In vitro metabolism of testosterone in the horse liver and involvement of equine CYPs 3A89, 3A94 and 3A95
Abstract
Testosterone (TES) 6-β-hydroxylation is a significant metabolic step in the biotransformation of TES in human liver microsomes and reflects cytochrome P450 (CYP) 3A4/5 specific metabolic activity. Several CYP3A enzymes have been annotated in the horse genome, but functional characterization is missing. This descriptive study investigates TES metabolism in the horse liver in vitro and the qualitative contribution of three CYP3A isoforms of the horse. Metabolism of TES was investigated by using equine hepatocyte primary cultures and liver microsomes. Chemical inhibitors were used to determine the CYPs involved in TES biotransformation in equine microsomes. Single CYPs 3A89, 3A94, and 3A95, recombinantly expressed in V79 hamster lung fibroblasts, were incubated with TES and the fluorescent metabolite 7-benzyloxy-4-trifluoromethylcoumarin (BFC). The effect of ketoconazole and troleandomycin was evaluated on single CYPs. Testosterone metabolites were analyzed by HPLC and confirmed by GC/MS. In hepatocyte primary cultures, the most abundant metabolite was androstenedione (AS), whereas in liver microsomes, 6-β-hydroxytestosterone showed the largest peak. Formation of 6-β-hydroxytestosterone and 11-β-hydroxytestosterone in liver microsomes was inhibited by ketoconazole, troleandomycin, and quercetin. Equine recombinant CYP3A95 catalyzed 11-β-hydroxylation of testosterone (TES). Metabolism of BFC was significantly inhibited by ketoconazole in CYP3A95, whereas troleandomycin affected the activities of CYP3A94 and CYP3A95. Both inhibitors had no significant effect on CYP3A89. Metabolic reactions and effects of inhibitors differed between the equine CYP3A isoforms investigated. This has to be considered in future in vitro studies.
Introduction
In humans, cytochrome P450 (CYP) 3A4 and CYP3A5 are responsible for the biotransformation of more than half of all commonly prescribed drugs and constitute an important CYP subfamily that plays a role in several drug–drug interactions. Four members of this subfamily are known, but only two are commonly expressed in the adult liver, namely CYP3A4 and CYP3A5 (Lamba et al., 2002). The substrate specificity of CYP3A4 and CYP3A5 are very similar corresponding to their similarity in protein sequences (Daly, 2006). In horses, the CYP3A subfamily contains significantly more members than in humans. Seven genes have been defined of which six seem to be regularly expressed in the equine (Schmitz et al., 2010). To date, three equine CYP3As, CYP3A89, CYP3A96, and CYP3A97, have been heterologously expressed in insect cells. CYP3A89 was not able to be expressed as a full-length protein in insect cells, and the suggestion was made that it might be a pseudogene (Knych et al., 2010). Expression levels of several members of the CYP3A subfamily were investigated using real-time PCR. In both studies CYP3A89 was among the most highly expressed CYPs, but variations between individual horses were high (Knych et al., 2010; Tyden et al., 2012).
Testosterone has been used for many years as a substrate to measure CYP activity (Lu et al., 1973a,b; Ford et al., 1975; Haugen et al., 1975; Shiverick, 1981; Ryan et al., 1984). It has many advantages including its good stability and the fact that a number of different CYPs metabolize TES to several metabolites making it a convenient ‘starting substance’ when nothing is known about substrate specificity of CYPs in a species. TES is metabolized in vitro to a variety of metabolites, predominantly products hydroxylated at different positions of the ring system (Ford et al., 1975; Miura et al., 1989).
In the late eighties Kawano et al. (1987) described that the 6-β-hydroxylation of TES was linked to one specific human CYP isoform called P450 human-1 at that time. Waxman et al. (1988) demonstrated that this CYP seems to be responsible for the 6-β-hydroxylation of several steroids pointing to a type of chemical reaction very specific to this isoform. Eventually, the 6-β-hydroxylation reaction was defined as a chemical reaction reflecting CYP3A4/5 activity and TES is today recommended as probe substrate for preclinical in vitro drug interaction testing (Yuan et al., 2002; FDA, 2006).
