Effect of benazepril and robenacoxib and their combination on glomerular filtration rate in dogs
Abstract
Combined use of angiotensin-converting enzyme inhibitors and nonsteroidal anti-inflammatory drugs may induce acute kidney injury, especially when combined with diuretics. The objective of this investigation was to evaluate the effect of benazepril, robenacoxib and their combination in healthy dogs. In each of two studies (studies 1 and 2), 32 beagle dogs were randomized into one of four groups in a parallel-group design. Groups received once-daily oral treatment for 7 days with placebo, benazepril, robenacoxib or benazepril plus robenacoxib. In study 2, all dogs received additionally 2 mg/kg furosemide orally twice daily. The primary endpoint was the glomerular filtration rate (GFR) estimated from the plasma clearance of iohexol. Secondary endpoints included standard clinical monitoring and, in study 2, plasma renin activity, urine volume, specific gravity and aldosterone concentration and water intake. Administration of furosemide induced diuresis, reduced GFR and activated the renin–aldosterone–angiotensin system. Benazepril and robenacoxib, administered alone or in combination, were tolerated well, did not decrease GFR with or without co-administration of furosemide and significantly reduced urinary aldosterone concentrations. No increased risk of acute kidney injury was identified with the combination of benazepril and robenacoxib in healthy dogs. Different effects might occur in dogs with heart or renal disease.
Introduction
Angiotensin-converting enzyme (ACE) inhibitors (ACEIs) are used in the management of various diseases in dogs, including congestive heart failure (CHF), chronic kidney disease (CKD) and hypertension (Lefebvre & Toutain, 2004). Nonsteroidal anti-inflammatory drugs (NSAIDs) are used to treat fever, inflammation and pain in dogs, notably related to osteoarthritis (OA) and surgery. Administration of an ACEI and an NSAID to the same animal may be considered in dogs suffering from both cardiovascular disease and pain/inflammation, for example CHF or CKD and OA. In addition, ACEIs and NSAIDs may have additive beneficial efficacy in certain diseases, notably proteinuric CKD (Vogt et al., 2010). Additive effects of ACEIs and NSAIDs in reducing proteinuria have been described in humans (Heeg et al., 1990).
Negative pharmacodynamic interactions between ACEIs and NSAIDs may occur, however (Whelton, 1999). First, by inhibiting the synthesis of vasodilatory prostaglandins, NSAIDs may reduce the systemic antihypertensive efficacy of ACEIs (Meune et al., 2003). Second, as a result of reduction in vasodilatory prostaglandins, NSAIDs can constrict the renal afferent arteriole leading in extreme cases to reduced glomerular filtration rate (GFR) and acute kidney injury (AKI). The risk of AKI with NSAIDs is increased by dehydration or reduced blood pressure, for example with general anaesthesia or the administration of ACEIs (KuKanich et al., 2012).
One of the main mechanisms of action of ACEIs in slowing the progression of CKD is the reduction in glomerular capillary hypertension, mediated via the inhibition of synthesis of angiotensin (ANG)-2 leading to a selective dilation of the efferent arterioles in the kidney (Lefebvre & Toutain, 2004). As a result of reduced glomerular capillary pressure, ACEIs may reduce GFR in patients with CKD. The benefit-to-risk ratio of ACEIs in CKD remains positive, however, provided that the reduction in GFR, usually evidenced by increased plasma creatinine concentrations, is only moderate in intensity (International Renal Interest Society, 2013). The combination of vasoconstriction of the renal afferent arteriole by an NSAID and dilation of the efferent arteriole by an ACEI carries the risk of excessive reduction in GFR potentially leading to AKI (Heeg et al., 1990). The risk of AKI is further increased by the concomitant use of diuretics that themselves reduce blood flow to the glomerulus via intravascular volume depletion (Cupp, 2013). The danger of the combination of ACEIs, diuretics and NSAIDs in humans was described as a ‘triple whammy’ (Thomas, 2000; Cupp, 2013).
Nevertheless, reports of adverse interactions between ACEIs and NSAIDs are rare in dogs. Since the ACEI benazepril (Fortekor®) was registered in dogs in 1995 and the NSAID robenacoxib (Onsior®) in 2009, reports of negative interactions or renal failure have been extremely rare in both cats and dogs.
The objective of this study was to evaluate the effect of benazepril, robenacoxib and their combination in healthy dogs. Two studies were conducted, without and then with the administration of the loop diuretic furosemide. The primary endpoint was the GFR, which was estimated from the plasma clearance of iohexol.
Materials and Methods
Two studies (termed study 1 and study 2) were conducted using similar designs, except that in study 2 the diuretic furosemide was administered and plasma renin activity (PRA), urine volume and water intake and urine variables including aldosterone were measured. Both studies followed a randomized and parallel-group design comparing three treatment groups (benazepril, robenacoxib and their combination) to the control group (Table 1).
| Group | Treatment (mg/kg) once daily | Study 1 | Study 2 | |
|---|---|---|---|---|
| A | None (control) | Cohort 1 | 4 f | 2 m & 2 f |
| Cohort 2 | 4 m | 2 m & 2 f | ||
| B | Benazepril (0.5–1.0) | Cohort 1 | 4 f | 2 m & 2 f |
| Cohort 2 | 4 m | 2 m & 2 f | ||
| C | Robenacoxib (1.0–2.0) | Cohort 1 | 4 f | 2 m & 2 f |
| Cohort 2 | 4 m | 2 m & 2 f | ||
| D | Benazepril (0.5–1.0) + robenacoxib (1.0–2.0) | Cohort 1 | 4 f | 2 m & 2 f |
| Cohort 2 | 4 m | 2 m & 2 f | ||
- f = female, m = male.
Studies 1 and 2 were performed, respectively, in compliance with the registered permit numbers 2011_11_FR and 2012_34E_FR approved by the Swiss Cantonal Animal Welfare Committee, and after approval of the protocols by the company Global Animal Welfare Officer. Study 2 was initiated only after positive tolerability results were obtained from study 1. The studies were designed to use the fewest number of animals possible while being consistent with the objectives. Dogs, the target species for the test items, were used as the current state of scientific knowledge did not provide acceptable alternatives, in vitro or otherwise, to accomplish the purpose of the studies. All dogs were returned to the site's animal facilities upon completion of the studies.
