Pharmacokinetic and pharmacodynamic evaluations of a 10 mg/kg enrofloxacin intramuscular administration in bearded dragons (Pogona vitticeps): a preliminary assessment
Abstract
Enrofloxacin (E) is commonly used in veterinary medicine. It is necessary to perform pharmacokinetic/dynamic studies to minimize the selection of resistant mutants of bacteria and extend the efficacy of antimicrobial agents. Eight healthy adult Pogona vitticeps were assigned into two groups of equal size and treated with a single intramuscular injection of E at 10 mg/kg. Blood samples were withdrawn at different scheduled times for each group, and rectal swabs were collected. E and ciprofloxacin (active metabolite) blood concentrations were quantified by an HPLC validated method, while the in vitro antimicrobial susceptibility was evaluated by the Kirby–Bauer disc diffusion susceptibility test. The pharmacokinetic profiles of E gave similar pharmacokinetic parameters irrespective of the collection time schedule. Bacteria isolation showed the presence of both E. coli, Salmonella enterica subspecies enterica and subspecies 3a, Proteus spp., and Pseudomonas spp. The majority of isolated colonies were sensitive to E, but the treatment did not reduce the number of bacteria in faeces. Results suggest that E is able to reach blood concentrations high enough to kill susceptible bacteria (MIC < 0.9 μg/mL), but at the same time does not significantly affect intestinal bacteria.
Introduction
The 6-fluorinated piperazinyl derivatives, fluoroquinolones (FQs), have a wide range of antibacterial activities and are being increasingly used in veterinary medicine because of both their effectiveness in treating bacterial infections and advantageous pharmacokinetic properties (McKellar et al., 2004). Veterinary drugs have become an integral part of livestock production and play an important role in maintaining animal welfare, mainly through disease prevention, treatment of infections, controlling the risk of zoonotic disease transmission and increasing the productive capacity of animals. Since the late 1980s, the FQs have become one of the most widely used groups of synthetic antimicrobials in veterinary medicine (Walker, 2000). One of the active ingredients belonging to this class, enrofloxacin (E), has become a commonly used (nonlicensed) antimicrobial in reptile medicine because of its excellent activity against both gram-positive and gram-negative aerobic bacteria and its favourable safety profile (Jacobson, 1999). The efficacy of E is enhanced in several species by the formation of an active metabolite, ciprofloxacin, which exhibits a similar potency and spectrum of activity (Walker, 2000). The pharmacokinetics of E have been investigated in several different species of reptiles (Lewbart et al., 1997; Young et al., 1997; Helmick et al., 2004; Giorgi et al., 2013; Salvadori et al., 2015). In these species, therapeutic blood concentrations of the drug were achieved, but disposition varied markedly among species, indicating that extrapolation between reptiles is likely to result in inaccurate dosing of E.
Bearded dragons, like other small exotic animal species may be difficult to sample for serially timed blood collections in a standard (ST) pharmacokinetic study. In a ST study, a small number of animals are used, but they are sampled intensively to collect multiple blood samples per animal. The pharmacokinetic parameters are determined for each individual, and then, the mean values are taken for the group in a two-step process. Variation in pharmacokinetic parameters can only be derived from the small group of animals used in the study, and it is difficult to understand and predict the pharmacokinetic behaviour of a drug in a larger population from this design.
When sample collection at each time point is not possible for a single animal because of size limitations or restraint difficulty, samples can be collected from a group of animals large enough to sufficiently cover the full range of times and subsequently estimate concentration–time relationships. The concentrations are then averaged for each time point to fit a pharmacokinetic model to the mean data. This approach is called the naive-averaged data approach (NAD). This approach is still in use but its main limitation is that it does not provide an estimate of the variability in the population.
The aim of the study was 3-fold: (i) to evaluate and to compare the pharmacokinetic parameters of E and its metabolite ciprofloxacin (C) with ST and NAD approach studies; (ii) to determine integrated PK–PD parameters (AUC/MIC) (Cmax/MIC) for bacteria of medium susceptibility to E; (iii) to determine the in vivo activity of the drug on the rectal bacterial population.