Testosterone 6-β-hydroxylation has been widely used for characterization of CYP3A-mediated reactions in humans. In addition, this metabolic reaction has also been applied to measure potential CYP3A-mediated activity in liver microsomes of other species (Chauret et al., 1997; Shou et al., 2003). However, knowing that substrate specificity can strongly differ between species, it is obvious that 6-β-hydroxylation does not necessarily reflect CYP3A-related activity in other species (Fink-Gremmels, 2008). Furthermore, it has been described that significant species differences exist in the pattern of TES metabolites (Yamazaki & Shimada, 1997; Langsch et al., 2009; Thorn et al., 2011). Therefore, to define new probe reactions in a veterinary species, we have to examine the metabolic pattern of TES in vitro in this species and to characterize the respective CYPs involved in the metabolism of TES. Yet, we have to consider that TES is not only metabolized by enzymes of the cytochrome P450 family but that other enzymes, namely hydroxysteroid-17beta-dehydrogenases might play a role (Blomquist et al., 1994; Blomquist, 1995).
A few publications described the 6-β-hydroxylation of TES in horse microsomes and found that 6-β-hydroxytestosterone (6-β-OH-TES) was formed by equine liver microsomes, but the reaction was only slightly inhibited by troleandomycin, a human CYP3A4-specific inhibitor (Komori et al., 1993; Chauret et al., 1997). Recently, 6-β-hydroxylation of TES was shown to be mediated by recombinantly expressed equine CYP3A96 but with a significantly higher Km and lower Vmax than in the human CYP3A4 (Knych et al., 2010). Yet, it still remains unclear whether this reaction is characteristic for the equine CYP3A96 or if it is mediated by other CYP3A enzymes of the horse as well. As human CYP3A4 and CYP3A5 show high similarities in substrate specificity and metabolic reactions, we hypothesize that the horse CYP3As expressed in the liver show an overlap in their substrate specificities and between the chemical reactions catalyzed.
The aim of this study was to define metabolites of TES produced in the horse liver in vitro and to investigate the CYP subfamilies involved in metabolite formation with special regard to members of the CYP3A subfamily. We used hepatocyte primary cultures and liver microsomes to investigate liver metabolism and screened for the involved CYPs using known chemical inhibitors for human CYPs. We further examined TES metabolism and the effect of two chemical inhibitors using recombinantly expressed equine CYP3A89, CYP3A94, and CYP3A95.
Materials and Methods
Hepatocyte primary cultures
Equine hepatocyte primary cultures were isolated as described by Stefanski et al. (2013). Briefly, 100–250 g fresh liver was obtained maximal 40 min after stunning. Cells were isolated using a two-step perfusion method. Equine hepatocytes were isolated from livers of two mares both aged 20 years. Horses were healthy and not under any drug treatment prior to slaughtering. The cells were counted and initial cell viability was determined by trypan blue exclusion. Cells were maintained in Dulbecco's Modified Eagle Medium (DMEM) without phenol red, supplemented with 5% FBS, 200 mm l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin.
Microsomes
Microsomes from human livers were purchased from BD Biosciences (Temse, Belgium). Equine microsomes were prepared as described elsewhere (Mossner et al., 2011). For the inhibition experiments, microsomes from the liver of one 13-year-old warmblood gelding were used. The horse was healthy and not under any drug treatment before slaughter. Immediately after removal of the liver, a piece from the dextral lobe was cut into cubes of about 0.5 cm diameter, placed on dry ice, and transported to the lab, where the liver pieces were stored at −80 °C for several days until microsomes were prepared. Microsomal fractions were stored at −80 °C. Protein content was measured with the Bradford assay using BSA as a standard and determined to be 7.24 mg/mL. Total CYP protein content, accounting for 235 pmol/mg total protein, was determined by spectrophotometric assessment using the method of Omura and Sato (1964).
V79 cells
V79 Chinese hamster lung fibroblasts (Mainzer subclone) were used for heterologous expression of the equine CYPs. V79 cells (a kind gift of J. Buters, TU Munich, Germany) were cultivated in DMEM supplemented with 10% fetal calf serum, 1 mm sodium pyruvate, 4 mm l-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin (all reagents were purchased from Gibco, Carlsbad, CA, USA).