This manuscript was prepared in compliance with the ARRIVE Guidelines Checklist for animal in vivo experiments (Kilkenny et al., 2010).
Animals
A total of 64 unneutered beagle dogs were used, with 32 (16 males and 16 nonpregnant and nulliparous females) included in each study. All dogs were healthy and had not received an ACEI or NSAID for at least 2 months prior to each study.
Housing and management
The acclimatization phase lasted approximately 2 weeks and started on day −15 (study 1, Table 2) or day −11 (study 2, Table 3). During this phase, the dogs were acclimatized to their housing and management and were confirmed to be healthy based on physical examination, feed consumption and the results of clinical chemistry, haematology and urinalyses.
| Category | Cohort 1 study days | Cohort 2 study days | ||
|---|---|---|---|---|
| Pretreatment | Post-treatment | Pretreatment | Post-treatment | |
| Treatment with test items | 0 to 6 | 1 to 7 | ||
| Body weight | −15, −8, −1 | 6 | −15, −8, −1 | 7 |
| Food consumption | −15 to −8, −7 to −1 | 0 to 8 | −15 to −8, −7 to 0 | 1 to 8 |
| Haematology & coagulation | −7 | 1, 7 | −7 | 2, 8 |
| Clinical chemistry | −7 | 1, 7 | −7 | 2, 8 |
| Iohexol concentration (0, 2, 3 & 4 h) | −12 | 6 | −11 | 7 |
| Category | Cohort 1 study days | Cohort 2 study days | ||
|---|---|---|---|---|
| Pretreatment | Post-treatment | Pretreatment | Post-treatment | |
| Treatment with test items and furosemide | 0 to 6 | 7 to 13 | ||
| Body weight | −11, −7, −1 | 7 | −11, −2, 6 | 14 |
| Food consumption | −11 to −1 | 0 to 7 | −11 to 6 | 7 to 14 |
| Haematology & coagulation | −9, −1 | 6 | −9, 6 | 13 |
| Clinical chemistry | −9, −1 | 3, 6 | −9, 6 | 10, 13 |
| Urinalysis and water consumption | −4 | 5 | −2 | 12 |
| Iohexol concentration (0, 2, 3, & 4 h) | −3 | 6 | −1 | 13 |
| Plasma renin activity (0, 1, 2 & 4 h) | 0 | 5 | 7 | 12 |
The dogs were housed in pairs (same sex and treatment group) in a climate-controlled building with artificial light from 6.00 to 18.00. The dogs were placed in individual pens for 2–4 h each day to record feed consumption and had access to outdoor runs daily for 0.5–2 h. In study 2, the dogs were placed in metabolic cages for 24 h on days −2/−4 and 5/12 to measure the water consumption and urine volume.
Each dog was offered approximately 150–430 g dried pelleted food (Biomill Adult Medium, Biomill SA, Herzogenbuchsee, Switzerland) once a day. Due to the possible impact of feeding on endpoints, the dogs were fed at 14.00 ± 1 h each day, except at weekends during the acclimatization phase when the dogs were fed at 10.00 for logistical reasons. On the days that blood samples were taken, dogs were fed either after the 4-h time-point for PRA or the 4-h time-point after iohexol administration. Drinking water was supplied ad libitum.
Randomization and blinding
The dogs were assigned at random to one of four groups (4/gender/group), while maintaining homogeneous distribution of body weight where possible (Table 1). Due to the experimental workload, the dogs in both studies were divided into two cohorts with the first day of dosing staggered by 1 (study 1) or 7 (study 2) days. In study 1, cohort 1 consisted of all females and cohort 2 all males. In study 2, each cohort consisted of 2 female and 2 male dogs of each treatment group. This is a weakness of study 1, introducing the risk of a ‘sex by time’ interaction. However, no evidence for a sex effect or ‘sex by treatment’ interaction occurred in either study. The randomization was carried out by the statistician using SAS/STAT® procedure plan (SAS® version 9.2.2, 2008, SAS Institute Inc. Cary, NC, USA).
The study was not blinded. This was judged acceptable because the primary and main endpoints were objective.
In study 2, the dogs received in addition furosemide twice daily (BID) on days 0 to 5 (cohort 1) or 7 to 12 (cohort 2) and then a single dose on the morning of day 6 (cohort 1) or 13 (cohort 2).
Test items and furosemide
Benazepril was dosed orally at a minimum dose of 0.5 mg/kg (as the hydrochloride salt) with a range of 0.5–1.0 mg/kg (Fortekor® Flavour 5 mg tablets, Elanco Animal Health, Huningue, France). The dose range is in the upper part of that (0.25–1.0 mg/kg) registered for the treatment of CHF in dogs in the EU (The BENCH Study Group, 1999).
Robenacoxib was dosed orally at a minimum dose of 1.0 mg/kg with a range of 1.0–2.0 mg/kg (Onsior® 10 mg flavoured tablets, Elanco Animal Health, Huningue, France). This is the registered dose of robenacoxib for the treatment of OA in dogs in the EU (Reymond et al., 2012).
In study 2, furosemide was administered orally at a target dose of 2.0 ± 0.2 mg/kg BID (Salix® tablets containing 12.5 mg furosemide, Merck Animal Health, Kenilworth, NJ, USA), the same as used by Lantis et al. (2015a,b) and within the range of 1 mg/kg BID to 6 mg/kg three times daily recommended for dogs with CHF (Atkins et al., 2009).
The 7-day treatment time for the test items was selected in order to reach steady-state effects for benazepril (steady-state concentrations achieved within 2–3 days with the maximal inhibition of ACE within 2–4 days, King et al., 1995) and robenacoxib (terminal half-life 1–2 h in the blood and ~24 h in peripheral sites of inflammation, Jung et al., 2009; King et al., 2009).
Except for unplanned medical treatments, additional medications were prohibited. In order to avoid multiple administrations, benazepril and robenacoxib were dosed together in combinations of whole and part tablets in a single gelatin capsule. The control group received a placebo consisting of an empty capsule. In study 2, furosemide was administered in a different capsule and in the morning was administered at a maximum of 5 min prior to the other treatments. All treatments were administered at 8.00 ± 1 h with the exception that, in study 2, furosemide was administered at 8.00 ± 1 h and 20.00 ± 1 h. After dosing, 5 mL of water was administered via a syringe to facilitate the passage of the capsules into the stomach, and the dogs were observed for several minutes to ensure that the capsules had been swallowed.
