykinin postchallenge. Excellent exposure, coupled with evidence of the presence of the B1 receptor during an acute inflammatory model of pain, indicates an inability of the receptor to mediate inflammatory pain in canines.
Introduction
Safe and efficacious replacements for nonsteroidal anti-inflammatory drugs (NSAIDs) for the treatment of chronic pain have been at the forefront of pain research for many years. Due to the inducible nature of the bradykinin 1 (B1) receptor at peripheral sites during inflammation, the B1 receptor has the potential to deliver the desired safety profile of an NSAID replacement, especially under chronic dosing conditions.
Following induction, the B1 receptor is expressed in cells that maintain and exacerbate pain and inflammation. B1 receptors in these cells in turn propagate and potentiate the inflammatory cycle through the induction of plasma extravasation and the release of inflammatory mediators that sensitize the primary nociceptors. B1 receptor activation results in the release of arachidonic acid, prostaglandin E2, leukotrienes, and nitric oxide in vascular tissues and sympathetic neurons as well as interleukin-1 beta (IL-1β) and TNF-α from neutrophils and macrophages. Interactions with mast cells and/or primary nociceptors also release histamine, substance P, and serotonin (Chen & Johnson, 2007).
The attractive nature of this target has led to the discovery of a wealth of highly potent B1 receptor antagonists (Gougat et al., 2004; Chen & Johnson, 2007; Kuduk & Bock, 2008; Huang & Player, 2010; Farkas & Éles, 2011; De Falco et al., 2013). Increased upregulation of both B1 and B2 mRNA has been shown to coincide with the observed efficacy of known antagonists, further validating the mechanism (Levy & Zochodne, 2000). Visualization of the bradykinin B1 receptor during inflammation has been accomplished in both porcine vascular tissues (Schremmer-Danninger et al., 1996) and human and guinea pig lung tissues (Mak & Barnes, 1991). It has also been shown that B1 receptors are upregulated in rabbit aortic cells upon treatment with IL-1β (Marceau, 1995; Schanstra et al., 1998). In addition to this work, it has been shown that levels of bradykinin in the knee correlate with markers of cartilage degradation (GAG) and inflammation markers (IL-6) in humans with osteoarthritis (OA) (Bellucci et al., 2013).
Furthermore, cytokine profiles of dogs with both immune-mediated polyarthritis (Hegemann et al., 2005) and OA derived from cranial cruciate ligament rupture (Hegemann et al., 2005; Maccoux et al., 2007) show elevated levels of IL-1β in articular tissues. Treatment of canine articular chondrocytes in three-dimensional culture (Cook et al., 2000) as well as synovial explants (Hanks et al., 2010) with IL-1β has also been shown to cause a marked increase in PGE2 and MMP-3 along with a drop in glycosaminoglycan (GAG) concentration, indicative of both cartilage degradation and inflammation.
Although compelling evidence for the efficacy of the target in rodent models has been pervasive in the literature, both via murine B1 and B2 receptor knockouts (Lecci, 2000; Ferreira et al., 2002) as well as antagonism of the receptor (Gougat et al., 2004; Chen & Johnson, 2007; Kuduk & Bock, 2008; Huang & Player, 2010; Farkas & Éles, 2011; De Falco et al., 2013), a lack of bradykinin receptor homology between species (Hess et al., 2002), coupled with the inducible nature of the receptor, has hindered translation of the efficacy data between species. The B2 receptor has been found to be highly selective for the kinin peptides kallidin (KD) and bradykinin (BK) across mammalian species; however, the B1 receptors have a preference for the kinin peptides des-Arg10KD and des-Arg9BK along with significant species differences in the binding of synthetic peptide and nonpeptide ligands (Hess et al., 2001). Amino acid sequence homology of the B1 receptor between rat and canine has been found to be 69%, human to rat 71%, and human to canine 76% (Pesquero et al., 1996; Hess et al., 2001). By correlating evidence of the presence of the B1 receptor during an inflammatory challenge with efficacy data in a nonrodent species, we hope to further bridge the gap between rodent and human efficacy.
