Alterations in serum concentrations of visfatin and betatrophin in dogs with hypothyroidism
Abstract
Background
Hypothyroidism in dogs is associated with obesity and altered lipid and carbohydrate metabolism. The adipokines, visfatin, and betatrophin, affect glucose tolerance. Betatrophin is involved in lipid regulation.
Hypothesis
Visfatin and betatrophin serum concentrations are altered in hypothyroid dogs.
Animals
Dogs with naturally occurring hypothyroidism (n = 25) and healthy dogs (n = 25).
Methods
Insulin, visfatin, and betatrophin serum concentrations were measured in all dogs and 19 of the hypothyroid dogs after 30 days of thyroxine treatment. Body condition score (BCS) was determined (1-9 scale).
Results
Visfatin concentrations were lower in hypothyroid compared with healthy dogs (mean, 95% confidence interval [CI]; 2.0 ng/mL, 1.2-3.3 vs 5.1 ng/mL, 3.3-7.8; P = .004) and increased post-treatment (3.1 ng/mL, 1.9-4.9 vs 2.6 ng/mL, 1.6-4.1; P = .05). Betatrophin concentrations were lower in lean to normal (body condition score [BCS], 3-5) hypothyroid dogs compared to lean to normal healthy dogs (52 pg/mL, 9-307 vs 597 pg/mL, 216-1648; P = .03), but were not different between overweight (BCS, 6-9) hypothyroid and healthy dogs (341 pg/L, 168-695 vs 178 pg/mL, 77-415; P = .26), and decreased post-treatment in overweight dogs (206 pg/mL, 87-488 vs 268 pg/mL, 112-640; P = .004). Visfatin concentrations were higher in overweight compared with lean to normal dogs (4.7 ng/mL, 3.3-6.6 vs 2.2 ng/mL, 1.2-4.2; P = .04). Betatrophin concentrations were positively correlated with BCS (r = .47, P = .02) and insulin concentrations (r = .48, P = .03) in hypothyroid dogs and negatively correlated with BCS (r = −.47, P = .02) and thyroid stimulating hormone concentrations (r = −.56, P = .01) in healthy dogs.
Conclusions and Clinical Importance
Hypothyroidism in dogs is associated with alterations in visfatin and betatrophin concentrations that partially resolve with thyroxine treatment.
Abbreviations
-
- BCS
-
- body condition score
-
- TSH
-
- thyroid stimulating hormone
-
- TgAA
-
- thyroglobulin autoantibody
1 INTRODUCTION
Hypothyroidism is a common endocrine disease in dogs.1, 2 It is associated with obesity and alterations in lipid and carbohydrate metabolism. Hyperlipidemia with increased concentrations of triglyceride and cholesterol is a main feature of the disease, occurring in 75% to 90% of hypothyroid dogs.1, 2 The occurrence of insulin resistance was suggested in dogs with experimentally-induced as well naturally occurring hypothyroidism.3-6 Adipose tissue secretes a variety of cytokines (ie, adipokines) that are involved in the pathophysiology of insulin resistance and lipid metabolism. Betatrophin and visfatin are 2 novel adipokines. Betatrophin is secreted primarily from the liver and adipose tissue. It stimulates glycogen synthesis and inhibits gluconeogenesis in cultured hepatocytes,7 and it also inhibits lipoprotein lipase in muscle, whereas it stimulates it in adipose tissue.8, 9 Visfatin is produced by various tissues in addition to adipose tissue, including liver and skeletal muscle. Its effect on glucose homeostasis is complex because it acts as an extracellular inflammatory mediator, and it also has an important insulin sensitizing effect,10 possibly by binding to the insulin receptor at a site distinct from insulin.11 Obesity results in alterations in adipokine secretion. Betatrophin concentrations are largely increased in obesity in rodents and humans,8, 12 whereas reports regarding visfatin in obesity are inconsistent.13 Most studies in humans describe increased visfatin concentrations,11, 14 whereas studies in mice show decreased adipose tissue expression.15 In dogs, visfatin has been investigated in mitral valve disease,16 pancreatitis,17 and diabetes mellitus,18 but the effect of obesity has not been reported. To the best of our knowledge, betatrophin has not been studied in dogs.