Materials and Methods
Animal treatment and sampling
Eight clinically normal bearded dragons (Pogona vitticeps) of both genders (two males, six females), with body weights ranging from 0.29 to 0.37 kg (mean 0.326 kg), supplied by a private owner, were used for the study. Animals were pooled and housed in glass containers and were numbered on the back for identification. The animals were acclimated to the new environment for a 2-week period prior to commencement of the study. The study was approved by the ethical committee of the University of Pisa (protocol number 15100). Animals were judged to be in good health based on physical examination at the time of acquisition and at the start of the study and through daily observation of behaviour and appetite. These observations were made by specialized veterinary personnel (M.S.).
A basking area was heated to 30 °C using an infrared lamp. Ambient temperature in the room varied from 24 to 26 °C (electronic temperature sensors assured constant temperature in both the room and basking area).
A commercial injectable formulation of E (Enrovet® 25 mg/mL, Bio98, Milan Italy) was diluted with saline to 10 mg/mL and then injected intramuscularly (IM) into the right femoral muscle at the dose of 10 mg/kg. Although the standard clinical approach of drug administration to lizard is in the muscles of the front leg, in this study, the injection in the femoral muscle was preferred for two reasons: (i) it has been speculated that the renal portal system is unlikely to be clinically significant (Holz et al., 1997); (ii) drug administration in the caudal area increases the operator safety as a result of greater distance from the subject's mouth, a greater muscle mass warrants the IM injection and if needed, the flexibility of being able to rotate repeated injections through sites on four limbs.
The study was conducted as a randomized block parallel design study (with sex as the blocking factor). Animals were equally divided into two groups. Blood samples (0.4 mL) were collected from the caudal vein at 0, 0.5, 2, 6, 24, 72 and 120 h in group 1 (n = 4) (early sampling) and 0, 1, 4, 10, 48, 96 and 144 h in group 2 (n = 4) (delayed sampling) after the drug administration.
Blood was immediately transferred into tubes containing citric acid (Microtainer; Becton Dickinson and Company, Franklin Lakes, NJ, USA) and then stored at −20 °C until use, within 30 days of collection. Sterile swabs were gently inserted in the first rectal tract of each bearded dragon at 0, 0.5, 1, 2, 4, 6, 10, 24, 48, 96 and 144 h after E administration following the same schedule reported for the blood collection (group 1 early sampling and group 2 delayed sampling). Swabs were immediately stored at −80 °C until use.
Chemicals and reagents
Pure E, C and sarafloxacin (internal standard [IS]) (standards purity >99.0%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile, trichloromethane and isopropanol were purchased from Merck (Darmstadt, Germany). Tetraethylamine was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Orthophosphoric acid, disodium hydrogenophosphate, sodium dihydrogen phosphate and potassium dihydrogenophosphate were purchased from Carlo Erba (Milan, Italy). Deionized water was produced by a Milli-Q Millipore Water System (Millipore, MA USA). All the other reagents and materials were of analytical grade and supplied from commercial sources. The aqueous and organic components of the mobile phase, degassed under pressure, were mixed by the HPLC. The LC mobile phases were filtered through 0.2-μm cellulose acetate membrane filters (Sartorius Stedim Biotech, Aubagne Cedex, France) with a solvent filtration apparatus.
Preparation of solutions
Singular stock solutions of E, C and IS in water were prepared using volumetric flasks at an individual concentration of 1000 μg/mL and were stored at −20 °C. To reach a final concentration of 100 μg/mL, appropriate dilutions of stock standard solutions were prepared by diluting 1 mL of each solution to 10 mL. The solutions of E and C were then diluted in glass tubes (10 mL) to reach final concentrations of 10, 5 and 1 μg/mL and were stored at 4 °C. This latter concentration (1 μg/mL) was then diluted to prepare a 5-point calibration curve of the analytes at the following concentrations: 0.200, 0.100, 0.050, 0.025 and 0.010 μg/mL. The two analytes were stable for at least 10 weeks if stored at 4 °C.
Instrumentation and chromatographic conditions
The HPLC system was an LC system (Jasco Inc., Easton, MD, USA) consisting of a high-pressure mixer pump (model PU 980 Plus), spectrofluorometric detector (model 2020 Plus) and a loop of 50 μL. Data were processed using Borwin software. Chromatographic separation assay, modified from Giorgi et al. (2013), was performed by a Gemini C18 analytical column (250 × 4.6-mm inner diameter, 5-μm particle size, Phenomenex, Torrance, CA, USA) maintained at 25 °C. The mobile phase consisted of acetonitrile:aqueous solution (20:80 v/v%) at a flow rate of 1 mL/min.
