The efficacy and safety of cannabidiol as adjunct treatment for drug-resistant idiopathic epilepsy in 51 dogs: A double-blinded crossover study
Abstract
Background
Approximately 30% of dogs with idiopathic epilepsy (IE) are drug-resistant. Recent studies have suggested cannabidiol (CBD) may be an effective anticonvulsant in dogs with IE.
Objective
To evaluate the addition of CBD to antiseizure drugs (ASDs) on seizure frequency and to report adverse events in dogs with drug-resistant IE.
Animals
Fifty-one dogs. Dogs having at least 2 seizures per month while receiving at least 1 ASD were included in the trial.
Methods
Double-blinded placebo-controlled crossover study. The 5 mg/kg/day dosage met futility requirements after 12 dogs, and a dosage of 9 mg/kg/day was used in the next 39 dogs. Dogs were randomly assigned to receive CBD or placebo for 3 months, with a 1-month washout period between oils. Total numbers of seizures and seizure days were recorded. Diagnostic testing was performed periodically throughout the trial.
Results
At the 9 mg/kg/day dose, the decrease in total seizure frequency was significant compared with placebo. A 24.1% decrease in seizure days occurred in dogs receiving CBD and a 5.8% increase occurred in dogs receiving placebo (P ≤ .05). No significant difference was found in the number of responders (≥50% decrease in total seizures or seizure days). Liver enzyme activities increased at both dosages. Decreased appetite and vomiting were more common in the CBD phase (P ≤ .05).
Conclusions and Clinical Importance
Cannabidiol decreased total seizures and seizure days compared to placebo when administered to dogs PO at 9 mg/kg/day. Liver enzymes should be monitored with administration of CBD in dogs.
Abbreviations
-
- AE
-
- adverse event
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine transaminase
-
- ASD
-
- antiseizure drug
-
- CBD
-
- cannabidiol
-
- CSF
-
- cerebrospinal fluid
-
- CSU
-
- Colorado State University
-
- CI
-
- confidence limits
-
- IE
-
- idiopathic epilepsy
-
- MRI
-
- magnetic resonance imaging
-
- SAS
-
- statistical analysis software
1 INTRODUCTION
Idiopathic epilepsy (IE) is a chronic neurological condition defined by recurrent seizures originating from suspected genetic origin, confirmed genetic origin, or unknown origin.1 It is the most common neurological condition in dogs, affecting an estimated 0.5% to 5.7% of the overall canine population.2 More severe manifestations of the disease often are associated with euthanasia because of quality-of-life concerns, emphasizing the need for adequate seizure control and management.3, 4 Various antiseizure drugs (ASDs) are used for management of IE, with the current American College of Veterinary Internal Medicine consensus statement recommending phenobarbital and potassium bromide as 1st-line management options.5 However, these medications may be ineffective in controlling seizures in up to 30% of epileptic dogs.6 Furthermore, these medications often are associated with adverse events (AEs),7 indicating a need for additional treatment approaches.
Cannabidiol (CBD), a nonpsychoactive component of the cannabis plant, has been described anecdotally as an effective management for seizures.8-11 Recently, new opportunities to study its use as a treatment for drug-resistant epilepsy have become available under the Hemp Farming Act of 2018,12 which descheduled hemp products containing <0.3% tetrahydrocannabinol. Cannabidiol has demonstrated anticonvulsant properties in vitro,13 as well as in vivo in rodents.14-18 In humans, several randomized controlled trials have demonstrated the efficacy of CBD on decreased seizure frequency in patients with drug-resistant epilepsy.19-22
Although in vitro and in vivo studies have demonstrated the effectiveness of CBD for seizures in humans and rodents, relatively little research has been done in companion animals.23 In dogs, 2 small studies were conducted to evaluate the effect of CBD oil in addition to standard ASD management on seizure frequency in dogs with IE.24, 25 Both studies identified a larger decrease in seizure frequency in dogs that received CBD compared with dogs that received placebo. One study also identified a correlation between plasma concentrations of CBD and decrease in seizures.24
Our aim was to further explore the role of CBD in the management of IE in a larger population of dogs with drug-resistant IE. We hypothesized that a significant decrease in seizures would occur in dogs receiving CBD compared with dogs receiving placebo, with no concomitant impact on blood concentrations of ASDs. We also hypothesized that administration of CBD would not be associated with moderate or severe AEs.