Doses were determined using the body weight on day 6 (study 2, cohort 2) and day −1 for the other cohorts.
Endpoints
The primary endpoint of the study was the GFR, which was estimated from the plasma clearance of iohexol (CLioh). The iohexol dose range was 88.2–93.2 mg iodine/kg.
Secondary endpoints included clinical monitoring via the results of clinical examination, body weight, feed consumption, clinical chemistry, coagulation and haematology variables. In study 2, additional secondary endpoints were water intake, urine volume, urine aldosterone/urine creatinine ratio (Uald:creat), fractional excretions of sodium (FENa) and potassium (FEk), urine specific gravity (USG) and plasma ANG-1 and PRA.
Clinical monitoring
Dogs were checked at least once daily. A detailed clinical examination was made once during the acclimatization, predose, 5 h and 72 h after the first dosing with the test items, 5 h after the last dosing with the test items and a final examination after the final dosing.
Dogs were weighed in the morning before feeding three times in the baseline period and again at the end of the study (Tables 2 & 3). During single housing phases, daily food consumption was determined as the difference between rations offered and left after approximately 2 h.
Blood samples for clinical pathology (standard haematology, coagulation and clinical chemistry variables) were taken from a cephalic or jugular vein after an overnight fast once or twice during the acclimatization phase (for animal selection and as baseline), 24 h (study 1) or 4 days (study 2) after the first treatment and on the last dosing day (study 2) or the following day (study 1) (Tables 2 & 3).
Glomerular filtration rate (GFR)
The GFR was estimated from the CLioh after intravenous (IV) administration of iohexol. This was performed once during the acclimatization phase prior to the administration of the test item(s) and again after the last treatment.
Feed was withheld for at least 12 h but the dogs were well hydrated before IV administration of iohexol (Accupaque® 350, GE Healthcare Buchler GmbH & Co., Braunschweig, Germany). A target dose of 90 mg of iodine/kg was administered via an IV catheter placed in a cephalic vein followed by flushing with physiological saline solution. Actual mean (range) doses administered were 90.3 (88.2–93.2) iodine/kg in study 1 and 90.39 (88.3–94.28) iodine/kg in study 2. Blood was sampled from a jugular or antebrachial vein (i.e. not a vein used for iohexol administration) in 1.2-mL heparin tubes before and 2, 3 and 4 h after the administration of iohexol. Samples were stored on ice until centrifugation at 3000 g at 4 °C for 15 min. Once centrifuged, plasma was collected and stored at −20 °C before analysis.
Due to the breakdown of equipment between studies, plasma iohexol concentrations were analysed using two different methods in studies 1 and 2. Both methods were validated in dog plasma in terms of selectivity, linearity, repeatability, reproducibility and stability according to the document ‘Guidance for Industry: Bioanalytical Method Validation’ (U.S. Department of Health and Human Services, 2001, http://www.labcompliance.com/info/links/methods/guidelines.aspx).
In study 1, the endo- and exo-iohexol isomers were quantified separately. Analyses were performed on a High Performance Liquid Chromatography system (Hewlett Packard 1100, Palo Alto, CA, USA) coupled with a UV detector (Hewlett Packard 1050). The method was linear over the calibration ranges 1 to 80 μg/mL and 2 to 320 μg/mL for endo- and exo-iohexol, respectively. Intraday and interday precisions were lower than 9% for both isomers. The accuracy ranged from 98 to 102%. The limit of quantification (LOQ) was 1 μg/mL with 8% precision and 99% accuracy for endo-iohexol and 2 μg/mL with 10% precision and 109% accuracy for exo-iohexol.
In study 2, total plasma iohexol concentrations were measured using an Ultra High Performance Liquid Chromatography system (Acquity UPLC, Waters, Milford, MA, USA) coupled with a triple quadrupole mass spectrometer (Xevo, Waters). The method was validated with a calibration curve ranging from 5 to 400 μg/mL. Intraday and interday precisions were, respectively, lower than 11% and 12%. The accuracy varied from 87 to 102%. The LOQ was 5 μg/mL with a precision of 7% and an accuracy of 91%.

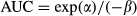

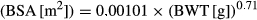
Urine variables
In study 2, dogs were housed in individual metabolism cages on days −4 and 5 (cohort 1) or −2 and 12 (cohort 2).
The total volume of water consumed over 24 h was calculated from the difference in volume of water provided and remaining in bowls that were suspended above the floor. The volume was checked repeatedly during the day.
Urine samples from 0- to 4-, 4- to 8-, 8- to 12- and 12- to 24-h periods were stored at below −70 °C before the analysis for aldosterone, creatinine, potassium, sodium and USG. Aldosterone was determined using a Symbiosis LC-MS/MS system (online SPE, Spark Holland, Emmen, Netherlands) after liquid–liquid extraction with ethyl acetate. Because urine was not collected in all periods in many dogs, data are presented as the weighted average for the interval of 0–24 h.
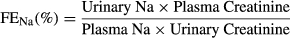
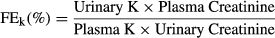
Plasma angiotensin 1 (ANG-1) and plasma renin activity (PRA)
In study 2, on the first and penultimate days of dosing (0 and 5 in cohort 1, 7 and 12 in cohort 2), approximately 1.2 mL of blood was collected (predose) in the morning before the administration of furosemide and 1, 2 and 4 h later (postdose). The blood was collected into precooled tubes containing K3 EDTA and aprotinin (500 KIU/mL of blood). The blood samples were transferred to ice, and the plasma was collected after centrifugation at approximately 3000 g at 4 °C for 15 min. The plasma was stored below −70 °C until analysis for ANG-1 concentrations using an enzyme immunoassay (Peninsula Labs, San Carlos, CA, USA). The PRA was calculated by comparing the concentration of ANG-1 obtained after incubation of the samples at +4 °C (the absence of renin activity) and +37 °C (the presence of renin activity).
Statistical analysis
The software SAS®, version 9.2 or higher, was used for all analyses (SAS 9.2 Help and Documentation, SAS Institute Inc., 2002–2009, Cary, NC, USA). The experimental unit was the results from each individual dog. Two-tailed P values less than 0.05 were defined as significant. Because no correction was made for multiple analyses, isolated events of statistical significance must be interpreted with caution. Data are presented as mean (±SD) or range.