The aqueous solution consisted of potassium dihydrogen phosphate (0.02 m) and 860 μL of tetraethylamine (0.012 m) in water adjusted to pH 4 with orthophosphoric acid (0.006 m). Excitation and emission wavelengths were set at 338 and 425 nm, respectively.
Sample extraction
Sample extraction was based on the technique previously described by Garcia et al., (1999) with slight modifications. For sample extraction, aliquots (0.2 mL) of blood were added to 0.1 mL of IS (1000 ng/mL) diluted with 800 mL of 0.1 m phosphate buffer at pH 7.1.
After adding 6 mL of a mixture of trichloromethane:isopropanol (5:1 v/v), the samples were shaken at 200 oscillations/min for 10 min and centrifuged at 4000 g for 5 min.
Five millilitre of the organic layer was transferred into a clean tube and dried at 40 °C under nitrogen stream. The residue was dissolved in 0.2 mL mobile phase, vortexed, and an aliquot was injected into the chromatographic system.
Quantification
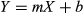
where Y = peak area, X = concentration of the standard in ng/mL, m = the slope of the curve and b = the intercept with Y axis. When unknown samples were assayed, a control and a fortified blank sample were processed along with each set for quality control. Linearity of the regression curve in the range 10–200 ng/mL was assessed on the basis of the residual plot, the fit test and the back calculation (within 20% of known amount). Limit of detection (LOD) and limit of quantitation (LOQ) were determined as analyte concentrations giving signal-to-noise ratios of 3 and 10, respectively. LOD was 3 ng/mL, while LOQ was 10 ng/mL.
Bacteria isolation and enrofloxacin efficacy
Samples were examined for the presence of Salmonella spp. with a four-step procedure: preenrichment, enrichment, selective and differential culture media and identification.
The swabs with cloacal or faecal material were subjected to preenrichment; each sample was soaked in 9 mL of buffered peptone water (BPW) medium for 30 sec then stored at −20 °C. BPW was incubated at 37 ± 1 °C for 18 ± 2 h.
The enrichment step involved 100 ± 10 mL of BPW dispersed in 10 ± 0.5 mL of Müller–Kauffmann tetrathionate broth with novobiocin (MKTTn) and incubated at 37 ± 2 °C for 24 ± 3 h. One mL of material from the opaque zone of the bacterial colonies was streaked on Xylose–Lysine–Deoxycholate agar with novobiocin (XLD + N) and Brilliant Green Agar (BGA) using a sterile disposable loop and incubated at 37 ± 2 °C for 24 ± 2 h. Colonies suspected as being Salmonella spp. on the XLD + N and/or BGA plates were inoculated into Triple Sugar Iron (TSI) and incubated at 37 °C for 24 h. Identification was performed using a commercial micro method (API 20 E; BioMerieux, Rome, Italy).
In vitro antimicrobial susceptibility test
Each strain was analysed to evaluate the antimicrobial susceptibility in agreement with the Kirby–Bauer disc susceptibility method (Bauer et al., 1966) described in Clinical and Laboratory Standard Institute (CLSI) guidelines.
Using a sterile inoculating loop or needle, four or five isolated colonies of organisms were touched to be tested; then, the colonies were suspended in 2 mL of sterile saline solution, and the tube was vortexed to create a smooth suspension (adjusting the turbidity to 0.5 McFarland standard). A sterile swab was dipped into the inoculum solution and rotated against the side of the tube using firm pressure, to remove the excess fluid. Mueller-Hinton Agar was inoculated by streaking the swab three times over the entire agar dried surface. Appropriate antimicrobial-impregnated discs were placed on the surface of the agar, using forceps to dispense each antimicrobial disc one at a time. Then, all plates were incubated at 37 °C for 18–24 h. The Kirby–Bauer test protocol provides for the measurement of the inhibition zone of microbial growth around the disc. Using a caliper, each zone was measured with the unaided eye while viewing the back of the Petri dish. In particular, the pharmacological activity of E against different bacteria was tested. For each isolate, the zone of inhibition around each disc was measured, after incubation at 37 °C. According to CLSI guidelines, depending on the inhibition zone size, the strain was classified as susceptible (S; >18 mm), intermediate (I; 14 < X < 18 mm) or resistant (R; <14 mm), compared to the reference values.