2 MATERIALS AND METHODS
2.1 Animals
The study was approved by the Colorado State University (CSU) Institutional Animal Care and Use Committee (Protocol #16-6790A). A target of 60 dogs was chosen to find an estimated difference of 50% in the episodes between the 2 groups. The calculation considered a statistical power of 80% and confidence of 95%. Dogs were prospectively enrolled from January 2018 to September 2020 after owner consent and fulfillment of enrollment criteria. Dogs were included if they met the tier II confidence level for diagnosis of IE, as defined by the International Veterinary Epilepsy Task Force Consensus Statement.26 Specifically, criteria included pretrial owner documentation of ≥2 seizures per month for at least 12 weeks while receiving at least 1 conventional ASD (phenobarbital, potassium bromide, levetiracetam, and zonisamide). Dogs receiving phenobarbital or potassium bromide were required to have blood or serum concentrations within the reported therapeutic range (20-40 μg/mL for phenobarbital and 0.88-3.0 mg/mL for potassium bromide).27-29 Those receiving levetiracetam or zonisamide were required to be within the accepted dose range for these medications (≥20 mg/kg PO q8h immediate-release levetiracetam; ≥30 mg/kg PO q12h extended-release levetiracetam; and ≥5 mg/kg PO q12h zonisamide). Alanine transaminase (ALT) and alkaline phosphatase (ALP) were considered abnormal if activities were >2.5× the high end of the reference range. If either ALT or ALP was abnormal, postprandial bile acid concentration was required to be normal (<30 μmol/L) for inclusion. Activity of ALP >2× the high end of the reference range in dogs receiving either zonisamide or phenobarbital was still acceptable if ALT activity or postprandial bile acid concentrations were normal because those medications are known to cause a clinically unimportant increases in ALP activity.30, 31 Infectious disease testing was performed if cerebrospinal fluid (CSF) analysis was abnormal and had to be negative for Bartonella spp., Ehrlichia spp., Anaplasma spp., Neorickettsia spp., Dirofilaria immitis, Wolbachia spp., Rickettsia spp., Toxoplasma gondii, Neospora caninum, Borrelia burgdorferi, and canine distemper virus. Other exclusion criteria included anticipation of owner noncompliance and dogs with documented comorbidities associated with a poor prognosis.
2.2 Study design
The study was conducted as a randomized, double-blinded, placebo-controlled crossover clinical trial comparing the effect of CBD versus placebo on seizure frequency in dogs taking concurrent ASD medication. A computer-based random number generator (Microsoft Excel, Microsoft Corporation, Redmond, Washington) was used to assign a treatment order to each dog. The owners and primary researchers were blinded to the treatment order until the end of the trial. Once a treatment order was assigned, owners were given either CBD-infused hemp seed oil with chicken flavoring (Applied Basic Science Corporation, Castle Rock, Colorado) or the placebo (hemp seed oil with chicken flavoring) to be administered PO at the same volume and timing throughout the trial. Dogs were given the CBD or placebo for 12 weeks and then, after a 4-week washout period, given the opposite oil. The first 13 dogs enrolled in the trial were given a dosage of 5 mg/kg/day (2.5 mg/kg q12h) of each oil. However, because of lack of improvement during either treatment phase, we elected to increase the dosage to 9 mg/kg/day (4.5 mg/kg q12h) for the next cohort of 48 dogs. During the baseline period (12 weeks before enrollment) and the study period, no alterations could be made to the ASD protocol without withdrawing from the study. However, 3 to 5 days of “rescue dosing” with levetiracetam, gabapentin, clorazepate, or a combination of these medications were permitted for cluster seizure or status epilepticus management before and during the trial.
At enrollment, baseline seizure frequency was recorded, and blood tests were obtained. These included ASD serum concentrations, CBD plasma concentrations, CBC, serum biochemistry, and postprandial bile acid concentrations. Each week, owners recorded behavioral and temperament variables using the previously validated Canine Behavioral Assessment & Research Questionairre (CBARQ) evaluation.32 Owners recorded observed seizure activity in their pets using seizure logs, noting the frequency and type of seizures (focal or generalized). Re-evaluation appointments were performed once monthly, at which time blood was collected for CBD plasma measurements, CBC, and serum biochemistry monitoring. Blood sampling was not performed at fixed time points postdose administration and instead was performed at appointment times more convenient for the owners. Owners also were told not to change their pet's diet for the duration of the trial.