Variables that were analysed only once post-treatment, including GFR, were analysed using analysis of covariance (ancova) with the pretreatment value as covariate and treatment as effect (Tables 5 & 8). Variables that were measured pretreatment and multiple times post-treatment were analysed using repeated-measures analysis of covariance (rmancova) (Tables 6 & 9). The pretreatment value was the covariate and the effects were treatment, time and ‘treatment by time’ interaction. Sex and its interaction with time and/or treatment were included as effects in all analyses, but these data are not shown in this paper because there was no evidence of a sex effect or ‘sex by treatment’ interaction for any variable. Data transformations were applied where appropriate. The adequacy of each statistical model was assessed by the evaluation of the normality of the residuals (Shapiro–Wilk test, SAS® procedure univariate). SAS® procedure mixed was used for the rmancova.
In the event that the P value was less than 0.1 for treatment or the ‘treatment by time’ interaction, individual groups were compared by linear contrasts with P < 0.05 defined as significant.
Results
Results from all dogs were included in all analyses.
Study 1
The 32 dogs were aged 5–38 months with body weight of 6–17 kg at baseline. Groups were evenly matched. The mean (range) doses administered once daily, respectively, alone and in combination were 0.79 (0.52–0.99) and 0.76 (0.61–0.93) mg/kg for benazepril hydrochloride and 1.61 (1.17–1.98) and 1.53 (1.23–1.85) mg/kg for robenacoxib.
P values are shown for change from baseline analyses (Table 4), ancova (Table 5) and rmancova (Table 6).
| Variable | Group A: Control | Group B: Benazepril | Group C: Robenacoxib | Group D: Benazepril + robenacoxib |
|---|---|---|---|---|
| Primary endpoint | ||||
| Iohexol clearance per BWT | 0.7812 | 0.1517 | 0.1608 | 0.3908 |
| Iohexol clearance per BSA | 0.8994 | 0.2342 | 0.1824 | 0.1946 |
| Secondary endpoints | ||||
| Red blood cell count | 0.0395 D | <0.0001 D | 0.0060 D | 0.0001 D |
| Haemoglobin | 0.1728 | <0.0001 D | 0.0772 (D) | 0.0014 D |
| Haematocrit | 0.0092 D | <0.0001 D | 0.0012 D | <0.0001 D |
| White blood cell count | 0.0329 D | 0.2167 | 0.5022 | 0.6183 |
| Prothrombin time | 0.0121 I | 0.2217 | 0.0196 I | 0.5451 |
| Activated partial thromboplastin time | 0.1071 | 0.4648 | 0.2351 | 0.1788 |
| Plasma albumin | 0.4985 | 0.0025 D | 0.0433 D | 0.0189 D |
| Plasma creatinine | 0.4001 | 0.0626 (D) | 0.9419 | 0.8170 |
| Plasma inorganic phosphorus | 0.0008 D | 0.0039 D | 0.0050 D | 0.2951 |
| Plasma total protein | 0.0483 D | <0.0001 D | <0.0001 D | <0.0001 D |
| Plasma urea nitrogen | 0.0516 (I) | 0.8719 | 0.3026 | 0.3587 |
| Plasma calcium | 0.0655 (D) | 0.0124 D | 0.3612 | 0.0747 (D) |
| Plasma chloride | 0.0156 I | 0.6543 | 0.6001 | 0.1256 |
| Plasma potassium | 0.0006 D | 0.0011 D | 0.0005 D | 0.0184 D |
| Plasma sodium | 0.0326 I | 0.2561 | 0.5127 | 0.0566 (D) |
- P values for change from baseline were calculated using ancova or rmancova. In the event of a significant ‘treatment by time’ interaction in the rmancova, P values are for day 7 after the onset of treatment (days 7/14) compared to baseline.
- I or D indicates a significant increase or decrease vs. baseline (P < 0.05, shown in boldface). Parenthesized (D or I) indicates borderline significant (P < 0.10).
| Variable | Exponential | P values in ancova | P value for normality | P < 0.05 post hoca | |
|---|---|---|---|---|---|
| Treatment | Baseline | ||||
| Iohexol clearance per BWT | Log | 0.2055 | 0.4239 | 0.2665 | None |
| Iohexol clearance per BSA | Log | 0.2043 | 0.4421 | 0.4525 | None |
- a Because there was no significant treatment effect, post hoc comparisons were not interpreted. BWT = body weight, BSA = body surface area.
| Variable | Exponential | P values in rmancova | P value for normality | P < 0.05 post hoca | |||
|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment by time | Baseline | ||||
| Red blood cells | None | 0.0004a | <0.0001a | 0.0275 a | <0.0001a | 0.0057a | B < A & D |
| Haemoglobin | None | 0.0003a | <0.0001a | 0.0400 a | <0.0001a | 0.0227a | B < A |
| Haematocrit | None | 0.0001a | <0.0001a | 0.0267 a | <0.0001a | 0.0074a | B < A |
| White blood cells | Log | 0.6045 | 0.5834 | 0.3522 | <0.0001a | 0.2096 | |
| Prothrombin time | None | 0.3828 | 0.1851 | 0.9360 | <0.0001a | 0.0002a | |
| Activated partial thromboplastin time | None | 0.9452 | 0.1507 | 0.6523 | <0.0001a | 0.0044a | |
| Plasma albumin | None | 0.0538 a | 0.0001a | 0.1409 | <0.0001a | 0.3874 | B & D < A |
| Plasma creatinine | None | 0.2518 | <0.0001a | 0.3123 | 0.0233a | 0.4200 | |
| Plasma inorganic phosphorus | None | 0.6880 | 0.6917 | 0.0029 a | <0.0001a | 0.8308 | B < A |
| Plasma total protein | None | 0.0055 a | 0.0035a | 0.3225 | <0.0001a | 0.5435 | B, C & D < A |
| Plasma urea nitrogen | None | 0.1381 | 0.0006a | 0.5383 | 0.0214a | 0.0191a | |
| Plasma calcium | None | 0.6788 | 0.0114a | 0.8510 | <0.0001a | 0.2699 | |
| Plasma chloride | None | 0.0424 a | 0.1625 | 0.4631 | <0.0001a | 0.6177 | B & D < A |
| Plasma potassium | None | 0.7450 | <0.0001a | 0.0447 a | <0.0001a | 0.1993 | |
| Plasma sodium | None | 0.0320 a | 0.0075a | 0.4329 | 0.0417a | 0.4952 | B & D < A |
- a In the event of P < 1.0 for treatment or ‘treatment by time’ interaction (value for the interpretation shown in boldface), post hoc comparisons were interpreted. In the event of a ‘treatment by time’ interaction, post hoc comparisons at day 7 after the onset of treatment (days 7/14) are shown.