The pathophysiological role of thyroid hormone in regulation of betatrophin and visfatin is still not completely understood. Betatrophin mRNA was induced by thyroid hormone in cultured hepatocytes of humans,19 and its circulating concentrations were increased in human patients with hypothyroidism.20 A non-linear in vitro effect of thyroid hormone on visfatin secretion from adipocytes was reported, with stimulation of secretion by low concentrations and suppression by higher concentrations of triiodothyronine.11, 21 Clinical studies regarding visfatin to date have yielded controversial results. Circulating visfatin concentrations in human patients with hypothyroidism were either increased or similar to those of healthy controls, and either decreased or further increased after treatment.11
Because hypothyroidism in dogs is associated with alterations in lipid and carbohydrate metabolism, and these alterations are consistent with deranged betatrophin and visfatin activity, we hypothesized that hypothyroidism would be associated with dysfunction of betatrophin, visfatin or both independent of any potential effect of obesity and would be manifested by alterations in serum concentrations of these adipokines. Our primary aims were to: (1) Compare serum concentrations of betatrophin and visfatin between dogs diagnosed with hypothyroidism and healthy control dogs, and (2) Determine the effect of thyroid hormone replacement on serum concentrations of betatrophin and visfatin in hypothyroid dogs. Additional aims were to determine associations between body condition, and serum concentrations of visfatin and betatrophin.
2 METHODS
The study included 25 newly diagnosed hypothyroid dogs and 25 healthy dogs. The hypothyroid dogs were identified from samples submitted to a referral Veterinary Medical Laboratory. Thyroid evaluation included measurement of free thyroxine (FT4) and thyroid stimulating hormone (TSH; Canine Free T4 and Canine TSH, Diagnostic Products Corporation Immulite, Siemens, New Jersey).22-25 The referring veterinarian of each newly diagnosed hypothyroid dog identified at the referral laboratory was contacted and with the dog's owner consent, the medical record was reviewed and a fasted pretreatment serum sample was collected. Dogs were included in the study group if the following inclusion criteria were met: (1) a combination of decreased serum FT4 concentration (reference range, 0.6-3.0 ng/dL) and increased TSH serum concentration (reference range, 0.03-0.40 ng/mL),22, 24 (2) clinical suspicion of hypothyroidism and absence of other illness based on clinical signs and clinicopathologic findings, including CBC and serum biochemistry, and (3) available fasted blood samples before initiation of thyroid hormone replacement. The presence of lymphocytic thyroiditis was determined by measurement of thyroglobulin autoantibody (TgAA; Canine Thyroglobulin Auto-Antibody Enzyme Immunoassay, Oxford Laboratories Inc, Oxford, Michigan). Treatment with thyroxine (0.022 mg/kg PO q12h) was evaluated in 19 of the hypothyroid dogs after 30 days by measurement of post-pill total T4 (TT4, Cobas Intergra 400 plus, Roche Diagnostics, Mannheim, Germany) and TSH concentrations. The control group included healthy dogs selected from the population of client-owned dogs from veterinary practices served by the referral laboratory during the same time period that were approximately matched by sex to the hypothyroid dogs, and their ages and BCS (1-9 scale)26 were within similar ranges of those of the hypothyroid dogs. The dogs were determined to be healthy and euthyroid based on the absence of any clinical signs or clinicopathologic abnormalities (CBC and serum biochemistry), and FT4 and TSH results within the reference ranges. Dogs treated with corticosteroids, trimethoprim-sulfonamide, or phenobarbital during the preceding month were excluded from the study.