The chemical structures and in vitro pharmacological profiles for compound A, (S)-5-amino-N-(3-((4-((4-methoxy-2-(trifluoromethyl)phenyl)amino)benzyl)carbamoyl)-tetrahydrofuran-3-yl)nicotinamide, have been reported. In brief, compound A is a potent and selective antagonist of the bradykinin B1 receptor. The human binding affinity constant (Ki) is 1 nm (Hauel et al., 2012). The canine binding affinity was found to be 16 nm, and the drug displays >1000× selectivity over the B2 receptor at 3 μm. The goal of the current study was to evaluate the pain-relieving effects of this antagonist in a canine acute inflammatory arthritis model.
Materials and Methods
Animal care and use
These studies were performed in accordance with the National Institutes of Health Guide to the Care and Use of Laboratory Animals and the Animal Welfare Act in association for the Assessment and Accreditation of Laboratory Animal Care Program. All activities utilizing animals occurred in Zoetis vivarium space which is an AAALAC-accredited institution.
FITC ligand binding to B1 receptor in canine CHOK1 cells
CHOK1 cells were transiently transfected with canine bradykinin receptors B1R or B2R (Hess et al., 2001) and grown in selective growth medium containing DMEM/F12 supplemented with 10% dialyzed FBS, 500 μg/mL G418 sulfate, 10 mm HEPES, and 1X antibiotic–antimycotic (Life Technologies, Waltham, MA, USA). qRT-PCR was performed on cell lysates to confirm appropriate gene expression against published sequences deposited in GenBank (data not shown). Cells were removed from the flask with cell dissociation medium (Life Technologies) and resuspended at 106 cells/mL in staining buffer containing DMEM/F12 supplemented with 10 mm HEPES, 0.1% BSA, and a cocktail of aminopeptidase inhibitors (1 μm amastatin, 1 μm phosphoramidon, and 1 μm captopril; Sigma-Aldrich, St. Louis, MO, USA). Cells were incubated at 37 °C/5% CO2 for 10 min with either 10 μm unlabeled B1R agonist ligand (Lys-[des-Arg9]-BK, Sigma) or vehicle (0.1% DMSO). Cells were then further incubated for 30 min with 650 nm fluorescently labeled B1R agonist ligand (FITC-C6-Lys-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe; American Peptide Company, Sunnyvale, CA, USA). Cells were washed in staining buffer and then fixed for 15 min in 4% paraformaldehyde (EMS, Hatfield, PA, USA) before acquisition on the FACSCanto II (BD, San Jose, CA, USA). Histograms were analyzed using FlowJo software (FlowJo, Ashland, OR, USA). Incubation with fluorescent ligand prior to washing and fixing ensured the plasma membrane was intact upon staining. For each sample, at least 10 000 cells were analyzed on a FACSCanto II flow cytometer. The fluorescence intensity of each cell was recorded, and the mean fluorescent intensity (MFI) of the cell population was calculated. For B1R-positive cells, cells were analyzed as above but instead of calculating MFI, the percentage of cells above a designated fluorescent threshold was determined. The B1R-CHO cells were also used for antagonist affinity measurements (data not shown).
Isolation of primary canine type B synoviocytes
Synovial tissue was dissected from a normal canine stifle and digested with Dispase II (Roche, Indianapolis, IN, USA). Synoviocytes were cultured in collagen-coated flasks (BD) in growth medium containing DMEM supplemented with 10% HI FBS and 1X Pen/Strep (Life Technologies). After two passages, cells displayed a fibroblast-like morphology consistent with type B synoviocytes and were frozen in cell recovery medium (Gibco, Grand Island, NY, USA).