Dogs that could not attend monthly appointments at CSU had blood samples collected by the primary veterinarian or a veterinary neurologist and shipped to CSU. At 0, 12, and 28-week time points all owners were required to bring their dogs to CSU for a neurological examination and to obtain additional blood samples for postprandial bile acids and ASD serum concentrations.
2.3 CBD oil and placebo information
The CBD was extracted from a crop that was registered as industrial hemp with the Colorado Department of Agriculture (#77046). The classification as industrial hemp certified that the plant contained a tetrahydrocannabinol concentration of ≤0.3%. Each batch of product was analyzed by at least 1 third-party laboratory for purity and cannabinoid concentrations (ProVerde Laboratories, Inc., Milford, Massachusetts; SC Labs [formerly Botanacor], Denver, Colorado; Aurum Labs, Durango, Colorado; (Table S1). The CBD oil was considered a full-spectrum product, containing approximately 100 mg/mL of CBD with trace amounts of other cannabinoids, varying slightly with different batches. The other ingredients were cold-pressed hemp seed oil and chicken flavoring. The placebo oil contained only the cold-pressed hemp seed oil and chicken flavoring. The CBD oil and placebo were masked in scent and appearance.
2.4 Laboratory analyses
Plasma concentrations of CBD were measured in canine plasma using a previously validated liquid chromatography-mass spectrometry technique33 at the Pharmacology Laboratory of the Drug Development & Discovery Shared Resource (University of Colorado Cancer Center). Phenobarbital and potassium bromide serum concentrations were measured at the CSU Veterinary Diagnostic Laboratory. Phenobarbital was measured according to the package insert for an Immulite 2000 analyzer (Siemens Medical Solutions USA Inc., Malvern, Pennsylvania). Levetiracetam and zonisamide serum concentrations were measured at Auburn University's Veterinary Diagnostic Laboratory using previously published methods.34, 35
2.5 Statistical analysis
A seizure day was defined as any 24-hour period during which a dog had at least 1 seizure, whereas total seizures were the total number of individual seizures reported for each dog. Percentage data were evaluated for normality using the UNIVARIATE procedure from SAS; it was not met for any of the endpoints, and thus, cubic root was used to approximate normal model assumptions. All endpoints were back-transformed for reporting in the original scale. The fixed effects included time (month), treatment, and their interaction term, which was not significant and, thus, removed from the model. Baseline absolute values for each percentage change endpoint were included as a covariate, and dog nested within treatment sequence was added as a random effect in the model. Pairwise treatment comparisons for all parametric data were conducted using the Tukey-Kramer test to protect against type I error.
Individual responsiveness to treatment was considered as an average monthly decrease of ≥50% in seizure numbers and seizure days. For that analysis, a 1-tailed t test was conducted comparing each treatment to a null hypothesis of 50% (SAS v 9.4). The number of AEs while on each treatment reported by the owner was analyzed as categorical data using Fisher's exact test wherein these were assigned a “yes” for each dog that had at least a single episode of each AE and a “no” if none was reported.
The ALP and ALT data were evaluated for normality using the Shapiro-Wilk test. If not normally distributed, the data were converted into logarithmic scale and GLIMMIX was constructed to evaluate the treatment and time point interaction effects. Tukey-adjusted differences and 95% confidence limits (CIs) were reported. A P value of .05 was used to determine significance. The program SAS v9.4 (SAS Institute Inc., Cary, North Carolina) was used for all statistical analyses.