All healthy dogs were examined once and all hypothyroid dogs were examined once at the time of diagnosis whereas 19 of the hypothyroid dogs were examined again after 30 days of thyroid hormone replacement. At each examination, sex, age, weight, BCS, and medical history were recorded and fasted serum samples were collected. Body condition score was determined by the attending clinician in the hypothyroid dogs and by 1 of the authors in the healthy dogs. Sera were separated and immediately frozen at −80°C until analysis. Serum concentrations of triglycerides, cholesterol, and glucose, and activities of alkaline phosphatase and alanine aminotransferase were measured using a biochemistry analyzer (Cobas Intergra 400 plus, Roche Diagnostics, Mannheim, Germany). Serum concentrations of insulin, visfatin, and betatrophin were measured using commercially available canine (Insulin ELISA kit, Mercodia, Uppsala, Sweden,27 and Visfatin ELISA kit, Tsz Biosciences, USA16, 18) or human (Betatrophin ELISA kit, MyBiosource Catalog No.: MBS761140) specific assays. For validation of the betatrophin assay, serial dilutions of canine serum were prepared, and the observed curves paralleled their respective standard betatrophin curves for humans. Serum samples were diluted 1:2 for the betatrophin assay. The dynamic ranges of the assays were as follows: insulin, 1.15 to 173 mU/L; visfatin, 0.1 to 10 ng/mL; betatrophin, 28 to 3000 pg/mL. Intra- and inter-assay coefficients of variation were: 4% and 5% for insulin, 5% and 7% for visfatin, 8% and 10% for betatrophin, respectively. The assays were conducted using an absorbance microplate reader (Infinite F50, Tecan, Switzerland) and a microplate washer (Hydro Flex, Tecan, Switzerland).
2.1 Data analyses
Linear correlations were assessed using Spearman (BCS) or Pearson's (all other variables) correlation coefficient tests. Associations between hypothyroidism and serum concentrations of insulin, visfatin, betatrophin, triglyceride, cholesterol, and glucose, and serum activities of ALT and ALP were evaluated using a general linear model including BCS groups (lean to normal, BCS 3-5; overweight, BCS 6-9) and sex as additional factors, and age as an additional covariate to the disease group (healthy or hypothyroid). The additional factors and covariates were included to control for potential confounding of the relationship between hypothyroidism and the outcome variables. The effect of treatment on outcome variables in the hypothyroid dogs was evaluated using generalized estimating equations including time (baseline and post-treatment) as a factor and body weight as a covariate, which was added to control for potential confounding of the effect of treatment. Interaction effects between disease group (general linear model) or time (generalized estimating equations) and each of the additional factors and covariates were evaluated. Factors, covariates, and interaction terms with a P value ≥.10 were removed from the models. Results were reported separately for each variable for which a significant interaction (P < .10) was observed. In addition, associations between TgAA groups (negative, inconclusive, and positive) and serum concentrations of insulin, visfatin, or betatrophin were evaluated using a general linear model including BCS groups and sex as additional factors, and age as an additional covariate. Bonferroni correction was applied for 3 multiple post-hoc comparisons among TgAA groups. Natural logarithmic transformations of the measured analytes were used and the log-transformed variables had a normal distribution (as confirmed by P-P plots) and constant variances. Adjusted means and 95% confidence intervals (CI) are presented, unless indicated otherwise. Data were analyzed using SPSS 26.0 for Windows and P ≤ .05 was considered significant.
3 RESULTS
The hypothyroid group included 21 mixed breed dogs and 1 of each of the following: American Pit Bull terrier, Cavalier King Charles spaniel, Giant schnauzer, and Labrador retriever. The healthy dog group included 14 mixed breed dogs, 3 Golden retrievers, 3 Border collies, and 1 of each of the following: Asian shepherd, Australian shepherd, Belgian malinois, Cocker spaniel, and Yorkshire terrier. Distribution of the hypothyroid and healthy dogs among sex, age, and body condition groups is presented in Table 1. Age ranged between 4 and 15 years in the hypothyroid dogs (median, 6 years) and between 1 and 14 years in the healthy dogs (median, 6 years). Body condition score ranged between 3 and 8 in the hypothyroid dogs (median, 7) and between 4 and 9 in the healthy dogs (median, 6). Biochemical and hormonal characteristics of the study groups are presented in Table 2. Of the 19 hypothyroid dogs that were reevaluated 30 days post-treatment, TSH concentrations were sufficiently suppressed (<0.10 ng/mL) in 16 dogs, of which overdose was present in 3 dogs with TT4 concentrations higher than the desired concentration for the specific post-pill interval (7.9, 8.3, and 12.4 μg/dL), and thyroxine replacement was considered adequate in the remaining 13 dogs. Treatment was considered inadequate in 3 dogs with insufficiently suppressed TSH concentration (0.41, 0.54, and 0.99 ng/mL).