Production of stable cell lines V79-CYP3A89, V79-CYP3A94, V79-CYP3A95
Experiments with equine CYPs 3A89, 3A94 and 3A95 were performed with V79 cell lines that were transduced with the respective CYPs. Sequences of these CYPs are published in the EMBL databank carrying the names: FN669293, NM_001190939, NM_001190940 (Schmitz et al., 2010). The cDNAs encoding CYPs 3A89, 3A94, and 3A95 were amplified using Expand High-Fidelity plus PCR System (Roche, Basel, Switzerland). Primers were designed manually including the sequence of an HA-tag at the 3′- end that can be used for detection as specific antibodies detecting this CYP isoform are not available. Subsequent sequencing was performed to verify the sequence of the inserts. The sequence of CYP3A89 showed two variants compared to the reference, namely p.S6T and p.S501N, and the sequence of CYP3A95 showed one variant p.V327A. PCR products were next digested with RsrII and subsequently cloned into the RsrII-cleaved lentiviral vector pRRL 2xRsrII TD (kindly provided by Patrick Salomon, University of Geneva, Switzerland) as described elsewhere (Dayer et al., 2007; Wyss-Fluehmann et al., 2010). HEK T293 cells (CRL-11268™, ATCC) were used for virus production and cultivated in DMEM supplemented with 10% fetal calf serum, 4 mm l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin. For virus production, HEK T293 cells were grown to 70% confluence in 150 mm dishes (TPP, Trasadingen, Switzerland) and were chemically transfected with FuGene HD Transfection Reagent (Roche) at a 3:1 ratio FuGene:DNA, with 5 μg of the pRRL vector containing the sequence of the respective CYP, 3 μg of the packaging protein-expressing vector (pPax2) and 2 μg of the envelope protein VSV-G-expressing vector (pMD2G). Virus stock was collected at day 2 and day 3 after transfection. For transduction, native V79 cells were grown to 30% confluence in 6-well-plates (TTP, Trasadingen, Switzerland) and transduced three times with 2 mL of virus stock, each containing the respective CYP. Transduction efficiency was monitored by immunocytochemistry with an antibody against the HA tag (mouse anti-HA, 1:100; Covance, Princeton, NJ, USA) and a goat anti-mouse as secondary antibody (Alexa Fluor® 488; 1:500; Rockland, Gilbertsville, PA, USA). To ensure a homogenous cell line, single-cell clones were selected based on a stable and intense fluorescence signal and were further cultivated. To ensure expression of the right enzyme, genomic DNA was isolated from the cells and sequenced. Activity of the CYPs was tested by incubation with 7-benzyloxy-4-trifluoromethylcoumarin (BFC) as described in a following paragraph.
Testosterone incubation with cells and sample preparation
For incubation of TES with equine hepatocyte primary cultures, cells were seeded in 6-well plates (TPP) at a density of 106 cells/well in duplicates. About 12 h after seeding, the medium was replaced with 4 mL medium containing 100 μm TES. Aliquots of the supernatant were removed after 0, 6, 15, and 24 h and frozen at −20 °C until analysis. To investigate the contribution of phase II metabolism, in one experiment, one sample of each time point was incubated with β-glucuronidase/arylsulfatase from Helix pomatia (Roche) for 2 h at 37 °C, and results were compared to nontreated samples.
For incubation of TES with V79-CYP cells, cells were seeded in 6-well plates in duplicates, such that they would reach 90% confluence at the time of sampling. Twenty-four hours after seeding, DMEM was replaced with 4 mL phenol red free DMEM containing 100 μm TES. After 0, 24, and 48 h, the reactions were stopped by removing the supernatant and freezing it at −20 °C until analysis. No external P450-NADPH-oxidoreductase was added, as the V79 cells show endogenous expression of this enzyme. One sample of each time point was incubated with β-glucuronidase/arylsulfatase from H. pomatia (Roche) for 2 h at 37 °C, and results were compared to non-treated controls. Untransduced V79 cells were incubated with TES alongside the transduced cells to investigate possible metabolism by the V79 cells. For analysis, 4 mL medium samples were spiked with the internal standard, TES-17-propionate. Subsequently, the samples were extracted with Chromabond™ octadecyl-modified silica gel cartridges (Machery-Nagel, Oensingen, Switzerland), and eluted using 1 mL methanol. The eluents were evaporated under nitrogen and dissolved in 1 mL 20% methanol after 5 min incubation and 1 min of vigorous vortexing. These samples were extracted a second time using new cartridges. Samples were again eluted with 200 μL methanol, once more evaporated under nitrogen, and dissolved in 200 μL 20% methanol. The samples were then analyzed by HPLC.
Testosterone incubation with liver microsomes and sample preparation
The microsomal incubation conditions used to study the metabolism of TES were adapted from former studies of our group (Mossner et al., 2011). Each incubation was performed in duplicates in a 100 mm phosphate buffer at pH 7.4 with NADPH regeneration system (BD Biosciences, Woburn, MA, USA) and 1 mg/mL microsomal protein. After 5 min preincubation at 37 °C, the reaction was started by addition of 100 μm TES. TES stock solutions were prepared in 100% methanol, such that the solvent content did not exceed 0.1% (v/v) in the incubation mix.