3 RESULTS
3.1 Enrollment
A total of 502 dogs were screened for eligibility for the study, and 61 met the inclusion criteria and were enrolled. Thirteen dogs initially were enrolled at 5 mg/kg/day, and 12 dogs completed this portion of the trial (which included crossover with placebo). The dogs receiving the 5 mg/kg/day CBD dose showed no evidence of a treatment effect (P = .36) on the percentage change of total seizures and seizure days. Consequently, the principal investigator decided to increase the CBD dosage of the remaining dogs to 9 mg/kg/day, based on epilepsy data in humans showing higher dosages (10-20 mg/kg) being effective for Lennox Gastaut syndrome.19, 20 Forty-eight dogs were enrolled at the 9 mg/kg/day CBD dosage, and 39 dogs completed the trial. Reasons for withdrawal from the 5 and 9 mg/kg/day groups included euthanasia because of worsening seizures or status epilepticus (5/61), acute kidney failure (1/61), intestinal perforation (1/61), accidental death (1/61), change in ASD management (1/61), and lost to follow-up (1/61). All dogs that completed both arms of the trial were included in the analysis.
Documented comorbidities at enrollment included allergies (5/61), hypoadrenocorticism (1/61), cranial cruciate ligament disease (1/61), suspected renal dysplasia (1/61), hypothyroidism (1/61), and low-grade heart murmur (2/61).
At the time of enrollment, 1 dog had a bromide concentration that was 16% below previously reported therapeutic margins. This dog also was receiving zonisamide and levetiracetam. The bromide concentrations were measured and found to be within the therapeutic range at the 12th- and 28th-week ASD concentration checks.
During enrollment, 3 dogs were considered to have increased nucleated cell counts on CSF analysis (118/μL, 45/μL, and 10/μL). The 1st had a small amount of suspected iatrogenic blood contamination (4297 RBC/μL) and was re-evaluated 1 week later and found to have a nucleated cell count of 0/μL. The 2nd had a large amount of suspected blood contamination (>200 000 RBC/μL). Given normal magnetic resonance imaging (MRI) findings and lack of interictal neurological deficits, the increased nucleated cell count was attributed to the large amount of blood in the sample, and CSF was not re-evaluated. The 3rd dog also had a large amount of suspected iatrogenic blood contamination (>18 000 RBC/μL). Again, given the normal MRI findings and lack of interictal neurological deficits, the increased nucleated cell count was attributed to blood contamination, and CSF was not re-evaluated. Two dogs had albuminocytologic dissociation with protein concentrations of 32 and 42 mg/dL. On MRI, 1 dog had cerebral microbleeding with normal CSF analysis and was negative for Anaplasma phagocytophilum, D. immitis, B. burgdorferi, and Ehrlichia ewingii on 4Dx testing. The microbleeding was not considered to be the source of seizures for this patient. All 6 dogs described were included in the trial.
During the trial, 1 dog in the 9 mg/kg/day CBD phase of the trial had its potassium bromide dosage decreased 8 weeks into the trial because of concern for possible bromism. This dog began the study with a bromide concentration of 2.2 mg/mL, and also was receiving phenobarbital and zonisamide during the trial. At approximately 8 weeks into the trial, the dog was hospitalized for lethargy and weakness and its bromide concentration at this time was 2.3 mg/mL. Without other findings to explain this change, it was elected to decrease the potassium bromide dosage by 25%. This dog was receiving placebo at the time and received CBD in the 2nd half of the trial. Three crossed over from the 1st trial oil to the 2nd at 6, 8, and 9 weeks without a washout period because of a large number of seizures at home. Of these 3 dogs, 2 were receiving CBD and 1 was receiving placebo during the 1st phase. Another dog was started on the 2nd trial oil after a 3-week washout. It was receiving CBD in the 1st phase and did not have detectable CBD in the plasma at the start of the placebo oil.
3.2 Animal demographics
Given the lack of significant effect at the 5 mg/kg/day dosage and small sample size, that data will not be reported here but can be made available upon request.
Of the 39 dogs in the 9 mg/kg/day dose group, the most represented breed was mixed breed (15/39), followed by golden retriever (6/39), French bulldog (3/39), Labrador retriever (2/39), Border collie (2/39), and Neapolitan mastiff (2/39), with 1 each of Australian shepherd, Australian cattle dog, Boston terrier, Gordon setter, Irish setter, Jack Russell terrier, Nova Scotia duck tolling retriever, Rottweiler, and Yorkshire terrier. The most represented sex was male castrated (24/39), followed by female spayed (13/39), and male intact (2/39). No female intact dogs were included in the study. The mean age at the start of the study was 3.5 ± 2.0 years. The mean body weight was 28.7 ± 13.0 kg, with a range of 2.9 to 60.4 kg.