Clinical monitoring
There were no significant changes from baseline in any group for body weight or feed consumption, and no treatment effect in the ancova (P = 0.35 and P = 0.84, respectively). One isolated event of loose stools on day 1 and vomiting on day 2 was observed in one dog in group D (benazepril + robenacoxib). This finding was not considered to be a result of the application of the test items. No other clinical signs were reported.
There were significant decreases from baseline in red cell count and haematocrit in all groups, possibly due to habituation of the dogs to the repeated sampling leading to less splenic contraction. In the control group, mean (±SD) haematocrit increased by 5.5% from 0.479 ± 0.042 L/L at baseline (day −7) to 0.507 ± 0.034 L/L after the first day of treatment and decreased by 3.7% to 0.462 ± 0.044 L/L after 7 days of treatment. There was a significant treatment effect, and in the post hoc comparisons, the values were lower in group B vs. A and D for red cell count and group B vs. A for haematocrit and haemoglobin. There was no significant treatment effect for white cell count or coagulation times.
There were significant changes from baseline for many of the clinical chemistry variables, with all groups represented. There was a significant treatment effect or ‘treatment by time’ interaction for the following variables, with lower values in the stated group compared to the control group (A): albumin (B & D); inorganic phosphorus (B); total protein (B, C & D), chloride (B & D) and sodium (B & D).
Glomerular filtration rate
The GFR was assessed from the CLioh. There was low variability in plasma iohexol concentrations (Fig. 1). There were no differences in conclusions when CLioh was expressed per unit BWT or BSA, but conclusions were based primarily using BSA because P values for normality of the distribution in the ancova were higher. There was no significant change from baseline in CLioh in any of the four groups, although mean CLioh expressed per unit BSA increased by 22.2% in group B (benazepril) compared to a 1.75% decrease in the control group A (Fig. 2). There was no significant treatment effect (P > 0.2) or the differences between groups in post hoc analyses for CLioh.
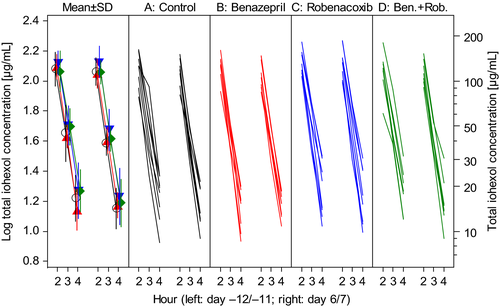
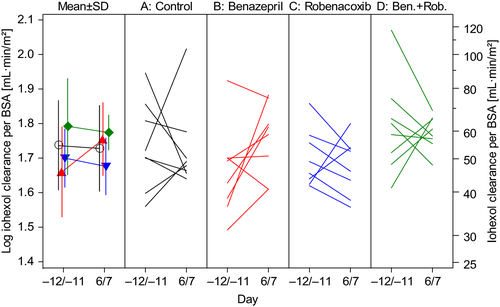
Study 2
The 32 dogs were aged 1–4 years with body weight of 8.6–13.9 kg at baseline. The groups were well matched. The mean (range) doses of the test items administered once daily, respectively, alone and in combination were 0.85 (0.54–0.98) and 0.76 (0.50–0.99) mg/kg for benazepril hydrochloride and 1.63 (1.01–2.0) and 1.51 (1.01–1.97) mg/kg for robenacoxib. The dose of furosemide administered was 1.99 (1.85–2.14) mg/kg BID.
P values are shown for change from baseline analyses (Table 7), ancova (Table 8) and rmancova (Table 9).
| Variable | Group A: Control | Group B: Benazepril | Group C: Robenacoxib | Group D: Benazepril + robenacoxib |
|---|---|---|---|---|
| Primary endpoint | ||||
| Iohexol clearance per BWT | 0.0108 D | 0.0003 D | 0.0015 D | 0.0003 D |
| Iohexol clearance per BSA | 0.0088 D | 0.0002 D | 0.0010 D | 0.0002 D |
| Secondary endpoints | ||||
| Red blood cell count | 0.0085 I | 0.8410 | 0.0957 (I) | 0.0255 I |
| Haemoglobin | 0.0065 I | 0.3606 | 0.0288 I | 0.0231 I |
| Haematocrit | 0.0186 I | 0.8566 | 0.0898 (I) | 0.0974 (I) |
| White blood cell count | 0.0652 (I) | 0.0577 (D) | 0.4383 | 0.0193 I |
| Prothrombin time | 0.2494 | 0.1357 | 0.0027 D | 0.7855 |
| Activated partial thromboplastin time | 0.0100 D | 0.2433 | 0.3943 | 0.7316 |
| Plasma albumin | 0.0061 I | 0.2808 | 0.0011 I | 0.0070 I |
| Plasma creatinine | 0.0082 I | 0.0590 (I) | 0.0385 I | 0.0031 I |
| Plasma inorganic phosphorus | 0.0947 (I) | 0.3981 | 0.6180 | 0.0842 (I) |
| Plasma total protein | 0.0038 I | 0.0229 I | 0.0001 I | 0.0003 I |
| Plasma urea nitrogen | <0.0001 I | 0.0117 I | 0.0012 I | 0.0228 I |
| Plasma calcium | 0.0033 I | <0.0001 I | <0.0001 I | <0.0001 I |
| Plasma chloride | <0.0001 D | <0.0001 D | 0.0001 D | <0.0001 D |
| Plasma potassium | 0.4182 | 0.0278 D | 0.0912 (D) | 0.0025 D |
| Plasma sodium | 0.6655 | 0.0221 D | 0.7088 | 0.0919 (D) |
| Water consumption | <0.0001 I | <0.0001 I | 0.0095 I | 0.0002 I |
| Urine volume | <0.0001 I | <0.0001 I | <0.0001 I | <0.0001 I |
| Urine specific gravity | <0.0001 D | <0.0001 D | <0.0001 D | <0.0001 D |
| Fractional excretion Na | 0.0366 I | 0.2482 | 0.1807 | 0.0962 (I) |
| Fractional excretion K | 0.6708 | 0.6525 | 0.7363 | 0.4422 |
| Urine aldosterone:creatinine | <0.0001 I | 0.0015 I | 0.0056 I | 0.0195 I |
| Angiotensin I (1, 2 and 4 h only) | <0.0001 I | <0.0001 I | <0.0001 I | <0.0001 I |
| Angiotensin I (predose 0 h only) | <0.0001 I | <0.0001 I | 0.0006 I | <0.0001 I |
| Plasma renin activity (1, 2 and 4 h only) | <0.0001 I | <0.0001 I | <0.0001 I | <0.0001 I |
| Plasma renin activity (predose 0 h only) | <0.0001 I | <0.0001 I | 0.0017 I | <0.0001 I |
- P values for change from baseline were calculated using ancova or rmancova. In the event of a significant ‘treatment by day/time’ interaction in the rmancova, P values are for day 6 after onset of dosing (days 6/13) compared to baseline.