Statistical analysis and pharmacokinetic evaluation
Noncompartmental pharmacokinetic evaluation time vs. blood concentration for each individual (ST) and for the averaged data set (NAD) was performed using WinNonlin v 5.3.1 software (Pharsight Corp., Sunnyvale, CA, USA). Maximum concentration (Cmax) of E in blood and the time required to reach Cmax (Tmax) were predicted from the data. The following variables were calculated: area under blood concentration–time curve to infinity (AUC0–∞), area under the first moment curve, mean residence time (MRT) and terminal half-life (HLλz). AUC was calculated by the linear trapezoidal rule (Gibaldi & Perrier, 1982). Terminal half-life was calculated by linear regression of the log concentration vs. time plots with at least 5 and 4 points in the group 1 and 2 data sets, respectively. The apparent volume of distribution (Vd) and clearance (Cl) were parameterized as Vd_F and Cl_F because of the extravascular administration of the drug.
The results were presented as mean ± standard deviation (SD). All the analyses were conducted using Graph Pad In Stat (Graph Pad Software, Inc., La Jolla, CA, USA). Differences in pharmacokinetic parameters between the groups were evaluated by student's t-test and considered significant if the associated probability level (P) was lower than 0.05.
The results obtained from the sensitivity tests were organized and analysed using Prism 5.0 (Graph Pad). Data are expressed as mean values ± SD. Data were analysed using Shapiro–Wilk normality test, and one-way anova with Tukey's multiple comparison test, assuming a significant value at P < 0.05.
Results
Animals
All animals survived the experiment and none of them demonstrated side effects due to E administration or the sample collection.
Pharmacokinetics
In group 1 (early), E was detectable in all blood samples from 0.5 up to 72 h, in one bearded dragon it was detectable at 120 h (Fig. 1a). In group 2 (delayed), E was detectable in all animals from 1 to 96 h (Fig. 1b). In both the groups, C blood concentrations were quite variable and much lower than those of the parental drug, but detectable in the same ranges of time. Excluding the parameters directly predicted from the data (Cmax and Tmax), the averaged calculated pharmacokinetic parameters for E in groups 1 and 2 (ST) and for the grouped samples (NAD) did not show significant differences (Table 1, Fig. 1c). E was rapidly absorbed and showed a half-life of about 20 h. The C concentrations fluctuated, preventing sound pharmacokinetic evaluation (Table 2). However, the average E/C AUCs ratio was 19.6 and 18.7 in group 1 and 2, respectively.
| Parameters | Units | Delayed | Early | Grouped | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | ||
| Rsq | 0.89715 | 0.148072 | 0.856225 | 0.057833 | 0.916 | |
| λz | 1/h | 0.0348 | 0.00455 | 0.03265 | 0.003586 | 0.0337 |
| HLλz | h | 20.17 | 2.64 | 21.43 | 2.013 | 20.54 |
| Tmax | h | 2 | 0 | 1 | 0 | 1 |
| Cmax | ng/mL | 9444 | 3473 | 11 916 | 5236 | 11 916 |
| AUC0–∞ | h·ng/mL | 146 042 | 35 150 | 163 670 | 50 469 | 152 297 |
| Vz_F | mL/kg | 1944 | 556 | 1912 | 555 | 1904 |
| Cl_F | mL/h/kg | 66.60 | 16.40 | 63.77 | 26.46 | 64.26 |
| AUMC0–∞ | h2·ng/mL | 4 016 724 | 1 197 078 | 4 898 131 | 1 839 479 | 4 752 120 |
| MRT | h | 25.71 | 5.07 | 27.65 | 2.34 | 30.54 |
- Rsq, correlation coefficient; λz, terminal phase rate constant; HLλz, terminal half-life; Tmax, time of peak; Cmax, peak plasma concentration; AUC0–∞, area under the plasma concentration–time curve extrapolated to infinity; Vz_F, appaent volume of distribution; Cl_F, apparent clearance; AUMC0–∞, area under the first moment curve from zero to infinity; MRT, mean resident time; SD, standard deviation.