3.3 Animal seizure characteristics and ASD medications: 9 mg/kg/day dose
During the pretrial period, 28/39 dogs were reported to have only generalized seizures, 8/39 dogs experienced both generalized and focal seizures, and 3/39 dogs experienced focal seizures alone. The most common ASD combination was phenobarbital and levetiracetam (26/39) and most dogs were receiving ≥2 ASDs (36/39). Three dogs were receiving monotherapy with either phenobarbital (n = 2) or levetiracetam (n = 1).
3.4 Percentage change in total seizures and seizure days from baseline: 5 and 9 mg/kg/day doses
No significant changes (P = .36) to total seizures or seizure days were observed in dogs receiving 5 mg/kg/day of CBD.
For dogs receiving 9 mg/kg/day, a post hoc power analysis assessment using the power procedure from SAS (v 9.4) for crossover designs, considering a 30% intra-dog correlation, indicated that percentage changes of total seizures had a power of 71.3%, whereas that of seizure days was 93.0%.
Dogs receiving CBD at the 9 mg/kg/day dose had a small increase in percentage change (3.31%) of total seizures from baseline, which was 10 times lower in magnitude compared with placebo (30.72%) and was significantly different (P = .04; Figure 1). Conversely, the percentage change of seizure days while on CBD decreased by 24.1% (P = .002), whereas dogs on placebo had a 5.81% increase (Figure 2). When analyzing the effect of treatment on the percentage change of seizure type, generalized seizure percent change was not different between CBD and placebo groups (P = .12). Model fitting of focal seizures was not feasible because of the large number of zero values. No significant differences related to the sequence of placebo and CBD oil for total seizures (P = .50) and seizure days (P = .94) were found.
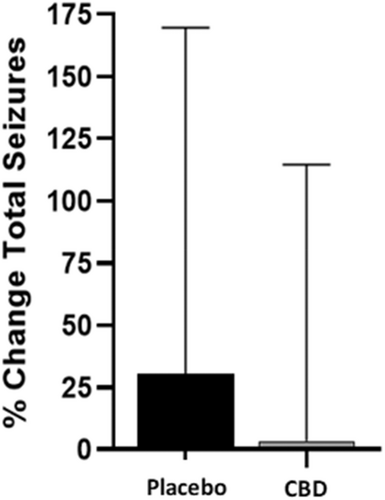

There were 9 responders (percentage change ≥ −50%) in the CBD group and 8 in the placebo group relating to total seizures (P = .78), and 13 versus 8 responders relating to seizure days for the CBD and placebo groups, respectively (P = .20). These differences were not significant.
3.5 Interaction between ASDs and CBD oil: 9 mg/kg/day dose
The interactions between phenobarbital, potassium bromide, zonisamide, and levetiracetam and treatment had no effect (P > .07) on the percentage change of seizure days or total seizures.
No evidence was found for differences between CBD administration and placebo for percentage change from baseline in ASD concentrations for any of the measured ASDs (P > .15). Large variation was found in all measured ASDs, which ranged from −42% to +144% for phenobarbital, −28% to +145% for potassium bromide, −45% to +174% for zonisamide, and −88% to +1311% for levetiracetam. Although not significant, the average percentage change of phenobarbital while receiving CBD was +11% and during the placebo phase was −1%.