| Healthy | Hypothyroid | |
|---|---|---|
| Sex | ||
| Spayed female | 14 | 17 |
| Neutered male | 8 | 6 |
| Intact female | 2 | 1 |
| Intact male | 1 | 1 |
| Age | ||
| <6 years | 12 | 11 |
| 6-10 years | 11 | 8 |
| >10 years | 2 | 6 |
| BCS | ||
| 3-5/9 | 8 | 3 |
| 6-9/9 | 17 | 22 |
- Abbreviation: BCS, body condition score.
| Healthy dogs (n = 25) | Hypothyroid dogs | Reference range | ||
|---|---|---|---|---|
| Baseline (n = 25) | Post-treatment (n = 19) | |||
| ALP (U/L) | 35 (32) | 54 (85) | 47 (112) | 21-170 |
| ALT (U/L) | 34 (21) | 56 (67) | 42 (141) | 19-67 |
| Cholesterol (mg/dL) | 230 (56) | 595 (574) | 199 (75) | 135-361 |
| Triglycerides (mg/dL) | 52 (31) | 285 (268) | 110 (61) | 19-133 |
| Glucose (mg/dL) | 94 (12) | 94 (32) | 90 (23) | 64-123 |
| Total T4 (μg/dL) | NA | NA | 4.5 (5.6) | 1.3-4.0 |
| Free T4 (ng/dL) | 1.3 (0.8) | 0.1 (0.0) | NA | 0.6-3.0 |
| TSH (ng/mL) | 0.15 (0.12) | 1.25 (1.62) | 0.01 (0.07) | 0.03-0.40 |
- Note: Presented are medians (interquartile range) of the activities or concentrations as measured.
- Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; NA, not available; T4, thyroxine; TSH, thyroid stimulating hormone.
Betatrophin concentrations and BCS were positively correlated in the hypothyroid dogs (r = .47, P = .02) and negatively correlated (r = −.47, P = .02) in the healthy dogs. Insulin concentrations were positively correlated with BCS in the hypothyroid dogs (r = .44, P = .04, respectively), but not in the healthy dogs. Betatrophin concentrations were positively correlated with insulin concentrations in the hypothyroid dogs (r = .48, P = .03) and negatively correlated with TSH concentrations in the healthy dogs (r = −.56, P = .005). No significant correlations between insulin and visfatin were found. Free T4 was negatively correlated with BCS (r = −.84, P < .001) and positively correlated with glucose (r = .79, P = .001) in the hypothyroid dogs, but were not significantly correlated with concentrations of insulin, betatrophin, or visfatin in any of the groups. Thyroglobulin autoantibody results in the hypothyroid dogs were positively correlated with TSH concentrations (r = .53, P = .02). Insulin concentrations were positively correlated with concentrations of cholesterol (r = .45, P = .03) and triglycerides (r = .48, P = .02) in the healthy dogs, and to concentrations of TT4 in the hypothyroid dogs post-treatment (r = .53, P = .03). Cholesterol and triglyceride concentrations were positively correlated (r = .59, P = .003) in the hypothyroid dogs.
Serum concentrations of insulin were higher (P < .001) in the hypothyroid (30.9 mU/L, 21.5-44.4) compared with the healthy (7.2, 5.4-9.7) dogs, after adjustment for BCS groups. No significant (P = .09) difference was found in insulin concentrations between lean to normal (13.2 mU/L, 8.4-20.5) and overweight (18.6 mU/L, 14.6-23.6) dogs after adjustment for disease group (Figure 1A). No significant effects on insulin of age (P = .83) and sex (P = .79) were found. Concentrations of insulin in the hypothyroid dogs decreased (P < .001) post-treatment (18.8 mU/L, 15.2-23.4 vs 33.3 mU/L, 22.5-49.3). No significant effect of weight (P = .75) on insulin concentration was found. In separate analyses for BCS groups, serum concentrations of insulin were significantly higher in hypothyroid compared with healthy dogs in overweight (P < .001) but not in lean to normal (P = .16) dogs, and significantly decreased post-treatment in overweight (P < .001), but not in lean to normal (P = .29) dogs.
