Updated ACVIM consensus statement on leptospirosis in dogs
Consensus Statements of the American College of Veterinary Internal Medicine (ACVIM) provide the veterinary community with up-to-date information on the pathophysiology, diagnosis, and treatment of clinically important animal diseases. The ACVIM Board of Regents oversees selection of relevant topics, identification of panel members with the expertise to draft the statements, and other aspects of assuring the integrity of the process. The statements are derived from evidence-based medicine whenever possible and the panel offers interpretive comments when such evidence is inadequate or contradictory. A draft is prepared by the panel, followed by solicitation of input by the ACVIM membership which may be incorporated into the statement. It is then submitted to the Journal of Veterinary Internal Medicine (JVIM), where it is edited before publication. The authors are solely responsible for the content of the statements. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC), the International Renal Interest Society (IRIS), or the International Society for Companion Animal Infectious Diseases (ISCAID).
[Correction added after first online publication on 17 November 2023. “Supportive” and “Confirmatory” headers were switched in section 3.1.2. SPECIFIC LABORATORY CRITERIA and article title has been corrected.]
Abstract
Since publication of the last consensus statement on leptospirosis in dogs, there has been revision of leptospiral taxonomy and advancements in typing methods, widespread use of new diagnostic tests and vaccines, and improved understanding of the epidemiology and pathophysiology of the disease. Leptospirosis continues to be prevalent in dogs, including in small breed dogs from urban areas, puppies as young as 11 weeks of age, geriatric dogs, dogs in rural areas, and dogs that have been inadequately vaccinated for leptospirosis (including dogs vaccinated with 2-serovar Leptospira vaccines in some regions). In 2021, the American College of Veterinary Internal Medicine (ACVIM) Board of Regents voted to approve the topic for a revised Consensus Statement. After identification of core panelists, a multidisciplinary group of 6 experts from the fields of veterinary medicine, human medicine, and public health was assembled to vote on the recommendations using the Delphi method. A draft was presented at the 2023 ACVIM Forum, and a written draft posted on the ACVIM website for comment by the membership before submission to the editors of the Journal of Veterinary Internal Medicine. This revised document provides guidance for veterinary practitioners on disease in dogs as well as cats. The level of agreement among the 12 voting members (including core panelists) is provided in association with each recommendation. A denominator lower than 12 reflects abstention of ≥1 panelists either because they considered the recommendation to be outside their scope of expertise or because there was a perceived conflict of interest.
Abbreviations
-
- ACVIM
-
- American College of Veterinary Internal Medicine
-
- AKI
-
- acute kidney injury
-
- ALP
-
- alkaline phosphatase
-
- ALT
-
- alanine aminotransferase
-
- aPTT
-
- activated partial thromboplastin time
-
- ARDS
-
- acute respiratory distress syndrome
-
- CBC
-
- complete blood count
-
- CDC
-
- Centers for Disease Control and Prevention
-
- CK
-
- creatine kinase
-
- CKDu
-
- chronic kidney disease of uncertain etiology
-
- COVID-19
-
- coronavirus disease of 2019
-
- CT
-
- computed tomography
-
- DGGR
-
- 1,2-o-dilauryl-rac-glycero-3-glutaric acid-(6′-methylresorufin) ester
-
- DNA
-
- deoxyribonucleic acid
-
- ECG
-
- electrocardiogram
-
- EDTA
-
- ethylenediaminetetraacetic acid
-
- EKST
-
- extracorporeal kidney support therapy
-
- IRIS
-
- International Renal Interest Society
-
- ISCAID
-
- International Society for Companion Animal Infectious Diseases
-
- LOA
-
- level of evidence
-
- LPHS
-
- leptospiral pulmonary hemorrhage syndrome
-
- LPS
-
- lipopolysaccharide
-
- MAT
-
- microscopic agglutination test
-
- NAAT
-
- nucleic acid amplification test
-
- OR
-
- odds ratio
-
- PCR
-
- polymerase chain reaction
-
- PT
-
- prothrombin time
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus 2