Inhibition experiments were carried out using 1 μm ketoconazole, 100 μm troleandomycin, 10 μm clopidogrel, 5 μm sulfaphenazole or 25 μm quercetin. High inhibitor concentrations were chosen according to the literature because inhibitors have not been extensively evaluated for the horse yet (Newton et al., 1995; Li et al., 2002; Mirghani et al., 2002; Richter et al., 2004; Sanchez et al., 2004; Ghosal et al., 2006; Polasek & Miners, 2006; Nebot et al., 2010; Greenblatt et al., 2011). Inhibitors were pre-incubated for 30 min prior to addition of 100 μm TES in order to account for possible mechanism-based inhibition. In the inhibition experiments, the organic solvent content did not exceed 0.2% (v/v). A sample containing the solvent instead of the inhibitor was used as control. To terminate incubations, an equal amount of ice-cold acetonitrile was given to the incubation mixture and the internal standard, TES-17-propionate, was added. The mixture was vigorously vortexed for 5 sec. Subsequently, samples were centrifuged for 10 min at 15 000 g at RT. Two hundred microlitre of the supernatant was taken directly for analysis by HPLC.
HPLC
Testosterone metabolites in incubations of TES with liver microsomes were quantified by HPLC after a method adapted from Chauret et al., 1997. TES and metabolites were determined by HPLC using an Ultimate 3000 System (Dionex, Reinach, Switzerland) with ultraviolet (UV) detection at 254 nm equipped with an EC 125/4 Lichrospher 100-5 RP-8 column (Macherey-Nagel, Dürren, Germany). The gradient mobile phase solvent A (methanol:water:acetonitrile, 39:60:1) was adjusted in a ratio of 90(A):10(B) with solvent B (methanol/water/acetonitrile, 80:18:2) and the flow rate to its maximum of 1 mL\min within 5 min before the HPLC program started. The gradient was then brought to a composition of 50(A):50(B) in 4 min, from 4 to 7 min 35(A):65(B), from 7 to 10 min 25(A):75(B), from 10 to 14 min 20(A):80(B), which was held for 4 min. From min 18 to 21, the initial condition of 90(A):10(B) was readjusted and held for two more min. The recorded peaks were labeled using the Dionex software Chromeleon 6.8 (Dionex) according to their retention times in relation to known standards.
In this setup, 6-β-OH-TES was detected at 5.3 min, 16-β-OH-TES at 6.45 min, AS eluted at 7.9 min, and TES eluted at 9 min. Quantitation was based on internal calibration with normalization of the peak area to an internal standard. The five calibration standards for TES ranged from 10 to 150 μm, the five calibrators for 6-β-OH-TES, 16-β-OH-TES, and AS ranged from 1 to 50 μm. Calibration curves were found to be linear (r2 ≥ 0.99). The detection limit of all substances was about 0.5 μm (S/N = 3) and the limit of quantitation between 1 and 2 μm (S/N = 10) depending on the metabolite. Intraday RSD data (n = 6, two concentrations) were between 2.2% and 22% and interday RSD data (n = 6, two concentrations) proved to be between 2.5% and 23.5%. Detection times within a day of analysis were stable.
GC/MS for peak determination
An unknown peak detected by HPLC at 6.6 min in incubations of TES with liver microsomes and V79-CYP3A95 cells was analyzed by GC/MS in order to reveal the chemical structure. In addition, the chemical structure of 6-β-OH-TES and AS in incubations with liver microsomes, defined by their retention times in the HPLC compared to standards, was confirmed by GC/MS. Samples from incubations with equine liver microsomes and single CYP3A95 were collected from the HPLC system and the eluent was evaporated. The samples were derivatized to form the methyloxime-trimethylsilyl ethers. One hundred microlitre of methoxyamine HCl 2% in pyridine was added, and the samples were heated at 60 °C for 1 h. After the evaporation of the solvent, 100 μL of trimethylsilyl imidazole was added and the samples were derivatized at 100 °C for 16 h. The derivatives were purified by gel filtration on Lipidex 5000 columns to remove the excess of the derivatisation reagent. The derivatives were then analyzed on a gas chromatograph 6890N equipped with a mass selective detector 5973N (Agilent Technologies, La Jolla CA, USA) in a scan run in the electron impact mode. The samples were chromatographed in a temperature-programmed run (210–265 °C) over 35 min. The mass range scanned was 50–800 Da. The ionization energy was 70 eV. The spectra obtained by this method were investigated for characteristic masses of TES metabolites. After a TES metabolite had been identified, it was compared with the spectrum of the standard compound. At least six ions had to correspond between the spectrum of the analyte and the spectrum of the standard compound as indicated in Table 1. By this procedure, AS, 6-β-OH-TES, and 11-β-hydroxytestosterone (11-β-OH-TES) were identified. Metabolites formed by hepatocyte primary cultures were not analyzed by GC/MS due to the scarcity of material.
| Metabolite | Specific ions analyzed |
|---|---|
| Androstenedione | m/z 344, 329, 313, 281, 267, 242 |
| 6β-hydroxy-testosterone | m/z 477, 462, 446, 387, 331, 313 |
| 11β-hydroxy-testosterone | m/z 477, 462, 446, 387, 372, 356 |
- Ions analyzed by GC/MS corresponding between the spectrum of the analyte and the spectrum of the standard compound (reference) at the correct retention time.