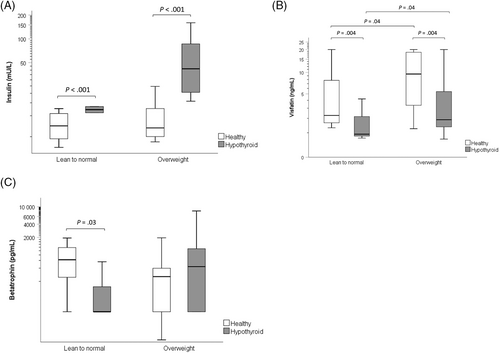
Serum concentrations of visfatin were lower (P = .004) in the hypothyroid (2.0 ng/mL, 1.2-3.3) compared with the healthy (5.1 ng/mL, 3.3-7.8) dogs, after adjustment for BCS groups, and higher (P = .04) in the overweight (4.7 ng/mL, 3.3-6.6) compared with the lean to normal (2.2 ng/mL, 1.2-4.2) dogs, after adjustment for disease group (Figure 1B). No significant effects of age (P = .59) or sex (P = .51) on visfatin concentrations were found. Concentrations of visfatin in the hypothyroid dogs increased (P = .05) post-treatment (3.1 ng/mL, 1.9-4.9 vs 2.6 ng/mL, 1.6-4.1). No significant effect of weight (P = .71) on visfatin concentration was found. In separate analyses for BCS groups, serum concentrations of visfatin were significantly lower in hypothyroid compared with healthy dogs in overweight (P = .02) but not in lean to normal (P = .21) dogs, and significantly increased post-treatment both in overweight (P = .02) and lean to normal (P < .001) dogs.
Significant (P = .04) interaction effect between disease and BCS groups was present in the analysis of betatrophin. Therefore, only separate analyses were performed for lean to normal and overweight dogs. In the lean to normal dogs, serum concentrations of betatrophin were lower (P = .03) in hypothyroid (52 pg/mL, 9-307) than in healthy (597 pg/mL, 216-1648) dogs, whereas the difference between hypothyroid (341 pg/L, 168-695) and healthy (178 pg/mL, 77-415) dogs was not significant (P = .26) in the overweight dogs (Figure 1C). No significant effects of age and sex were found on betatrophin concentrations in the separate analyses of BCS group (lean to normal, P = .42, P = .36; overweight, P = .73, P = .60, respectively). Concentrations of betatrophin decreased post-treatment in the overweight hypothyroid dogs (n = 16; 206 pg/mL, 87-488 vs 268 pg/mL, 112-640; P = .004). No significant effect of weight was found (P = .79). A significant (P < .001) interaction effect between time and weight in the analysis of the lean to normal dogs precluded further analysis of the effect of treatment on betatrophin concentrations in this group because the small number of lean to normal dogs did not allow stratification into weight groups.
Serum concentrations of triglycerides and cholesterol were higher in the hypothyroid compared with the healthy dogs (309 mg/dL, 232-411 vs 56 mg/dL, 42-74; P < .001 and 640 mg/dL, 543-755 vs 228 mg/dL, 194-268; P < .001, respectively) and decreased post-treatment (105 mg/dL, 86-128 vs 345 mg/dL, 232-513; P < .001 and 208 mg/dL, 184-236 vs 670 mg/dL, 528-852; P < .001, respectively). No significant differences were found between lean to normal and overweight dogs in serum concentrations of triglycerides (P = .54) and cholesterol (P = .18), and no significant effect of weight was observed (P = .53 and P = .26, respectively). Serum concentrations of glucose did not differ significantly between hypothyroid and healthy dogs (100 mg/dL, 88-113 vs 92 mg/dL, 82-105; P = .72), and did not significantly change post-treatment (94 mg/dL, 83-108 vs 100 mg/dL, 81-122; P = .60). No significant (P = .73) difference between lean to normal and overweight dogs in serum concentrations of glucose and no significant (P = .16) effect of weight was observed. In separate analyses for BCS groups, serum concentrations of triglycerides and cholesterol were higher in hypothyroid compared with healthy dogs both in overweight (P < .001 and P < .001, respectively) and lean to normal (P = .002 and P = .02, respectively) dogs, and significantly decreased post-treatment in overweight (P < .001 and P < .001, respectively) and lean to normal (P = .02 and P < .001, respectively) dogs. No significant differences were found in serum concentrations of glucose between hypothyroid and healthy dogs in overweight (P = .82) or lean to normal (P = .22) dogs, and no change post-treatment was observed (P = .42 and P = .60, respectively).