-
- SDMA
-
- symmetric dimethyl arginine
-
- SDS-PAGE
-
- sodium dodecyl sulfate-polyacrylamide gel electrophoresis
-
- ST
-
- sequence type
-
- SWGA
-
- selective whole genome amplification
-
- UPC
-
- urine protein-to-creatinine ratio
1 INTRODUCTION AND EPIDEMIOLOGY
Leptospirosis is caused by the spirochete Leptospira, a zoonotic bacterial pathogen that infects a wide variety of mammals and poikilothermic animals worldwide.1-12 Pathogenic leptospires can be serotyped into over 300 different serovars based on their outer lipopolysaccharide antigens.13 Serovars are organized into antigenically related serogroups. However, serogroup classification is confusing because the same serovar can be found in multiple different species, and each serogroup often contains a serovar with the same name (eg, serovar Grippotyphosa, serogroup Grippotyphosa). Therefore, there has been a move to classify Leptospira strains based on DNA sequence composition (sequence types [STs]).13, 14 Based on sequence information, the 68 known species15 of Leptospira are grouped into 2 pathogenic subclades, P1 (Pathogens 1, pathogenic species) and P2 (Pathogens 2, intermediately pathogenic), and 2 saprophytic subclades S1 and S2.13 The P1 subclade is further divided into “high virulence” and “low virulence” pathogenic species. Most leptospirosis in dogs results from infections by P1-virulent species such as Leptospira interrogans, Leptospira borgpetersenii, and Leptospira kirschneri, although occasionally P2 species have been associated with severe disease in dogs.16
Infections with Leptospira spp. occur when mucous membranes or abraded skin are exposed to pathogenic strains that are shed from the renal tubules of infected reservoir hosts. Worldwide, the most important reservoir hosts are rodents, especially Rattus norvegicus, in which a worldwide prevalence of infection of 30% has been identified, with prevalence exceeding 80% in some regions.17 Other wildlife and domestic animal species are also important in the epidemiology of disease (LOA 12/12). Organisms contaminate soil and water and can remain viable in the environment for weeks to months when conditions are optimal.18 Biofilm formation contributes to the ability of the spirochete to persist in the environment and in renal tubules of reservoir hosts.19, 20 The spirochete can replicate in water-saturated soil,18 which might contribute to accumulation of organisms in the environment. Leptospirosis is especially prevalent in regions with higher annual rainfall and warm climates. To some extent, seasonal leptospirosis incidence varies geographically depending on local rainfall patterns and periods of freezing temperatures that decrease spirochete viability.8, 21 However, because transmission also occurs after direct contact with reservoir hosts, including predation, after bite wounds, and through venereal and placental transfer, disease can occur in urban regions, and at times of year when organisms survive poorly in the environment.8, 22-25 The presence of backyard poultry, composting, or poor sanitation (eg, hoarding), can increase rodent populations and exposure risk to leptospires outside and inside homes. Outbreaks also have been recognized in regions with semi-arid climates in association with dog daycare or kennel environments,26-28 possibly because of direct dog-to-dog or rodent-to-dog transmission. All dogs are at risk of leptospirosis, regardless of signalment, geographic location, lifestyle, and the time of year (LOA 12/12).
There is widespread serologic evidence of infection of domestic cats by Leptospira spp., but based on rare reports of clinical disease, cats are considered disease-resistant when compared with other animal species.29, 30 Pathogenic leptospires have been detected in the urine of up to 20% of apparently healthy cats using PCR and culture,31-34 and thus cats may act as reservoir hosts. However, other studies worldwide have shown lower prevalence of leptospiruria (0% to 5%), even in free-roaming cats.35, 36 Cats may be an under-recognized source of pathogenic leptospires in some regions and should be considered in One Health investigations that employ sequence typing methods to advance knowledge of disease epidemiology (LOA 11/11).