FITC ligand binding to B1 receptor in canine type B synoviocytes
Type B synoviocytes were cultured in 6-well collagen-coated plates (1.25 × 106 cells/well) overnight. Growth medium was then replaced with serum-free medium containing DMEM supplemented with 0.1% BSA and Pen/Strep. Cells were then treated with canine IL-1β (5 or 10 ng/mL, R&D, Minneapolis, MN, USA) or LPS (1 or 10 μg/mL, Sigma) and incubated at 37 °C/5% CO2 for 4 h. Cells were removed from the plate with cell dissociation medium and resuspended in serum-free medium containing aminopeptidase inhibitors. Cells were then stained with FITC-B1R agonist ligand and analyzed on the FACS as previously described. To determine the specificity of the FITC ligand for the B1R receptor in synoviocytes, cells treated with 10 ng/mL IL-1β were pre-incubated for 15 min prior to staining with 20 μm unlabeled ligand or vehicle (0.1% DMSO).
Pharmacological studies conducted in a canine experimental model of acute inflammatory arthritis
All animals utilized in the following experiments were maintained on a 12-h light:dark cycle. A certified dog chow (Purina 5007, PMI Nutrition International, LLC, Brentwood, MO, USA) and water were offered ad libitum. All animals were individually housed during the experiments.
Oral PK in the dog
Oral doses of compound A (3 mg/mL in 10% ethanol, 50% polyethylene glycol 400, 40% of 20% beta-cyclodextrin sulfobutyl ether, 7 sodium salt (SBECD) in phosphate-buffered saline (PBS, Gibco, Grand Island, NY, USA)) were administered at 3, 9, and 27 mg/kg via gavage followed by 10 mL of water to adult male beagle dogs. Blood was collected at 0.25, 0.5, 1, 2, 4, 8, 12, and 24 h postdose and examined for drug plasma concentrations using plasma protein precipitation analyzed on a LC-MS/MS.
Measurement of compound A in the CNS
Adult male beagle dogs (N = 3) were dosed at 1.0 mg/kg i.v. (3 mg/mL compound A in 10% ethanol, 50% polyethylene glycol 400, 40% of 20% beta-cyclodextrin sulfobutyl ether, 7 sodium salt (SBECD) in PBS). A 2.0-mL jugular blood sample was collected just prior to drug administration via an i.v. injection into the cephalic vein using a 21-gauge needle. At 2 h post administration, a jugular vein blood sample was collected and the animals were euthanized using a barbiturate overdose (Fatal Plus, Vortech Pharmaceutical, Dearborn, MI, USA) and the skin and musculature was reflected back from the skull to aid in identifying the location of the cisterna magnum (CM). A ‘clean’ (no blood) CSF sample was collected from the CM using a 21-gauge butterfly infusion set and syringe. The blood samples remained on wet ice until the plasma was separated by centrifugation (1200 g × 15 min), placed into labeled plastic vials, and stored at ≤ −20 °C until analysis.
Effect of compound A following IL-1β challenge
These studies were conducted to determine the analgesic effects of compound A (9 and 27 mg/kg, p.o.) on lameness in a once-daily dosing paradigm for 2 days in dogs. Purpose-bred beagle dogs (N = 16 adult males; eight per treatment; 1–2 years old) had lameness induced by intra-articular injection of recombinant canine IL-1β. The recombinant canine IL-1β was freshly prepared approximately 1 h before synovitis induction by dilution with sterile PBS to a final concentration of 0.68 μg/mL. One hour prior to the first administration of placebo or compound A, Dexdomitor® was administered intravenously in the forelimb to produce a brief period of anesthesia. The stifle was shaved and cleaned using 2X alcohol wipe followed by Betadine scrub. While the animal was unconscious, 1.5 mL of canine IL-1β (1.0 μg total) was administered into the stifle to induce lameness, with the dose chosen such that it induced a consistent level of lameness (VAS 8-9) over a 12- to 24-h period which could be significantly reduced with carprofen and induced production of pro-inflammatory cytokines in the synovial fluid. Antisedan® was administered intramuscularly immediately following synovitis induction. Lameness was assessed at 3, 5, 7, 24, 27, and 30 h following synovitis induction. Dogs were moved to an area out of their general housing area and allowed to move about spontaneously. Each dog was observed, and lameness was assessed using a visual analog scale (VAS) by a single observer masked with regard to treatment. This is a continuous 10-cm scale anchored by two endpoints: lameness scores range from 0 cm (corresponding to no deviation from normal movement) to 10 cm (corresponding to the worst possible lameness; e.g., 3-legged lameness). Intermediate scores correspond to varying degrees of lameness such as limping, hip drop, hitching gait, toe touching.