Serum activities of ALP and ALT were higher in the hypothyroid compared with the healthy dogs (75 U/L, 49-115 vs 40 U/L, 27-59; P = .03 and 69 U/L, 54-89 vs 33 U/L, 26-41; P < .001, respectively) and did not change significantly post-treatment (P = .28 and P = .79, respectively). In separate analyses for BCS groups, serum activities of ALP were higher in the hypothyroid compared with the healthy lean to normal dogs (P = .02) and decreased post-treatment (P = .004), but no difference was observed in overweight dogs (P = .16) and no change was detected post-treatment (P = .51), whereas serum activities of ALT were higher in hypothyroid compared with healthy overweight (P = .002) and lean to normal (P = .03) dogs, but did not change post-treatment (P = .90 and P = .29, respectively).
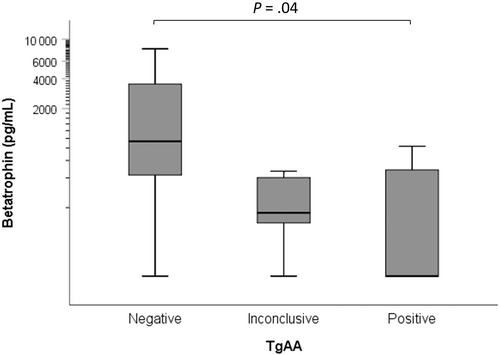
Thyroglobulin autoantibody results were available for 20 hypothyroid dogs, of which 8 were negative (<10%), 7 were positive (>25%), and 5 were inconclusive (10%-25%). Concentrations of betatrophin were significantly (P = .04) lower in dogs with positive TgAA results (126 pg/mL, 39-404) compared with those with negative TgAA results (945 pg/mL, 317-2819). Concentrations of betatrophin in dogs with inconclusive TgAA results (180 pg/mL, 45-715) were not significantly different from those with positive (P > .99) or negative (P = .19) TgAA results (Figure 2). No significant differences were found between dogs with positive, inconclusive, or negative TgAA results in concentrations of insulin (41.1 mU/L, 20.0-84.6, 26.1 mU/L, 11.8-57.6, or 42.0 mU/L, 21.5-81.8, respectively, P = .58) and visfatin (2.3 ng/mL, 0.8-6.4, 4.3 ng/mL, 1.4-13.1, or 2.5 ng/mL, 1.1-6.2, respectively, P = .66). No significant effects of BCS were identified in the analyses of betatrophin (P = .55), visfatin (P = .10), and insulin (P = .24).
4 DISCUSSION
We found decreased serum concentrations of visfatin in dogs with naturally occurring hypothyroidism, that partially resolved after thyroid hormone replacement for 30 days, as well as decreased serum concentrations of betatrophin in lean to normal hypothyroid dogs, and reduction in betatrophin concentrations in overweight hypothyroid dogs after thyroid hormone replacement for 30 days.
The decrease in concentrations of visfatin in the hypothyroid dogs might be the result of reduction in visfatin expression and secretion from ≥1 of its tissue sources, including adipose tissue and skeletal muscle. A nonlinear regulation of visfatin expression by T3 was demonstrated in adipocyte culture (3T3-L1), where visfatin expression was induced by T3 at concentrations up to 0.5 nmol/L, and suppressed at higher concentrations.21, 28 A potential positive effect of T3 on visfatin expression in dogs might support the possibility of a decrease in visfatin secretion from adipose tissue in hypothyroidism. In addition, visfatin is suggested to act as a myokine,29 and in chickens, its secretion is higher from skeletal muscle than from adipose tissue.30 Muscle atrophy associated with thyroid dysfunction in dogs31 might result in a decrease in visfatin secretion from skeletal muscle in hypothyroidism.