| Delayed | Early | ||||
|---|---|---|---|---|---|
| Time (h) | ng/mL | SD | Time (h) | ng/mL | SD |
| 1 | 209.59 | 160.99 | 0.5 | 105.97 | 29.98 |
| 4 | 277.23 | 106.00 | 2 | 185.54 | 92.51 |
| 10 | 201.39 | 99.41 | 6 | 118.29 | 78.34 |
| 48 | 205.75 | 237.47 | 24 | 89.98 | 36.56 |
| 96 | 121.41 | 130.78 | 72 | 63.94 | 45.38 |
| 144 | / | / | 120 | 24.25 | / |
Sensitivity test
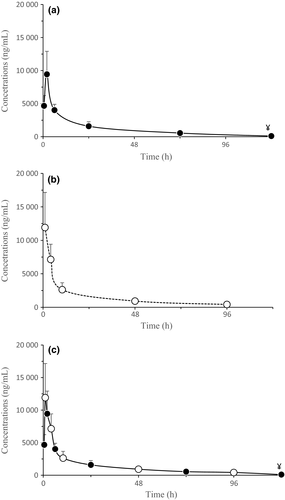
Sensitivity test facilitated the isolation and recognition of Salmonella enterica subspecies enterica, S. enterica subspecies 3a, E. coli, Pseudomonas spp. and Proteus spp. Moreover, it was possible to identify the susceptibility profile for each bacterium: resistant, intermediate or sensitive. Overall, it was possible to identify 38 colonies of S. enterica (including both subspecies), 19 of E. coli, 12 of Pseudomonas spp. and 2 of Proteus spp. (Fig. 2), and most of them showed sensitivity to E (diffusion > 18 mm).
Among the sensitive colonies, S. enterica subspecies enterica had a zone of inhibition of 28.80 ± 0.8367 mm (mean ± SD), S. enterica subspecies 3a had 28.50 ± 3.609 mm, E. coli had 27.00 ± 3.256 mm, Proteus spp. had 0 mm, and Pseudomonas spp. had 22.89 ± 3.551 mm.
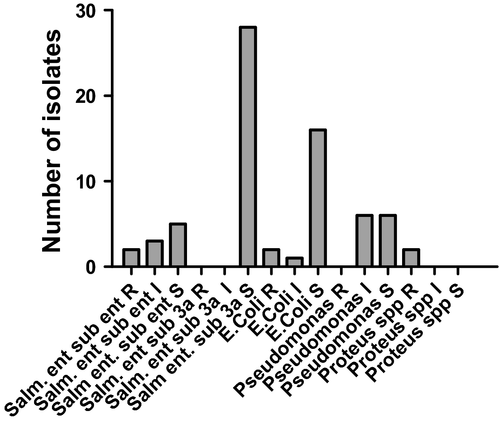
Data were organized dividing the results into the two groups: group 1 (early sampling) and group 2 (delayed sampling).
Early sampling group (Fig. 3a): at 0 h, it was possible to isolate E. coli and S. enterica sub. 3a strains sensitive to enrofloxacin. Thirty minutes after the drug administration, it was possible to isolate colonies of E. coli, S. enterica sub. 3a and sub. enterica, all sensitive to E. At the following experimental time points, S. enterica sub. 3a sensitive to E were isolated until the last collection point without showing statistically significant differences between any of the experimental time points. E. coli was isolated maintaining its sensitivity to E up to 24 h. No further E. coli were isolated between this time point and completion of the sample collection. Salmonella enterica sub. enterica demonstrated intermediate resistance at 24 and 72 h. At 120 h, it was possible to isolate a colony of S. enterica sub. enterica that demonstrated complete resistance to E.