3.6 Bile acid monitoring and change in liver enzyme activities: 9 mg/kg/day dose
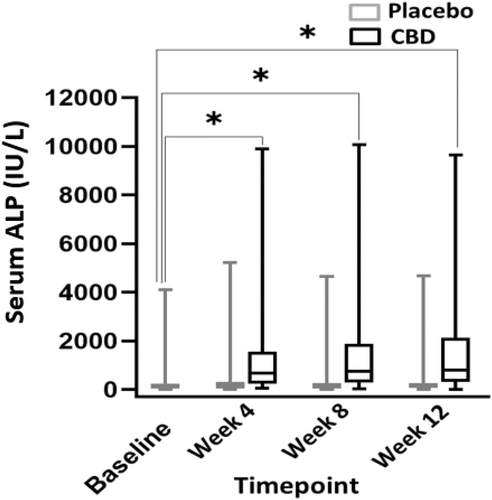
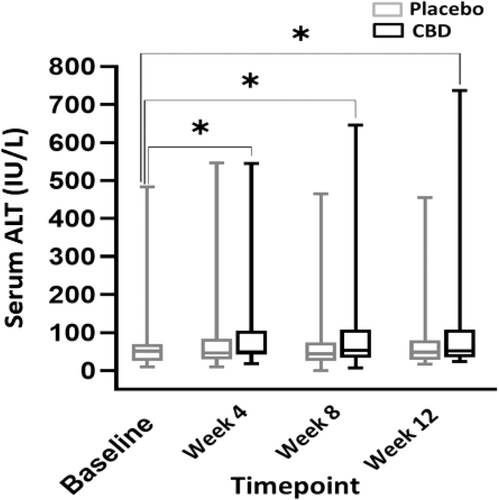
When comparing liver enzyme activities between months, a significant difference was found when comparing CBD and placebo treatment, and CBD-treated dogs consistently had higher ALP activities (P ≤ .001). Also, a significant difference in ALP activity was found when comparing CBD treatment during all 3 months and baseline (P ≤ .001). No difference was found between placebo and baseline for any month (P ≥ .88; Figure 3). A significant difference was found in mean serum ALT activity between CBD and placebo groups (P = .003; Figure 4). The mean baseline ALP activity was 278 (9-4112) IU/L. The mean ALP activity after the 3rd month of CBD treatment was 1585 (16-9642) IU/L. Of 30 dogs with ALP activity ≤2× the high end of the normal range at baseline, 24 (80%) had activities above twice the high end of the normal reference range when receiving CBD (95% CI, 368.7-849.9). Two of these dogs also had a single-month result >2× the high end of the reference range during placebo administration. The mean baseline ALT activity was 64.4 (9-483) IU/L. The mean ALT activity after the 3rd month of CBD treatment was 114.5 (7-646) IU/L. In 5 dogs (12.8%), ALT activity increased >2× the high end of the reference range during CBD administration (95% CI, 52.1-75.4). One dog had ALT activity >2× the high end of reference range only during the placebo phase. Two dogs had ALT activity at ≥2× high end of the reference range during both the placebo and treatment phases. Of these 2 dogs with increased ALT activity during both phases, both had baseline ALT activity above the high end of the reference range with 1 above twice the high end of the reference range.
Postprandial bile acid concentrations were increased in 2 dogs at the end of week 28. One had received CBD in the 1st phase (52 μmol/L) and the other had received CBD in the 2nd phase (81 μmol/L). All other bile acid concentrations at weeks 12 and 28 were normal.
3.7 Adverse events
Owner-reported AEs during 9.0 mg/kg/day dosing included decreased appetite, vomiting, soft feces or diarrhea, foraging, anxiety, increased appetite, increased activity, decreased activity, ataxia, and aggression (Table 1). Of these, only decreased appetite (P = .01) and vomiting (P = .05) were more frequent when dogs received the CBD treatment compared with placebo (Table 1). No differences were found between CBARQ scores for trainability, aggression, fear and anxiety, separation-related anxiety, excitability, attachment/attention seeking, or other behavioral problems while dogs were on CBD compared with placebo in both dosing groups.
| Adverse event | CBD | Placebo | P |
|---|---|---|---|
| Decreased appetite | 14 | 4 | .01 |
| Vomiting | 9 | 2 | .05 |
| Soft feces or diarrhea | 10 | 4 | .14 |
| Foraging | 8 | 5 | .55 |
| Anxiety | 9 | 6 | .57 |
| Increased appetite | 2 | 2 | 1.00 |
| Increased activity | 3 | 4 | 1.00 |
| Decreased activity | 1 | 5 | .20 |
| Ataxia | 3 | 2 | 1.00 |
| Aggression | 1 | 2 | 1.00 |
- Abbreviation: CBD, cannabidiol.
3.8 Plasma CBD concentration
The mean plasma CBD concentration at the 5.0 mg/kg/day dosage was 173.6 ± 101.5 ng/mL. Plasma CBD concentrations in the 9 mg/kg/day treatment group ranged from 226.7 to 2833.7 ng/mL with a mean ± SD of 1207.4 ± 571.3 ng/mL.