The finding of decreased visfatin concentrations in hypothyroid dogs is in contrast to reports from most studies in human hypothyroid patients, where visfatin concentrations were found to be increased.28, 32-35 One potential explanation for this discrepancy is the fact that whereas most cases of hypothyroidism in humans result from autoimmune thyroiditis,36 that disease accounts for only approximately 50% of the cases in dogs.37 Indeed, contrary to most studies in humans and similar to the findings in the dogs of our study, decreased visfatin concentrations were reported in a study on hypothyroidism that resulted from thyroidectomy in human patients with differentiated thyroid cancer.38 In addition, visfatin concentrations were positively correlated with autoimmunity in human patients with thyroiditis,38 whereas no association between visfatin and thyroglobulin autoantibody was found in dogs in our study. Therefore, it is possible that the increase in visfatin in patients with lymphocytic thyroiditis results from the inflammatory disease rather than thyroid dysfunction. Another possible explanation might be species differences in visfatin origin as a myokine or an adipokine, with potentially higher secretion from skeletal muscle than adipose tissue in dogs compared with humans. Visfatin expression in skeletal muscle in dogs was found to be second highest after the liver, although adipose tissue expression was not reported in this study.39 Nevertheless, the higher visfatin concentrations in overweight compared with lean to normal dogs, similar to findings in obesity in humans,14 imply adipose tissue as a source for visfatin secretion in dogs as well. Concentrations of visfatin increased after 30 days of thyroid hormone replacement, but did not reach the concentrations in the healthy dogs, suggesting this period of time was insufficient to completely resolve the alterations attributed to hypothyroidism. This conclusion might be supported by the observation that other biochemical alterations had not completely resolved in some of the dogs within 30 days of treatment and the fact that not all treated dogs were euthyroid after 30 days.
Differential alterations in betatrophin concentrations were present in hypothyroid dogs of various body condition. The findings of decreased betatrophin in lean to normal hypothyroid dogs are in agreement with the reported induction of betatrophin mRNA expression by thyroid hormone in hepatocytes in vitro (HepG2 cells).40 Nevertheless, the finding suggesting increased betatrophin concentrations in overweight hypothyroid dogs, although it did not reach significance, might be consistent with current reports of increased serum betatrophin concentrations in human patients with overt and subclinical hypothyroidism.20, 41 The significant decrease in betatrophin concentration with thyroid hormone replacement supports this finding. An explanation for the potential opposite alterations in betatrophin in lean to normal compared with overweight dogs is not apparent, but potentially could lie in the effect of obesity on betatrophin. Obesity and insulin resistance are associated with increased betatrophin in most studies in humans and rodents,8 and positive associations between betatrophin and BCS and insulin concentrations also were present in the hypothyroid dogs in our study. It is possible that the negative effect of thyroid dysfunction on betatrophin was apparent in the lean to normal dogs, but the positive effects of obesity and insulin resistance on betatrophin prevailed in the overweight dogs. The results of the lean to normal dogs in our study should be interpreted with caution, because only 3 hypothyroid dogs were included and therefore any misclassification might markedly affect the results. In addition, although unlikely, the presence of a concurrent disease cannot be completely ruled out in a thin hypothyroid dog.