Inhibition of equine CYP3As by ketoconazole and troleandomycin
To test if the inhibitors of human CYP3A4 ketoconazole and troleandomycin inhibit the activity of equine CYP3A89, CYP3A94, and CYP3A95, we incubated CYP expressing V79 cells with 100 μm BFC instead of TES because not all isoforms showed detectable metabolism of TES, and monitored its biotransformation to the fluorescent metabolite 7-hydroxy-4-trifluoromethylcoumarin (HFC). The fluorescence-based assay was performed as described previously with minor modifications (Donato et al., 2004). Cells were seeded in 24-well plates so that they reached 90% confluence at the end of incubation with BFC. Medium containing 1 μm ketoconazole dissolved in methanol or 100 μm troleandomycin dissolved in DMSO was added to the cells 30 min before addition of 100 μm BFC. Quercetin could not be tested due to its high background fluorescence. After a 5-h incubation, the supernatant was diluted 1:2 with a quenching solution (0.25 m Tris in 60% (v/v) acetonitrile). The 5-h incubation time was chosen based on previous experiments showing linear HFC formation over time up to an 8 h incubation period. The metabolism from BFC to the fluorescent HFC was measured with a plate reader HT Synergy (Biotek, Winooski, VT, USA) using excitation at 400/30 nm and emission at 530/25 nm. Incubation time was within the linear phase of metabolite formation. Experiments were done in triplicates on three different days (n = 9).
Statistical analysis
Data from experiments comparing CYP activity in CYP-expressing V79 cells incubated with solvent versus inhibitor were analyzed using the Wilcoxon Rank-Sum Test. Data were analyzed with NCSS statistical software (version 2007, Kaysville, UT, USA). P values < 0.05 were considered significant.
Results
Testosterone metabolites formed in equine hepatocyte primary cell culture
Two independent primary hepatocyte preparations were incubated with 100 μm TES, and metabolite formation was monitored for 24 h (Fig. 1). AS (assigned by retention time) was found to be by far the most prominent peak in both livers. A much smaller peak, likely representing 6-β-OH-TES and some small unidentified peaks were also seen (Fig. 1). Metabolite peaks did not increase after deglucuronidation of the samples. Due to the scarce sample material, it was not possible to determine the unknown peaks by GC/MS.
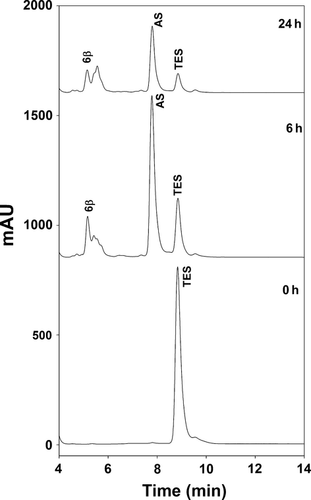
Testosterone metabolites formed in equine and human liver microsomes
Equine liver microsomes incubated with 100 μm TES produced 6-β-OH-TES and AS in similar amounts. The chemical structure of these peaks was confirmed by GC/MS. In addition, another peak appeared at 6.6 min. This peak was collected and analyzed by GC/MS and thereby determined to be 11-β-OH-TES (Fig. 2a). The peak identity of 11-β-OH-TES was confirmed by spiking of the samples with a standard.
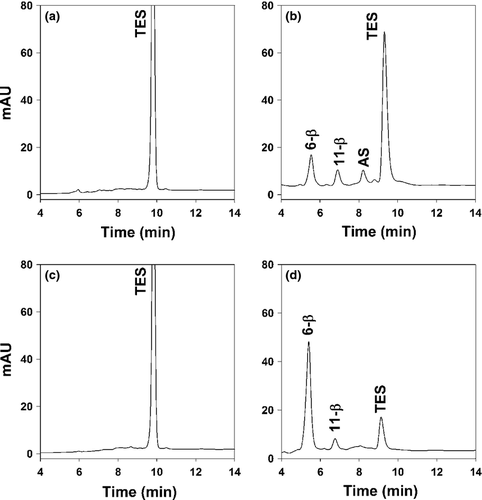
Incubations with human liver microsomes showed one major peak at the retention time of 6-β-OH-TES and a second much smaller peak at 6.6 min, presumably 11-β-OH-TES. In contrast to equine microsomal incubations, AS was not detected (Fig. 2b).