Statistics
Prior to initiation of the IL-1β challenge experiments, animals were allocated to treatment groups and housing location according to a randomized complete block design with one-way treatment structure. Blocking was based on housing location. A block consisted of two individually housed animals in adjacent pens with each treatment occurring once within each block. Animals were housed in a single room, and the experimental unit was the animal.
VAS lameness data were analyzed using a general linear mixed model for repeated measures. The model included the fixed effects of treatment, time, and the treatment by time interaction. Random effects include block, the interaction between block and treatment, and residual. No variance stabilizing transformation was required for this model. Least-squares means were used as estimates of treatment means. Standard errors of least-squares means were estimated, and 90% confidence intervals were constructed. Treatment differences were assessed at the 10% level of significance (P < 0.10) if any treatment related effects are significant using Fisher's protected LSD.
Drugs
Drug doses refer to their respective free bases. The selective B1 antagonist compound A was synthesized by Zoetis Laboratory Sciences Medicinal Chemistry Laboratories.
Results
FITC binding to canine CHOK1 cells and type B synoviocytes
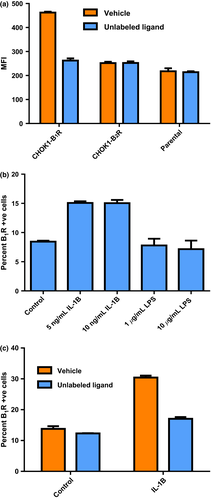
A fluorescently labeled B1-selective agonist ligand (FITC-Lys-[des-Arg9]-BK) was used to demonstrate binding to CHOK1-B1 receptor transients (Fig. 1a). No ligand binding to CHOK1- B2 receptor transients was observed, nor to the parental line. The fluorescent ligand was successfully displaced from the B1 receptor by excess unlabeled ligand (Lys-[des-Arg9]-BK, 10 μm). The lack of B2 receptor binding coupled with the displacement with unlabeled ligand indicated specificity of the fluorescent ligand for the B1 receptor. To explore the expected upregulation of the B1 receptor in canine synovial tissues, primary canine type B synoviocytes isolated from synovial tissue were treated with both LPS and IL-1β. Cells treated with IL-1β demonstrated upregulation of the B1 receptor via binding of the fluorescent ligand; however, there was no dose dependency in the concentration range tested (Fig. 1b). Cells treated with LPS did not exhibit any B1 receptor upregulation (Fig. 1b). The specificity of the binding of the fluorescent ligand to the synoviocytes was further demonstrated by displacement with unlabeled agonist ligand (Fig. 1c).
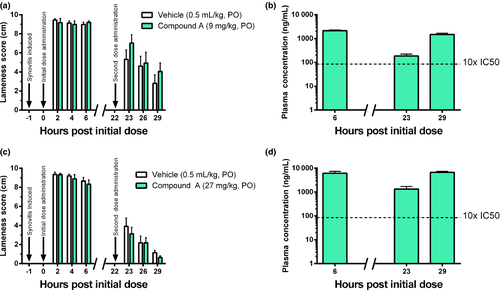
PK assessment, measurement of compound A in the CNS and synovial fluid, and effect of compound A following IL-1β challenge in canines
Compound A was dosed at 1 mg/kg i.v. as well as at 3, 9, and 27 mg/kg p.o. (t1/2 5.6 h, Vd 804 mL/kg, Cl 228 mL/kg/h, %F 89) to evaluate both linearity of the dosage regimen and the tolerability of the antagonist. Doses of 9 and 27 mg/kg were selected to maintain blood levels in excess of 10x and 100x the IC50 for 24 h, respectively. Cerebrospinal fluid was also drawn at 2 h postdose (1 mg/kg, i.v.) of compound A to determine CNS exposure relative to plasma levels. CNS exposure was found to be 100x lower than plasma exposure in each subject (356–469 ng/mL plasma (free) vs. 3.26–4.64 ng/mL CSF). Dogs were administered an initial dose of compound A (9 mg/kg, p.o.) 1 h postsynovitis induction with IL-1β and a second dose at 22 h. Lameness was measured via VAS lameness score assessment at 2, 4, 6, 23, 26, and 29 h.