Because immunity induced by vaccination with current Leptospira bacterins is serogroup-specific, knowledge of serogroups that commonly cause disease within a particular geographic region remains important for vaccine design. After introduction of 4-serovar vaccines in North America in the mid-2000s and subsequently in Europe and South America, leptospirosis has been recognized predominantly in unvaccinated dogs or dogs vaccinated with 2-serovar vaccines.6, 37 Although traditionally the dog has been considered the reservoir host for serovar Canicola, this paradigm has been challenged by the identification of chronic subclinical infections in dogs with other serovars, the detection of serovar Canicola in dogs with acute disease, and the identification of other host species, such as rodents, horses, and pigs, as the source of serovar Canicola strains.17, 38, 39 In addition, 1 model indicated that rodents served as the main source of environmental contamination even when dog-adapted strains were present in a population of humans, rodents, and free-roaming and owned dogs.40 Given this observation, until additional evidence is available, dogs should not be assumed to be the reservoir host when serovar Canicola is identified or suspected as the cause of disease (LOA 12/12). Because the pattern of seroreactivity identified by microscopic agglutination test (MAT) serology does not reliably predict the infecting serogroup,41 and many STs belong to a serogroup, accurate identification of potential sources of infection requires sequence typing. For example, a combination of sequence typing and serotyping was used to identify black rats as the likely source of infection of a dog in Japan with L. interrogans serogroup Australis.42
2 PATHOPHYSIOLOGY AND CLINICAL MANIFESTATIONS
After entering the host through mucous membranes, skin lesions, or macerated skin, pathogenic leptospires enter the bloodstream and rapidly disseminate throughout the body.43 In circulation, leptospires use multiple strategies to evade innate immune recognition and innate host defense and killing mechanisms.44 Utilizing corkscrew motion, leptospires efficiently invade host tissues at gel-liquid borders such as vascular walls,45 emigrating from the vascular space by binding to vascular endothelial cadherin and weakening endothelial cell barriers.46 Breakdown of the endothelial cell barrier also may contribute to the development of leptospiral pulmonary hemorrhage syndrome (LPHS) in dogs and other host species.47-49 The molecular pathogenesis of leptospirosis has been reviewed recently.46 Although the most prominent manifestations of leptospirosis reflect acute tubulointerstitial nephritis and liver dysfunction, the disease is multisystemic, and many other organs are affected (Table 1; LOA 11/11).1, 50 Associations between infecting strain and clinical manifestations of disease have not been clearly identified.51 Kidney and hepatic manifestations usually occur together, but occasionally are recognized in isolation.52 Liver dysfunction results from disruption of hepatocyte intercellular junctions by spirochetes, with leakage of bile into the circulation which is reflected biochemically as a cholestatic hepatopathy. Pathogenic leptospires have direct cytotoxic effects on platelets, with platelet destruction and de-adherence noted in vitro.53 Aseptic meningitis has been described in up to 25% of humans with leptospirosis,54 and appears to occur in dogs.55 Histopathological lesions of LPHS lung tissue are characterized by various degrees of intra-alveolar hemorrhage in the absence of a marked inflammatory cell infiltrate or vasculitis. Intra-alveolar edema, fibrin, and hyaline membranes, which are characteristic of disorders with diffuse alveolar damage such as acute respiratory distress syndrome, also can be present, but are not a predominant feature.1 Although the pathogenic mechanisms of LPHS are poorly understood, they likely are multifactorial, with both host- and pathogen-related factors playing a role.56
| Organ involvement | Possible clinical signs | Diagnostic investigation |
|---|---|---|
| Acute kidney injury (tubulointerstitial nephritis) | Vomiting, diarrhea, dehydration, lethargy, inappetence, polyuria, polydipsia, oliguria, anuria, abdominal pain | Azotemia, electrolyte abnormalities, isosthenuria, glucosuria, proteinuria, pyuria, cylindruria, hyperechoic renal cortices |
| Cholestatic hepatopathy | Vomiting, diarrhea, dehydration, lethargy, inappetence, icterus | Increased liver enzymes, hyperbilirubinemia, hypoalbuminemia |
| Leptospiral pulmonary hemorrhage syndrome | Tachypnea, hemoptysis, increased breath sounds | Anemia, hypoxemia, diffuse or patchy interstitial to alveolar patterns |
| Coagulopathy | Petechiae, ecchymoses, hematuria, melena, hematemesis, epistaxis | Anemia, hypoalbuminemia, thrombocytopenia, hyperfibrinogenemia |