- I or D indicates a significant increase or decrease vs. baseline (P < 0.05, shown in boldface). Parenthesized (D or I) indicates borderline significant (P < 0.10).
| Variable | Exponential | P values in ancova | P value for normality | P < 0.05 post hoca | |
|---|---|---|---|---|---|
| Treatment | Baseline | ||||
| Primary endpoint | |||||
| Iohexol clearance per BWT | None | 0.7069 | 0.0048a | 0.0322a | |
| Iohexol clearance per BSA | None | 0.6910 | 0.0045a | 0.0413a | |
| Secondary endpoints | |||||
| Red blood cell count | None | 0.2874 | 0.0017a | 0.0463a | |
| Haemoglobin | None | 0.5163 | <0.0001a | 0.0947 | |
| Haematocrit | None | 0.4225 | 0.0001a | 0.2462 | |
| White blood cell count | None | 0.0185 a | 0.0003a | 0.8398 | B < A & D |
| Prothrombin time | None | 0.1113 | <0.0001a | 0.2834 | |
| Activated partial thromboplastin time | None | 0.1976 | <0.0001a | 0.3887 | |
| Water consumption | None | 0.1409 | 0.0010a | 0.8205 | |
| Urine volume | None | 0.1085 | 0.0011a | 0.4932 | |
| Urine specific gravity | None | 0.0907 a | 0.0019a | 0.1966 | |
| Fractional excretion Na | None | 0.8918 | 0.6721 | 0.0415a | |
| Fractional excretion K | None | 0.7898 | 0.0123a | 0.2143 | |
| Urine aldosterone:urine creatinine | None | 0.0359 a | 0.0028a | 0.1907 | B, C & D < A |
- a In the event of P < 1.0 for treatment effect (value for the interpretation shown in boldface), post hoc comparisons were interpreted.
| Variable | Exponential | P values in rmancova | P value for normality | P < 0.05 post hoca | |||
|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment by time | Baseline | ||||
| Plasma albumin | None | 0.3976 | 0.0003a | 0.0523 a | <0.0001a | 0.0984 | |
| Plasma creatinine | Log | 0.7722 | 0.3079 | 0.1995 | <0.0001a | <0.0001a | |
| Plasma inorganic phosphorus | None | 0.2214 | 0.3879 | 0.3714 | 0.0001a | 0.9039 | |
| Plasma total protein | None | 0.5532 | <0.0001a | 0.0440 a | <0.0001a | 0.2960 | |
| Plasma urea nitrogen | None | 0.3839 | 0.2535 | 0.4581 | 0.0008a | 0.5570 | |
| Plasma calcium | None | 0.4185 | 0.0065a | 0.0049 a | <0.0001a | 0.6485 | A < D |
| Plasma chloride | None | 0.0284 a | 0.0010a | 0.4950 | 0.0058a | 0.7245 | B < A |
| Plasma potassium | None | 0.3531 | 0.0747 | 0.5426 | 0.1880 | 0.3042 | |
| Plasma sodium | None | 0.1938 | 0.8725 | 0.8325 | 0.0655 | 0.4909 | |
| Angiotensin I (t1, 2 and 4 h only) | Log | <0.0001 a | <0.0001a | 0.9160 | <0.0001a | 0.0190a |
A < B & D C < A & D |
| Angiotensin I (predose t0 h only) | Log | 0.2287 | n. a. | n. a. | 0.0016a | 0.9287 | |
| Plasma renin activity (t1, 2 and 4 h only) | Cube root | <0.0001 a | <0.0001a | 0.5370 | <0.0001a | 0.0949 |
A < B & D C < A & D |
| Plasma renin activity (predose t0 h only) | Cube root | 0.0794 a | n. a. | n. a. | 0.0185a | 0.7871 | C < D |
- a In the event of P < 1.0 for treatment or ‘treatment by day/time’ interaction (value for the interpretation shown in boldface), post hoc comparisons were interpreted. In the event of a ‘treatment by day/time’ interaction, post hoc comparisons at day 6 after onset of dosing (days 6/13) are shown. n.a, not applicable.
Furosemide was effective in promoting diuresis as indicated by highly significant (P < 0.0001) increases in mean estimated water consumption by 55% (694 ± 206 to 1076 ± 263 mL), urine volume by 205% (235 ± 94 to 718 ± 253 mL), PRA at Emax by 185% on day 0 (682 ± 1159 to 1943 ± 2515 pg/mL/h) and by 302% on day 5 of dosing (2802 ± 885 pg/mL/h at Emax on day 5) and Uald:creat by 223% (0.135 ± 0.071 to 0.436 ± 0.211, unit 10−6), and decreases in USG by 4.3% (1.062 ± 0.034 to 1.016 ± 0.004). The differences between the results for estimated water intake and urine production suggest that water consumption data are less reliable (dogs could play with the water bowls).
Clinical monitoring
No relevant clinical findings were observed during the biological phase of the study. There were no significant changes from baseline or differences between the groups for body weight or feed consumption.
Haematocrit increased significantly from baseline in the control group (by 4.3% from 0.46 ± 0.021 L/L at day −1/6 to 0.48 ± 0.034 L/L at day 6/13), but not significantly in groups B, C and D. There was no significant treatment effect in the ancova.
White cell counts increased significantly from baseline in group D. There was a significant overall treatment effect, and in the post hoc comparisons, the values were significantly higher in groups A and D vs. group B.