The negative association between betatrophin concentrations and BCS in the healthy dogs is different from reported increased betatrophin in obesity in most reports in humans,12 although decreased concentrations in obesity or no difference between lean to and obese individuals were reported in a few studies in humans,42, 43 and it was suggested that this heterogeneity partially could be explained by glycemic status.12 In non-obese human subjects, betatrophin concentrations were increased with impaired glucose tolerance,44 suggesting the increase in betatrophin in obesity might be attributed, at least partially to glucose intolerance. Therefore, the different alteration in betatrophin in obesity in dogs might be attributed to the fact that obese dogs do not become glucose intolerant despite long-standing insulin resistance.45
Concentrations of betatrophin were lower in dogs with positive TgAA results, indicative of the presence of lymphocytic thyroiditis, compared with those with negative TgAA results. Betatrophin is considered unrelated to autoimmunity in humans with hypothyroidism, as its circulating concentrations are reported to be increased in overt and subclinical patients, but not in those with positive antibody accompanied by normal thyroid function.20 Whereas lymphocytic thyroiditis occurs in about approximately 50% of all hypothyroid dogs,37 it accounts for practically all cases in human patients.36 Accordingly, all of the hypothyroid human patients in that study had lymphocytic thyroiditis and the effect of thyroid dysfunction on betatrophin was demonstrated. In our study, lymphocytic thyroiditis was diagnosed in about 35% the dogs, and additional 33% had inconclusive results. Although the absence of autoantibodies usually is associated with thyroid atrophy, it also might indicate a late stage of lymphocytic thyroiditis.37 Therefore, betatrophin concentrations could potentially be higher with a disease that is more severe or of longer duration, in agreement with the positive association of betatrophin with disease severity in human hypothyroid patients.20
Concentrations of insulin were higher in the hypothyroid dogs, similar to a previous report in dogs with naturally occurring hypothyroidism.6 Experimentally-induced hypothyroid dogs were shown to be markedly insulin resistant, whereas glucose tolerance was maintained by increased insulin secretion, and it was suggested that increased concentrations of growth hormone and insulin-like growth factor-1 are contributing factors.5 The positive association between concentrations of betatrophin and insulin in the hypothyroid dogs suggests that betatrophin also might play a role in insulin resistance. This finding is in agreement with reports of increased betatrophin in conditions associated with insulin resistance in human.46, 47 Over-expression or knockdown of betatrophin was demonstrated to impair or improve insulin signaling in hepatocytes, respectively.46 Our study also demonstrated a decrease in insulin concentrations that was accompanied by a decrease in betatrophin concentrations after thyroid hormone replacement, suggesting at least partial recovery of insulin sensitivity. In view of the improvement in insulin sensitivity in the hypothyroid dogs with treatment, the positive association between insulin and thyroxine concentrations post-treatment was unexpected. However, because therapeutic concentrations of thyroxine are dependent on absorption and post-pill time interval, measured thyroxine concentrations might not correctly represent the overall adequacy of treatment.
As expected, hyperlipidemia in the hypothyroid dogs resolved in most dogs with thyroid hormone replacement. Although this finding was concurrent with partial resolution of the alterations in visfatin and betatrophin concentrations, a causal role for these adipokines in hypothyroidism-associated hyperlipidemia in dogs cannot be supported because no correlations between the adipokines and lipids were identified in our study. In contrast, a study on human hypothyroid patients identified a positive association between concentrations of betatrophin and concentrations of cholesterol and triglycerides, and it was suggested that betatrophin mediated the occurrence hyperlipidemia in hypothyroidism by inhibition of lipoprotein lipase.41
Our study had some limitations. First, the small number of dogs might have prevented identification of significant differences and associations on one hand, and on the other hand, any misclassification might have had a substantial impact on the results, and potential spurious results might have occurred. Second, the BCS system that was used as an indicator of body condition is subjective and may not accurately account for visceral adiposity, which is considered a major source for visfatin in other species. Third, only dogs with increased TSH were included in the hypothyroid group, although TSH within the reference range is expected in approximately 30% of all hypothyroid dogs. Therefore, an important fraction of the hypothyroid dog population was not included. In addition, free T4 was measured using an analogue immunoassay rather than by equilibrium dialysis.48 Fourth, a bias because of inclusion of multiple dog breeds cannot be ruled out. And lastly, as a cross-sectional study, cause and effect relationships cannot be determined.
In conclusion, compared with healthy dogs, visfatin concentrations are lower in hypothyroid dogs, whereas betatrophin concentrations are lower in lean to normal hypothyroid dogs and might be higher in overweight hypothyroid dogs. Post-treatment, concentrations of visfatin increase, whereas concentrations of betatrophin decrease, concurrent with a decrease in insulin concentrations. These alterations might play a role in insulin resistance and hyperlipidemia associated with hypothyroidism in dogs, and further investigation is warranted to determine the potential benefit of therapeutic modulation of these adipokines.
ACKNOWLEDGMENT
Supported by a grant from the Clinical Studies Fund of the Veterinary Teaching Hospital, The Hebrew University of Jerusalem.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the institutional ethics review committee of The Hebrew University of Jerusalem (reference number: HU-NER-2020-030_A).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