4 DISCUSSION
Ours was a double-blinded crossover trial to evaluate the role of CBD in the management of drug-resistant IE in dogs. Although no differences were found in number of responders (≥50% reduction in total seizures or seizure days) between treatment groups, there was a 24.1% decrease in seizure days in dogs receiving CBD compared with 5.8% increase in dogs receiving the placebo (Figure 2). Although the total number of seizures increased in both treatment groups, a significant difference was found between the CBD and placebo groups (3.31% and 30.72%, respectively; Figure 1). The increase of total seizure number in both groups could be a consequence of some dogs having large numbers of clusters within each seizure day.
It is unclear why the 5 mg/kg/day dose in the first 13 participants failed to show a treatment effect as it had in a previous study.24 However, the crossover design of our study may have better elucidated the individual treatment effect in comparison to the previously low-powered pilot study.24
The mean CBD concentration was approximately 7-fold higher in the 9 mg/kg/day dosing group compared with the 5 mg/kg/day dosing group despite the 0.8-fold difference in dosage. However, these results are difficult to evaluate because of the nonstandardized approach to the timing of blood collection for CBD concentration analysis in relation to the time of dose administration. Given the short half-life of CBD, CBD concentrations likely fluctuate throughout each day of dosing. The timing of blood collection could contribute to variability.33, 36-41 In a previous placebo-controlled study, a negative correlation was found between increasing plasma CBD concentrations and seizure decreases.24 A study investigating the use of CBD for epilepsy in humans also found more decrease in seizures with increasing plasma concentrations.42 In future studies, standardized timing between dose administration and blood collection will be important to elucidate a correlation between CBD plasma concentration and seizure control.
No statistically significant changes to ASD concentrations were found with CBD administration. Although not significant, large individual variation was found in all ASDs from baseline. Without standardized timing of blood collection after dose administration and relatively short half-lives of zonisamide and levetiracetam, the serum concentrations of these medications may have been affected, but it would not explain changes in bromide concentrations. Other explanations for variable concentrations could include owner noncompliance (including diet changes) or a change in drug manufacturing during the trial. When switching from 1 generic medication to another as much as a 40% difference in bioavailability can be observed for drugs not considered to be narrow therapeutic index drugs.43 Given the lack of significant differences in ASD concentrations between CBD and placebo, these variations in ASD concentrations may reflect a normal change seen with ASD administration in dogs.
Although no significant effect of CBD on serum phenobarbital concentrations was observed, an 11% increase in phenobarbital concentrations during the CBD phase occurred compared with a −1% change during the placebo phase. This difference may represent normal physiologic fluctuations or instead may indicate a pharmacokinetic interaction without sufficient power to detect significance. A previous study showed that the highest plasma concentrations (Cmax) of CBD increased in conjunction with chronic phenobarbital administration.38 Evaluation of phenobarbital pharmacokinetics with chronic administration of both CBD and phenobarbital together has not been evaluated previously. The interactions between chronic CBD and phenobarbital administration warrant further investigation.
Increases in ALP activity are seen consistently in veterinary literature with chronic dosing of CBD.24, 36-38, 44 Our study also found an increase in ALP activity in dogs receiving CBD (P ≤ .001) in addition to increases in ALT activity in dogs receiving CBD (P ≤ .003). Increases in ALT activity have not been reported in previous veterinary studies, but increases in AST and ALT activity are the most common AE related to CBD administration in human patients with drug-resistant epilepsy.45, 46 This finding may represent hepatic damage and could highlight interactions between CBD and concurrent ASDs. The dosage of CBD in our study was higher than in previous studies and this increase in ALT activity may represent a risk factor with chronic administration of larger doses of CBD. Two dogs with increases in ALT activity at the end of the trial also had increased bile acid concentrations, indicating impaired hepatic function. All other bile acids test results were normal. This finding may further indicate the potential for hepatic injury with the administration of CBD in conjunction with other ASDs.