For coagulation variables, the activated partial thromboplastin time decreased significantly from baseline in the control group and the prothrombin time decreased significantly from baseline in group C. There were no significant treatment effects in the ancova.
For the clinical chemistry variables, there were significant decreases from baseline to days 6/13 in the control group for plasma chloride and significant increases in albumin, calcium, creatinine, total protein and urea. There were also significant decreases from baseline in chloride (groups B, C and D) and significant increases from baseline in albumin (groups C and D), calcium (B, C and D), creatinine (C and D), total protein (B, C and D) and urea (B, C and D). Only for chloride was there a significant treatment effect, and in the post hoc comparisons, the values were significantly lower in group B than in group A.
Glomerular filtration rate
The GFR was assessed from the CLioh. There were no differences in conclusions when CLioh was expressed per unit BWT or BSA. In all four groups, CLioh decreased significantly at day 6/13 vs. baseline due to the action of furosemide (Fig. 3). In the control group, mean CLioh per unit BSA decreased by 18.6%. There was no significant effect of benazepril, robenacoxib or the combination of benazepril and robenacoxib on GFR, evidenced by P values >0.6 for the overall treatment effect in the ancova (Table 8) and the lack of significant differences between groups in the post hoc comparisons. However, there was variability between dogs in the GFR results (Fig. 3).
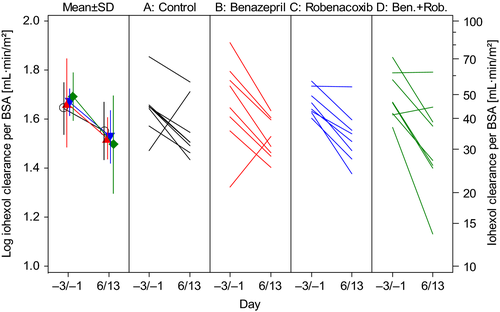
Urine variables
In the control group, there were highly significant (P < 0.0001) increases from baseline for water consumption, urine volume (Fig. 4) and Uald:creat (Fig. 5), and a decrease in USG. There were significantly similar changes from baseline for all four variables also in groups B, C and D.

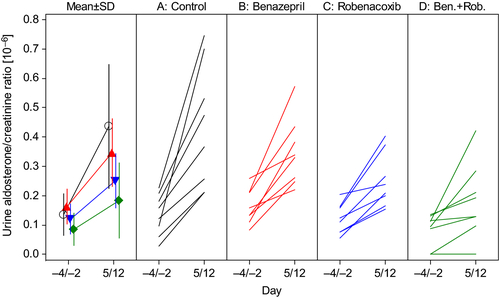
The only urine variable with a significant treatment effect was Uald:creat (P = 0.036), and in the post hoc analyses, the values in groups B, C and D were significantly lower than in group A.
ANG-1 and PRA
Administration of furosemide produced an increase in PRA in all groups, as evidenced by the highly significantly (P < 0.0001) higher values at time 0 on days 5/12 compared to 0/7 in the control group (Fig. 6, Table 7). Baseline values were also higher at time 0 on days 5/12 compared to baseline in groups B, C and D.

There was a highly significant (P < 0.0001) treatment effect for PRA (Table 9), and PRA values were significantly higher in groups B and D compared to the control group. PRA values were also significantly higher in groups A and D compared to group C.
The results for ANG-1 were qualitatively similar to those for PRA (Tables 7 and 9).
Discussion
The combined use of ACEIs, diuretics and NSAIDs in humans has a potential ‘triple whammy’ adverse effect on kidney function leading to an increased risk of AKI (Thomas, 2000). In this study, benazepril, robenacoxib and their combination were tolerated well and did not decrease GFR, either with or without the concomitant administration of furosemide. Similar findings were found recently in healthy cats (J.N. King, A. Panteri, M. Graille, W. Seewald, G.M. Friton and C. Desevaux, unpublished data). No negative effects of robenacoxib on GFR were reported previously with a high dose of robenacoxib (30 mg/kg) in rats (King et al., 2009).
In study 2, furosemide was administered at a dose of 2 mg/kg BID for 7 days, and this was effective in inducing diuresis, evidenced by a mean increase of 205% in urine volume and a 4.3% decrease in USG. In addition, furosemide caused a reduction in GFR (by 18.2%) and the activation of the renin–angiotensin–aldosterone system (RAAS) as observed by increases in PRA (by 185–302%) and urine aldosterone concentrations (Uald:creat, by 223%). Reduction in GFR is an established action of diuretics and is mediated via a reduction in perfusion pressure (Surdyk et al., 2011).
Benazepril had the anticipated effects of an ACEI, effectively inhibiting the RAAS as evidenced from significantly increased PRA and decreased urine aldosterone in study 2. These effects are consistent with the beneficial actions of ACEIs in heart and kidney disease (Lefebvre & Toutain, 2004). The increase in PRA is presumed to be a consequence of benazepril's action in inhibiting ANG-2 production, thereby leading to the reduction in the negative feedback of ANG-2 on renin release or synthesis. A marked increase in PRA and a decrease in plasma aldosterone with a modest reduction in urine aldosterone with benazepril in dogs were also observed by Mochel et al. (2013), although Lantis et al. (2015a,b) reported no significant inhibition of urine aldosterone by either benazepril or enalapril in dogs administered furosemide.
As noted in the introduction, ACEIs as a group have the potential to reduce GFR in patients with heart or kidney disease (Lefebvre & Toutain, 2004). However, no evidence for increased plasma creatinine concentrations with benazepril was observed in placebo-controlled field studies of dogs with CHF (The BENCH Study Group 1999) or CKD (J.N. King, A. Font, J.F. Rousselot, R.A. Ash, U. Bonfanti, C. Brovida, I.D. Crowe, D. Lanore, D. Pechereau, W. Seewald & G. Strehlau, unpublished data). In this study, no significant effects of benazepril on GFR, with or without robenacoxib, were noted, although GFR increased by 17.7% from baseline in the benazepril group in study 1 compared to a 3.2% decrease in the control group. In cats, benazepril has been shown to significantly increase GFR (Brown et al., 2001; J.N. King, A. Panteri, M. Graille, W. Seewald, G.M. Friton and C. Desevaux, unpublished data). The inhibition of RAAS combined with no reduction in GFR with benazepril in this study is consistent with the demonstrated efficacy and tolerability of this drug in dogs with CHF and CKD (The BENCH Study Group 1999; Pouchelon et al., 2004; J.N. King, A. Font, J.F. Rousselot, R.A. Ash, U. Bonfanti, C. Brovida, I.D. Crowe, D. Lanore, D. Pechereau, W. Seewald & G. Strehlau, unpublished data).