Inhibition of testosterone metabolite formation in equine liver microsomes
Equine liver microsomes were incubated with TES and five different inhibitors, all known to specifically inhibit certain isoforms of human CYPs.
Ketoconazole an established inhibitor for human CYP3A4 (Ghosal et al., 2006; Greenblatt et al., 2011) led to a decrease in 6-β-OH-TES formation by about 65% (Fig. 3a) and to a complete inhibition of the formation of 11-β-OH-TES (Fig. 3b). Formation of AS increased to around 150% after addition of ketoconazole (Fig. 3c). Troleandomycin, another inhibitor specific for human CYP3A4 (Newton et al., 1995; Polasek & Miners, 2006; Nebot et al., 2010), decreased 6-β-OH-TES formation by 30% and 11-β-OH-TES by 38% and increased AS formation slightly, thus showing a similar pattern to the effects of ketoconazole. Quercetin, an inhibitor of human CYP2C8 (Li et al., 2002; Ghosal et al., 2006; Nebot et al., 2010) inhibited the formation of 6-β-OH-TES to about half and the formation of 11-β-OH-TES to 60%. In contrast, AS formation increased after addition of quercetin to 142%. Clopidogrel, a substance declared acceptable by the FDA to inhibit human CYP2B6 (Richter et al., 2004) and proven to inhibit equine CYP2B6 with an IC50 of about 6 μm (Peters et al., 2013), had no significant effect on the formation rate of any of the observed metabolites. Sulfaphenazole, an inhibitor of human CYP2C9 (Newton et al., 1995) did not change the formation of the three metabolites when compared to solvent controls (Fig. 3).

Metabolites of testosterone produced by recombinant equine CYPs
Untransduced V79 cells were incubated with TES as a negative control. In these untransduced cells, several very small peaks appeared after 24 h of incubation (Fig. 4a). Two hours of deglucuronidation did not increase peak sizes. In CYP3A89 and CYP3A94-expressing cells, peak patterns and peak size were the same as in nontransduced V79 cells. Deglucuronidation had no significant effect on the peak pattern or peak areas. In contrast, apart from the small peaks seen in the other CYP3A incubations, V79 cells transfected with CYP3A95 produced significant amounts of 11-β-OH-TES as was confirmed by GC/MS analysis (Fig. 4b).
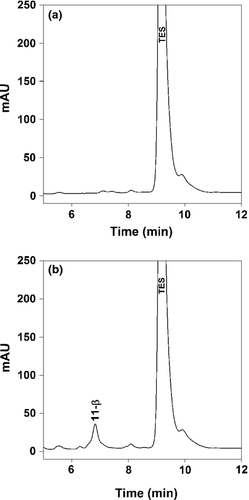
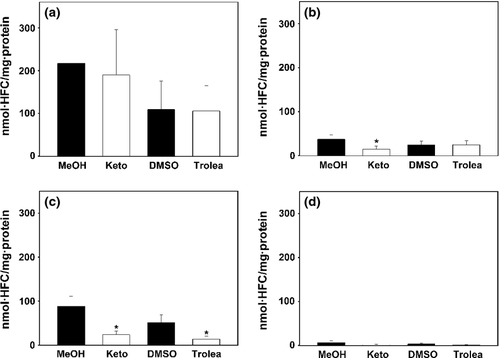
Inhibition of equine CYP3As by ketoconazole and troleandomycin
To investigate whether the different CYP3A isoforms of the horse are inhibited by the same chemical inhibitors as human CYP3A4, all three equine CYP3A isoforms expressed in V79 cells were incubated with the substrate BFC for 5 h. Testosterone was not a suitable substrate due to no detectable biotransformation by CYP3A94 and CYP3A89. Subsequently, the amount of the fluorescent metabolite HFC was measured with the two inhibitors, ketoconazole and troleandomycin, and compared to their respective solvent controls containing methanol or DMSO. The investigated inhibitors had different effects on the different CYP3A isoforms: CYP3A89 was not significantly inhibited by either inhibitor (Fig. 5a). In contrast, BFC metabolism in CYP3A94 was significantly inhibited to 60% through addition of ketoconazole, but not by troleandomycin (Fig. 5b). The activity of CYP3A95 was significantly inhibited to 73% and 75% by ketoconazole and troleandomycin, respectively (Fig. 5c). Untransduced cells showed a background activity six times less than the lowest activity in transduced cells (Fig. 5d).