In the vehicle-treated dogs, IL-1β produced marked lameness at 2, 4, and 6 h after injection. Lameness severity reached 9 of 10 cm on the VAS score. In dogs treated with 9 mg/kg of compound A, free drug exposures reached 3000 ng/mL (free), which is more than 100-fold above the IC50 (Fig. 2b). Despite these high exposures, no reduction in lameness was observed at 2, 4, or 6 h after dosing (Fig. 2a). Clinically significant lameness was still present at the 23, 26, and 29 h timepoints in the vehicle-treated dogs. In the drug-treated group, exposures were 10- to 100-fold above the IC50 (Fig. 2b); however, no significant reduction in lameness was observed (Fig. 2a). In a subsequent study utilizing a 27 mg/kg p.o. dose, compound A also failed to result in a measurable improvement of lameness (Fig. 2c), despite reaching exposures of 9000 ng/mL(free) (Fig. 2d).
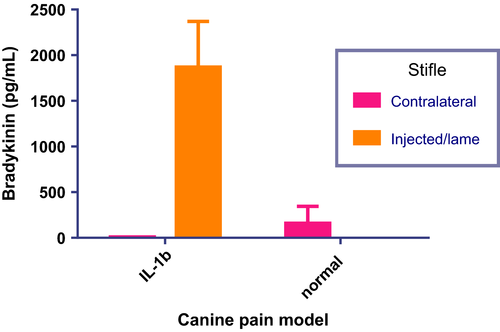
Measurement of the levels of bradykinin present in canine synovial fluid was also obtained, from both the lame ipsilateral (i.e. injected) and the contralateral (i.e. noninjected) stifle of IL-1β synovitis model dogs from the dosing experiments. Results of the assay which fell within the linear range of the standard curve were used to obtain group means which are plotted in the bar graph shown in Fig. 3. The synovial fluid from the injected/lame stifle showed a marked increase in bradykinin concentrations upon treatment with IL-1β when compared with the contralateral stifle. Bradykinin was not detected in synovial fluid from the contralateral stifles.
Discussion
The present studies are the first to characterize the effects of selective bradykinin B1 receptor antagonists in a canine model of acute inflammatory arthritis. The lack of homology of the B1 receptor across species is well established (Hess et al., 2002) and therefore complicates the translation of observed efficacy in arthritis models across species. The transient nature of B1 receptor expression and its upregulation only under inflammation also challenge the assessment of efficacy, or the lack thereof.
The major focus of this article relates to the understanding of the nature of the role that the B1 receptor plays in the perception of pain in canines in an acute arthritic state. IL-1β is a pro-inflammatory cytokine that induces additional pro-inflammatory cytokines, stimulates pro-angiogenic factors, and induces chondrocyte apoptosis (for review, see Sutton et al., 2009). Additionally, IL-1β is found in the synovial fluid of dogs with various pathological joint conditions, such as hip dysplasia, cranial cruciate ligament rupture, and osteoarthritis (Fujita et al., 2005, 2006; Maccoux et al., 2007). Based on these findings, it stood to reason that direct injection of IL-1β would produce an experimental model of joint pain with a measurable lameness. Indeed, injection of IL-1β has produced a lameness that lasts up to 48 h and is reproducible over multiple studies, while intra-articular injection of PBS does not produce an observable lameness (unpublished data). Additionally, this lameness is sensitive to treatment with carprofen (Pfizer Animal Health, New York, NY, USA) (4.4 mg/kg, p.o.), an NSAID currently utilized to treat OA in dogs (unpublished data). These criteria support the utilization of this model to determine the efficacy of potential analgesic compounds, such as B1 antagonists.