Robenacoxib inhibited the increases in PRA and urine aldosterone induced by furosemide. Similar effects were reported previously with other NSAIDs in rats and humans (Kammerl et al., 2001; Besen et al., 2009) and recently with robenacoxib in cats (J.N. King, A. Panteri, M. Graille, W. Seewald, G.M. Friton and C. Desevaux, unpublished data). Many of the renal effects of furosemide are mediated by prostaglandins, and it has been shown in rats and humans that some NSAIDs inhibit the diuretic, natriuretic and chloruretic effects of furosemide at the level of reabsorption of sodium and chloride in the loop of Henle (Paterson et al., 2011). In this study, there was only weak evidence that robenacoxib inhibited the diuretic action of furosemide; water consumption and urine volume were numerically lower in the robenacoxib groups (C and D) compared to the control, but differences did not approach significance. In addition, there were no significant differences between robenacoxib- and placebo-treated dogs for plasma protein or albumin, red blood cell variables or USG.
Although robenacoxib did not significantly inhibit the diuretic and GFR-reducing actions of furosemide, it did markedly and significantly attenuate the increases in urine aldosterone concentrations induced by furosemide. Because aldosterone is linked to the progression of CHF or CKD, these findings provide a rationale for the therapeutic use of benazepril and robenacoxib in combination, notably in proteinuric CKD (Bianchi et al., 2006; Bernay et al., 2010).
The results of this study cannot be simply extrapolated to other ACEIs and NSAIDs because the pharmacology of individual agents differs. Of specific relevance to this study is the fact that the active metabolite of benazepril, benazeprilat, is excreted approximately equally via biliary and renal routes in dogs (Waldmeier & Schmid, 1989), and therefore its clearance is maintained even when renal function declines (Lefebvre et al., 1999). Similar conclusions might therefore not be obtained for other ACEIs that are mainly or exclusively excreted by the kidneys. In addition, robenacoxib has a short residence time in the central compartment (Jung et al., 2009), and therefore, its pharmacodynamic actions in the healthy kidney should be of short duration. Similar conclusions might therefore not be obtained with NSAIDs with longer half-lives. In addition, robenacoxib is a highly selective inhibitor of cyclo-oxygenase (COX)-2 (King et al., 2010), although the evidence is mixed if the risk of AKI is lower with selective COX-2 inhibitors compared to nonselective NSAIDs (Lafrance & Miller, 2009).
Mixed results have also been reported on the effects of other NSAIDs on GFR in dogs. The preferential COX-2 inhibitor carprofen and the nonselective inhibitor indomethacin both reduced GFR to a similar extent when administered in combination with furosemide (4 mg/kg BID) for 8 days to beagle dogs (Surdyk et al., 2011). Administration of carprofen and ketoprofen at the time of induction of anaesthesia was associated with significant reductions in creatinine clearance in dogs undergoing castration surgery (Forsyth et al., 2000). In dogs undergoing ovariohysterectomy, all of the three NSAIDs tested had negative effects on renal variables and the effect was greater with ketorolac and ketoprofen compared to carprofen (Lobetti & Joubert, 2000). However, no significant adverse effects on renal function, including GFR, were reported for carprofen or meloxicam in dogs undergoing general anaesthesia (Boström et al., 2002, 2006; Frendin et al., 2006).
No negative effects on GFR were also observed in healthy beagle dogs administered the ACEIs benazepril or enalapril in combination with the NSAID tepoxalin (Fusellier et al., 2005). Those results may not be predictive for our study, however, as the mechanism of action of tepoxalin (reported to be mainly the inhibition of COX-1 and lipoxygenase) differs from the COX-2 selective action of robenacoxib.
The principal limitation of the studies is the use of healthy and relatively young beagle dogs. The results cannot therefore be simply generalized to dogs of different ages and breeds or with clinical diseases such as CHF, CKD and OA. For example, the residence time of robenacoxib in tissues is markedly prolonged by the presence of inflammation, and therefore its duration of action in the kidney might be longer if renal inflammation is present as compared to the healthy dogs used in this study (King et al., 2009). Second, the main endpoints in this study were orientated to safety, including GFR to assess the risk of AKI with the combination of the ACEI and NSAID. We did not study the possible interaction of robenacoxib inhibiting the antihypertensive efficacy of benazepril. Third, the dogs were fed a standard diet and were dosed with the test items in the morning at approximately 8.00. Chronological differences in RAAS have been reported in dogs, notably related to the timing of feeding (Mochel et al., 2014), and therefore, different results might be obtained with other diets or different timings of feeding or dosing. Last, multiple statistical analyses were made with no adaptation of the P values. Therefore, isolated events of statistical significance of secondary endpoints need to be interpreted cautiously.
In conclusion, the ACEI benazepril and the NSAID robenacoxib, administered alone or in combination, were tolerated well and produced no reduction in GFR in young healthy beagle dogs, with or without co-administration of furosemide. Benazepril and robenacoxib, and especially their combination, reduced urinary aldosterone concentrations induced by furosemide, and therefore, combination therapy might be beneficial in clinical cases, for example proteinuric CKD. However, it is not known whether the same actions would be present in dogs with the activation of RAAS induced by CHF or CKD, but not receiving furosemide. There was between-animal variability in GFR results, and different results might be obtained with different breeds and/or dogs with CHF or CKD. Further studies are therefore required to test the tolerability and/or efficacy of the combination of benazepril and robenacoxib in dogs with clinical disease.
Acknowledgments
We thank François Aleman for the clinical pathology, Sabine Cardinali for the plasma renin activity, Christophe Chardonnens for the aldosterone and Marlène Lacroix for the iohexol analyses. We also thank Arnaud Lainé for study coordination.
Competing Interests
At the time of the study, all authors were employed by Novartis Animal Health, now owned by Elanco Animal Health (a division of Eli Lilly and company), which distributes and markets benazepril (Fortekor®) and robenacoxib (Onsior®).