The most common owner-reported AEs related to CBD administration were decreased appetite and vomiting (Table 1). Other reported AEs did not show significant differences between treatment groups. This finding is contrary to a previous study evaluating escalating doses of CBD and vehicle administration (medium-chain triglyceride oil), which found similar AEs in both groups.47 One possible explanation may be the difference in delivery oil chosen for this study. No previous studies have evaluated AEs associated with hemp seed oil in dogs.
Epidiolex is a highly purified CBD oil (isolate) approved by the FDA for the management of Lennox-Gastaut and Dravet syndrome in humans.48, 49 The responder rate in multiple meta-analyses evaluating CBD in these diseases along with other drug-resistant epilepsies ranges from approximately 37% to 40%.50-52 Our study did not use genetic testing to differentiate among different subtypes of drug-resistant epilepsy in dogs. Given the multitude of genetic causes for epilepsy in humans,53 there are likely multiple genetic factors affecting dogs with IE in the general population and in our study. Genetic testing may play an important role in the future of CBD use in IE.
Further research into the efficacy of CBD as an adjunctive ASD or as monotherapy is warranted given our results. Although there was no difference between the CBD and placebo groups for responders, this finding should consider the fact that most dogs included in our study were already drug-resistant to multiple anticonvulsants. Numerous studies in drug-resistant epilepsy in humans have suggested that after failure of the 1st or 2nd anticonvulsant, odds are lower for responding to each subsequent anticonvulsant.54-56 Therefore, the next step for the evaluation of CBD in the management of IE could be to compare CBD to phenobarbital in drug-naïve idiopathic epileptic dogs.
Limitations of our study include reliance on owner observation for seizure reporting. Some seizures could have been missed and others may have been misreported. In addition, veterinarians and support staff evaluating patients and blood test results at each appointment may have been inadvertently unblinded when seeing increased ALP activity in 1 group. However, owners and those performing statistical analysis were blinded without access to blood test results during the trial. An inherent limitation of any epilepsy trial is possible regression to the median for some patients.57 We attempted to lessen the chances of this possibility by verifying seizure frequency without any changes in ASDs for 3 months before entering the trial. Longer trials in the future would help to counteract this phenomenon. Although no significant differences were observed in ASD concentrations between CBD and placebo administration and all patients received consistent doses of ASDs (unless used as a rescue treatment), large fluctuations in serum concentrations of all ASDs still occurred during the trial and likely related to normal variation of serum concentrations over time. This finding is an unavoidable limitation in our study design. It is also uncertain how subtle differences in CBD oil analysis among batches could have affected seizure control (Table S1). Batches with detectable tetrahydrocannabidiol would have led to the administration of approximately 2 mg/day in a 10 kg dog. It is unclear if such an amount could have affected seizure control. In addition, this situation emphasizes the difficulty in maintaining consistency in a full spectrum product derived from plants.
Post hoc analysis of the final number of dogs included in the trial indicated a power of 71.3% relating to total seizures and 93% relating to seizure days. This calculation suggests that the study was adequately powered to evaluate percentage change from the baseline of seizure days. However, our study was underpowered to detect the percentage change from the baseline of total seizures.
5 CONCLUSION
We showed that administration of 9 mg/kg/day of CBD achieved a 24.1% decrease in seizure days from baseline. This medication generally was well tolerated without moderate or severe AEs. There was no evidence that CBD had a drug interaction with any of the ASDs administered during the trial, but potential interactions between CBD and phenobarbital warrant continued investigation. Although our study did not establish a therapeutic plasma concentration of CBD, further investigation would be beneficial, especially considering the wide interindividual variability in pharmacokinetics of CBD in dogs.33, 36-41
Given the potential of an “entourage” effect with CBD-rich oil51 (oil containing other measurable phytocannabinoids and terpenes), evaluation of other mixtures of phytocannabinoids as well as other phytocannabinoids alone should be completed in the future. Dose escalation also may provide better seizure control given the results of previous studies in humans.19, 20
Cannabidol shows promise as an anticonvulsant and warrants further investigation. Care should be taken to monitor liver enzyme activity and bile acid concentrations when this drug is administered chronically to dogs.
ACKNOWLEDGMENT
Funding provided by the American Kennel Club Canine Health Foundation.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by Colorado State University (CSU) IACUC (Protocol #16-6790).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