Discussion
Numerous studies exist, characterizing metabolism of anabolic steroids in the horse in vivo and in vitro. These publications are written with the focus on detecting abuse of anabolic steroids in equine sports and therefore mostly employ TES derivatives as substrates (Ho et al., 2007a,b; Soma et al., 2007; Scarth et al., 2010). In contrast, our work focusses on the potential use of TES as a substrate for pharmacological characterization of recently identified horse CYP3A isoforms. The 6-β-hydroxylation of TES has been defined as marker reaction to identify human CYP3A4/5 activity. The first step of this descriptive study was to identify metabolites formed in the horse liver in vitro by using equine hepatocyte primary cultures and liver microsomes.
Our incubations with equine hepatocyte primary cultures showed a very large peak for AS and no 11-β-OH-TES formation in contrast to liver microsomes where AS formation was equally important in the formation of other metabolites. This is consistent with the presence of hydroxysteroid-17-beta dehydrogenases as cytosolic and membrane-bound enzymes responsible for conversion of the 17-β groups in different steroids (Blomquist et al., 1994; Blomquist, 1995).
A recently published study, investigated metabolite formation in liver microsomes compared to homogenized horse liver (Wong et al., 2011). Interestingly, in that study, the only metabolite detected in incubations of TES with horse liver microsomes by GC/MS was AS, whereas in incubations with homogenized horse liver, 11 different metabolites were detected. In our study, AS was also found in incubations with equine liver microsomes, but, in addition, at least two hydroxylated TES metabolites were detected as well. Except for AS, none of the metabolites found in homogenized horse liver was detected in our incubations with equine hepatocyte primary cultures or microsomes. This might be due to differences in sample preparation and analytical techniques or it may be the consequence of interindividual variations.
A study by Scarth et al. (2010) demonstrated that incubation of stanozolol (17β-hydroxy,17α-methyl-5α-androstano[3,2-c]pyrazole), a synthetic TES derivative, with equine liver microsomes and S9 fraction rendered all the metabolites found in equine urine samples. In our study, we used hepatocyte primary cultures and microsomes to model the in vivo situation. AS, 6-β-OH-TES, and 11-β-OH-TES were found. None of these metabolites had been described for the in vivo TES metabolism in the horse before (Houghton, 1977; Houghton & Dumasia, 1979). It is therefore possible that the studies on in vivo metabolism of TES are incomplete regarding the metabolite spectrum.
In the samples from hepatocyte primary cultures and V79 cells, we tried to identify the extent of glucuronidation/sulfatation of TES and its metabolites by incubation of samples with H. pomatia β-glucuronidase and sulfatase. No significant difference was seen between samples with and without deglucuronidation. This is unlike studies of TES metabolism in vivo where some metabolites were only detected after hydrolysis (Houghton & Dumasia, 1979). Yet, it has been described that H. pomatia β-glucuronidase/sulfatase does not hydrolyze all steroid sulfates completely (Mauvais-Jarvis & Baulieu, 1965; Shackleton et al., 1968). Additionally, it is possible that the expression of UDP-glucuronosyltransferase and sulfatase enzymes is too low in the above-mentioned systems to make a relevant contribution to the overall metabolism.
When comparing metabolites produced from human liver microsomes and horse liver microsomes, it is evident that 6-β-OH-TES is the most abundant metabolite in human microsomes, whereas in horses 11-β-OH-TES and AS are important metabolites as well. A recent investigation with bovine liver microsomes detected 6-β-, 2-β- and 16-β hydroxylase activity in these microsomes (Pegolo et al., 2010). We did not find any 16-β hydroxylase activity in our horse liver microsomes although the assay would have been able to detect 16-β-OH-TES. It has been demonstrated by several investigations that the spectrum of metabolites formed by liver preparations of different species displays a broad variation. The metabolites of TES vary qualitatively and quantitatively (Martignoni et al., 2004). Therefore, our findings meet these expectations.