Through the utilization of a labeled, B1R-specific ligand, we were able to show that synovial cells, when treated with IL-1β, upregulate the B1 receptor. We were also able to show that high levels of bradykinin were present in the synovial fluid of dogs treated with IL-1β, similar to levels seen in those of dogs clinically diagnosed with OA, and therefore, catabolites of bradykinin should also be present due to the rapid degradation of bradykinin that is known to occur (Bhoola et al., 1992). The presence of bradykinin as well as its catabolites, such as [des-Arg9]-BK and [des-Arg10]-kallidin, is known to upregulate both B2 and B1, respectively (Schanstra et al., 1998; Bertram et al., 2009; De Falco et al., 2013). The sum total of these observations further supports the likelihood that the B1 receptors were present over the course of the IL-1β inflammation model.
Upon treatment with the selective B1 antagonist compound A postchallenge with IL-1β, one can see that the inhibition of the bradykinin B1 receptor does not appear to ameliorate the pain associated with synovitis in dogs. Two doses of compound A spread over a 2-day period allowed ample opportunity for therapeutic intervention and upregulation of the B1 receptor, which is believed to occur anywhere from 2–6 h postchallenge (Schanstra et al., 1998; Bertram et al., 2009). Although lameness scores were high on day 1 of the study and may have overwhelmed the effect of compound A, the scores on day 2 were less severe and compound A was still incapable of producing an effect. As previously mentioned, carprofen (4.4 mg/kg, p.o.) significantly reduced the same levels of lameness as observed in this study (unpublished data). The lack of efficacy was observed despite reaching blood levels 1000x the IC50 (16 nm) of the antagonist in plasma as well as 10x the IC50 in the CNS; neither peripheral nor central exposure was impactful on perceived pain. Central B1 receptor agonism following peripheral noxious stimulation has been implicated in the painful response to heat and chemical irritants (Lecci, 2000); thus, adequate exposure of central B1 receptors to compound A was necessary to rule out a lack of central exposure in measured efficacy. While concentrations of compound A were not measured in the synovial fluid, it is unlikely that the drug failed to distribute to this tissue compartment (Owens et al., 1995). These findings contrast those of IL-1β-induced mechanical hyperalgesia models in rats, where intra-articular injection of the B1 receptor antagonist peptide, [des-Arg9Leu8]-BK, reduced the level of hyperalgesia at 1, 4, and 6 h postadministration (Davis & Perkins, 1994), further highlighting the observed species disconnect in the efficacy of bradykinin B1 antagonists.
The lack of response to treatment of the canine synoviocytes with LPS may have been a product of incubation time. In previous unpublished studies, a full LPS-induced cytokine response required 21–24 h of incubation to manifest, whereas the IL-1β-induced responses were well developed by 5 h. The staining work conducted in the current study occurred at 4 h, possibly being insufficient to allow full upregulation and visualization of the B1 receptors.
In summary, our studies demonstrate the lack of efficacy of a potent and selective bradykinin B1 receptor antagonist in an IL-1β-induced canine arthritis model. Despite the model replicating the expression of IL-1β observed in dogs clinically diagnosed with OA as well as the visualization of the B1 receptor in synovial cells challenged with IL-1β in vitro, high exposures of compound A failed to affect lameness in dogs. Currently, there is a need for the development of anti-inflammatory analgesics with superior safety profiles to those of NSAIDs. Bradykinin B1 receptor antagonists do not show promise as potential therapeutics for the treatment of pain in dogs.