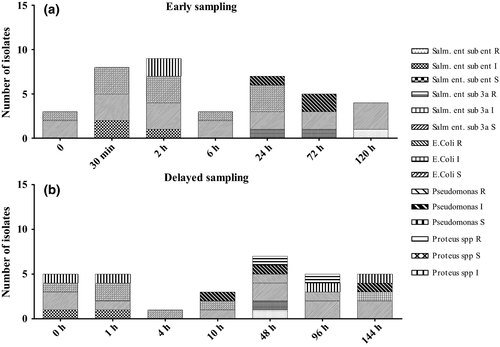
Delayed sampling group (Fig. 3b): at 0 h, prior to the drug administration, it was possible to isolate Pseudomonas spp., E. coli, S. enterica sub. 3a and sub. enterica, all sensitive to E. The same pattern was found 1 h after the drug administration. Four hours after the administration, it was possible to isolate a colony of E. coli sensible to E. Starting from 10 h after E injection, the isolated colonies of Pseudomonas spp. demonstrated an intermediate resistance, this remained the case at 48 h. At this time point, Proteus spp., E. coli and S. enterica sub. enterica demonstrated resistance to E. Salmonella enterica sub. enterica showed also intermediate resistance. Colonies of E. coli and of Proteus spp. resistant to E were isolated at 96 h, while S. enterica sub. 3a. and Pseudomonas spp. demonstrated consistent sensitivity to the drug. At 144 h, S. enterica sub. 3a maintained its sensitivity, while E. coli demonstrated intermediate resistance. Pseudomonas spp. variably showed intermediate resistance and sensitivity.
Results obtained by matching both groups were analysed with one-way anova and Tukey's multiple comparison test: no statistically significant differences were demonstrated comparing the different time points.
Discussion
No adverse effects such as development of local irritation and soft tissue necrosis were detected at the injection sites. This is in line with a recent study in turtles using a 10 mg/mL diluted E (Giorgi et al., 2013), but in disagreement with studies using more concentrated drug solutions (James et al., 2003; Jacobson et al., 2005). Therefore, it is possible that the concentration of the injectable solution plays a pivotal role in preventing tissue irritation after intramuscular administration of E.
In the present study, two kinds of sampling time schedules (ST) were used: neither demonstrated significantly different pharmacokinetic parameters. In addition, the NAD approach did not produce statistically significant differences in pharmacokinetic parameters compared to the ST study. It is suggested that the 7 blood sampling times between 0 and 120 or 144 h are enough to give a satisfactory description of the pharmacokinetic trend of E in bearded dragons. An alternative design, when frequent sample collection is prohibitive, is a population pharmacokinetic modelling approach. Population pharmacokinetic modelling attempts to obtain an estimate for a population of animals and to seek the sources of variation in the dose–concentration relationships. The population approach was also attempted: unfortunately, due to the small animal sample size, the results were not reliable (data not shown).
The pharmacokinetics of E has previously been studied in several animal species (López-Cadenas et al., 2013). Wide pharmacokinetic differences have been reported, especially between mammals and reptiles. However, marked differences have also been reported within the reptile order (Papich, 1999). The present study has shown an average HLλz of E that is shorter than that reported in Trachemis turtles (47 h) (Giorgi et al., 2013) and vipers (27 h) (Waxman et al., 2014), similar to that reported in alligator (21 h after iv administration) (Helmick et al., 2004), but higher than in pythons (6 h) (Young et al., 1997), highlighting once again the importance of the species-specific pharmacokinetic studies.
In most organisms, E is metabolized to C through a de-ethylation reaction: both molecules have similar potency and spectrum of activity (Brown, 1996). Given the extensive marketing and use of FQs in the veterinary field, several pharmacokinetic studies have been performed on E and C in different animal species. 35% of E is reported as being metabolized to C in mammals (Reo et al., 2001); hence, the antibiotic action can mostly be attributed to the parental drug rather than its metabolite. The same also applies for aquatic species where C appears to contribute only 15% of the antibiotic action (James et al., 2003; Guanghong et al., 2006). A smaller amount of C has been found in bearded dragons (about 5%). This is in line with a recent study on Trachemis turtles (Giorgi et al., 2013), showing that C contributed only 5% of the antibiotic action of the parental drug. This behaviour might been accounted for the slower metabolism of bearded dragons and turtles, as compared to other species (Ertl & Winston, 1998) and to the fact that CYP450 3A, the enzyme that metabolizes E to C, (Vaccaro et al., 2003) appears to be poorly expressed in reptiles (Ertl & Winston, 1998).