To get some evidence for the CYP isoforms that metabolize TES in horses, we incubated liver microsomes together with chemical inhibitors known to be selective for certain human CYP isoforms. Chemical inhibitors, inhibiting the formation of 6-β-OH-TES and 11-β-OH-TES in incubations with equine liver microsomes had the contrary effect on AS formation: AS formation increased when the other two metabolites decreased. The reason for this phenomenon could be an increased availability of the substrate TES after inhibition of some metabolizing enzymes. We speculate that AS is formed by a different enzyme compared to the other two metabolites, which is likely to be a member of the hydroxysteroid-17-beta dehydrogenase family known to be present in many tissues and to contribute significantly to steroid metabolism in both sexes. However, using human liver microsomes and single recombinant CYPs, AS formation was attributed mainly to CYP2C19 (Yamazaki & Shimada, 1997). Another explanation would be that 6-β-OH-TES and 11-β-OH-TES negatively influence the activity of the AS-forming enzyme.
Inhibitors known to be selective for human CYP3A enzymes inhibited the formation of 6-β-OH-TES and 11-β-OH-TES the most. The only other inhibitor blocking metabolism of TES was quercetin. These findings correspond to a study by Scarth et al. (2010) investigating in vitro metabolism of stanozolol. In this study, ketoconazole and quercetin were the only inhibitors that blocked metabolism of stanozolol to its 6-β-OH and 16-β-OH stanozolol metabolites. Interestingly, sulfaphenazole, an inhibitor for human CYP2C9, had no impact on the metabolism of TES in equine liver microsomes, although in humans CYP2C9 contributes to TES metabolism (Yamazaki & Shimada, 1997). In contrast, in bovines, CYP3A and CYP2B isoforms seem to be responsible for hydroxylation of TES (Pegolo et al., 2010).
Studies with single equine CYP3A89, 94, and 95 were carried out using heterologously expressed single CYPs. To our knowledge, this is the first time that functional expression of these CYPs has been demonstrated. In 2010, equine single CYPs 3A96, 3A97, and 3A89 were expressed in insect cells. However, in this study, equine single CYP3A89 was not expressed as a functional enzyme and post-translational modification was postulated as the reason (Knych et al., 2010). In our system, CYP3A89 was functional and even showed the highest activity towards the substrate BFC when compared to CYPs 3A95 and 3A96.
The other two heterologously expressed equine CYP3A enzymes were proven to be functional as well. Although the sequence of all equine CYP3As is very similar, the metabolic reaction using TES as a substrate differed between the isoforms. CYP3A95 was the only isoform metabolizing TES to 11-β-OH-TES. This is noteworthy as the human isoforms CYP3A4 and CYP3A5 share the ability to hydroxylate TES at the 6-β position.
In the study by Knych et al. (2010), CYP3A96 was shown to biotransform TES to 6-β-OH-TES. As, in our study, 6-β-hydroxylation was inhibited through inhibitors known for human CYP3A4, there is now more evidence that CYP3A96 could be an important CYP responsible for 6-β-hydroxylation of TES. However, this still has to be verified using other equine single CYPs. Our results indicate the possibility that different hydroxylation reactions of TES could serve as model reactions defining activity of specific CYP3A isoforms. It seems as if there are more significant differences between the chemical reactions of the equine CYP3A isoforms than between human CYP3A4 and CYP3A5. We found that ketoconazole and troleandomycin inhibited 11-β-hydroxylation and 6-β-hydroxylation of TES in equine liver microsomes and interpreted this as inhibition of CYP3A isoforms. Ketoconazole significantly inhibited CYPs 3A94 and 3A95, but no significant inhibition was obtained in equine CYP3A89. Troleandromycin did not inhibit CYPs 3A89 and 3A94 significantly, but a significant inhibition was seen in CYP3A95. The inhibition potency of ketoconazole and troleandromycin was similar in CYP3A95. Yet, our inhibition experiments using single CYPs revealed that there are differences between the responses of the equine CYP3A isoforms to inhibitors. These differences have to be taken into account when conducting inhibition studies. Therefore, to determine the contribution of a certain CYP3A isoform in a microsomal incubation, it is necessary to use the inhibitor proven to be most specific for this isoform. More research must be undertaken to find the right inhibitors for selected horse CYP3As.
This study gives data on the in vitro metabolism of TES in the equine liver and the contribution of three equine CYP3A isoforms to the metabolism of TES. In addition, the response to inhibitors has been investigated for the same three CYP3A isoforms. Defining CYP-specific metabolic reactions and inhibitors will help to establish preclinical testing of newly developed substances for horses in the future. Knowing more about the TES metabolizing enzymes may also help to explain interindividual differences in TES metabolism and to improve interpretation of doping tests.
Acknowledgments
The authors thank Prof. Wolfgang Thormann and Regula Theurillat for fruitful discussions. Furthermore, they thank Dr. Philippe Plattet for cloning the CYPs and providing the HEK293T cells.