Historically, the minimum inhibitory concentration (MIC) of a drug has been used to recommend antimicrobial dosing regimens. However, basing dose rate on MIC alone could be misleading, because previous studies have determined that bactericidal concentrations for FQs are only reached at 8–10 times the MIC (Forrest et al., 1993). Recently, three pharmacodynamic parameters have been identified as the best way to accurately predict therapeutic outcome: (i) length of time that simulated drug concentrations remain above the MIC of the susceptible organism (t > MIC); (ii) the maximum drug concentration to MIC ratio (Cmax/MIC); and (iii) the area under the simulated blood time curve to MIC ratio (AUC/MIC) (Mac Gowan et al., 2000; Wright et al., 2000; Toutain & Lees, 2004). Because quinolone antibiotics are generally considered to have concentration-dependent bactericidal activity rather than time-dependent affect, Cmax/MIC and AUC/MIC ratios have been identified as possible pharmacodynamic predictors of clinical and microbiologic outcome as well as bacterial resistance (Wright et al., 2000). Data from in vitro models indicated that high Cmax/MIC values are important in preventing emergence of resistance, whereas AUC/MIC is the best predictor of antibacterial effect (Mac Gowan et al., 2000). It has been suggested that Cmax/MIC ratios > 10 or AUC/MIC ratios of 125 are required for quinolones to have clinical and microbiologic success and to limit the development of bacterial resistance, respectively (Wright et al., 2000; Liu et al., 2002). MIC values of bacteria isolated from reptiles may be different. They can be classified as susceptible (MIC < 1 μg/mL), of intermediate susceptibility (1 μg/mL > MIC > 2 μg/mL) and resistant (MIC > 4 μg/mL). The bacteria isolated from bearded dragons with the highest MIC is the Devriesea agamarum MIC = 2.0 μg/mL (Hellebuyck et al., 2009; Devloo et al., 2011). In the present study, considering a bacterium with an MIC value of 2.0 μg/mL, the Cmax/MIC ratio was 4.7. Considering this pharmacodynamic value, the administered dose of 10 mg/kg via the intramuscular route would not be efficacious for a bacterium with MIC of 2.0 μg/mL. In addition, the average AUC/MIC is lower than the requested safety value (125). These pharmacodynamic parameters are not exceeded even if the AUC and Cmax values of the metabolite C are added to those of E. From this study, it seems that a 10 mg/kg intramuscular administration of E might be effective against bacteria with MIC values of 0.8–0.9 μg/mL but not sufficient for treating infections of bacteria with MIC over 0.9 μg/mL.
Considering the sensitivity results from cloacal swabs, it was possible to recognize a plethora of different bacteria at all time points. Most of isolated colonies were S. enterica sub. 3a (39.4%) and E. coli (22.5%) sensitive to E. A minor component was represented by sensitive colonies of S. enterica sub. enterica and of Pseudomonas spp. (7.0% and 8.4%, respectively). The resistant colonies represented the smallest part of all isolations: S. enterica sub. enterica, E. coli and Proteus spp. resistant colonies accounted each for 2.8%. These results are encouraging for many reasons: the first is that E could be considered an efficient antimicrobial drug in bearded dragons that, after a single administration, might only rarely induce intermediate or full resistance. Second, considering that these animals are becoming widely popular as pets, increasing their interactions with people, it is very important that specific bacteria in bearded dragons do not develop antibiotic resistance and pass this on to their owners. In both humans and animals, positive associations between consumption of antimicrobials and corresponding resistance in bacteria were observed in most cases (WHO, 2011).
The limitation of the present study was the small animal sample size. Despite this, this study suggests that E is a safe drug that can be administered intramuscularly in bearded dragons without inducing side effects. A single intramuscular administration of 10 mg/kg E produced blood concentrations that should be effective against susceptible bacteria and did not reduce the amount of cloacal bacteria. Only a small percentage of isolated bacteria showed resistance to E, and this argues in favour of its use without a high risk of inducing inter- or intra- species antimicrobial resistance. Laboratory tools need to be developed, protocols standardized and guidelines established to provide accurate detection and reporting practices that will enable more effective treatment strategies for difficult infections. The present study gave more information oriented to a rational use of E in bearded dragons, not only to reach a therapeutic target but also to avoid antibiotic resistance for reptiles, other pet species and humans. Further studies with a larger animal sample size are warranted to confirm these data.
Acknowledgments
This work was equally supported by athenaeum funds (ex 60% University of Pisa, Fondi per la Farmacovigilanza Regione Piemonte). Any external funding did not support the preparation of manuscript. Authors acknowledged to Dr E. Owen (University of Queensland, Australia) the English editing of the manuscript.
Conflict of Interest Statement
None of the authors have any financial or personal relationships that could inappropriately influence or bias the content of the paper.