| Vasculitis | Peripheral edema, mild ascites, pleural effusion | Pleural effusion, mild ascites/retroperitoneal fluid |
| Pancreatitis | Vomiting, diarrhea, abdominal pain | Hyperbilirubemia, increased liver enzyme activities, increased canine pancreas-specific lipase activity, increased DGGR lipase |
| Ocular involvement | Uveitis, conjunctivitis, retinal hemorrhages | Fundoscopic examination |
| Myocarditis | Cardiac arrhythmias | Increased serum troponin, ECG abnormalities |
| Enteritis | Vomiting, diarrhea, abdominal pain | Thickened intestinal walls, evidence of intestinal intussusception |
| Myositis | Reluctance to move | Increased CK activity |
| Reproductive tract | Abortion, infertility | |
| Skin | Calcinosis cutis |
Once the host mounts an acquired immune response, leptospires are cleared from the blood but may persist as biofilm in the eye57 or the renal tubules.19 Progression of tubulo-interstitial nephritis to renal fibrosis has been described in dogs.58 Leptospira infection has been associated with acute interstitial nephritis of uncertain etiology in humans, which may be a precursor to chronic kidney disease of uncertain etiology.59 More evidence is needed before ascribing a causative role to leptospires in chronic hepatitis in dogs in the absence of kidney disease (LOA 11/11).60, 61
The initial febrile phase of the disease is often non-specific. Some dogs may be evaluated by veterinarians in this phase, before there is biochemical evidence of organ dysfunction. Affected dogs may show inappetence, lethargy, vomiting, increased thirst and urination because of non-oliguric renal dysfunction, fever, or some combination of these signs. Because leptospirosis can progress rapidly to acute kidney injury (AKI), the disease should be considered in dogs with acute onset of febrile illness, especially if unvaccinated for leptospirosis. The owners of such dogs should be informed that clinical re-assessment and biochemical testing are recommended should their dog's condition fail to improve within 24 hours. If the regional incidence of leptospirosis is high or the history otherwise supports the possibility of leptospirosis, nucleic acid amplification testing (NAAT) should be offered (see Section 3). Based on studies in humans,62, 63 empirical treatment with doxycycline for 7 days also could be considered, but more evidence is needed to support the latter recommendation to optimize antimicrobial stewardship (LOA 11/11).
Clinicopathologic alterations observed in subsequent phases of the disease reflect its multisystemic nature, including direct organ injury and secondary complications such as aspiration pneumonia, pancreatitis, intestinal bacterial translocation, or sepsis. Observed changes are influenced further by the timing of presentation, the severity of the disease, and previous treatments.
The most common CBC findings include neutrophilia (27%-94%), increased band neutrophils (3%-81%), lymphopenia (2%-29%), monocytosis (29%-68%), thrombocytopenia (14%-73%), and mild to moderate, non-regenerative anemia (18%-92%).48, 64-79 Uncommonly, severe anemia occurs, which may follow gastrointestinal or pulmonary hemorrhage.80 Serum biochemistry alterations48, 64-68, 71-79 reflect organ dysfunction and may show different profiles depending on the geographic origin or the timing of presentation of reported cases. Acute kidney injury is associated with increased blood urea nitrogen (54%-100%), creatinine (55%-100%), and phosphate (42%-100%) concentrations. Hepatic injury manifests as increased liver enzyme activities, dominated by increased ALP (19%-94%), and to a lesser extent increased ALT (22%-87%) and AST (28%-69%). Hyperbilirubinemia (15%-94%) typically occurs in the absence of other clinicopathologic signs of liver failure. Mild to moderate hypoalbuminemia frequently is observed in dogs with leptospirosis (18%-88%). C-reactive protein has been moderately increased in all dogs with Leptospira-induced AKI,72, 77 but this was not different from dogs with AKI from other causes.72 Electrolyte abnormalities may relate to gastrointestinal or kidney dysfunction and may be aggravated by direct inhibition of the tubular Na+-K+-ATPase by leptospiral endotoxin.81 Frequently reported electrolyte abnormalities include hyperkalemia (12%-53%), hypokalemia (17%-41%), hyponatremia (12%-64%), and hypochloremia (12%-46%).64, 66, 71-73, 76, 77 In humans, leptospires usually induce hypokalemic, non-oliguric AKI with impaired tubular sodium reabsorption,82 and the same may occur in dogs. However, severe hyperkalemia may be encountered in dogs with oliguric or anuric AKI. In these dogs, hyperkalemia often is a limiting factor for successful conservative management. Serum creatine kinase activity was increased in 44% of dogs with leptospirosis, suggesting myositis.77 Increased serum troponin I concentration in 69%-94% of dogs supports the presence of myocardial injury.77, 83 Its concentration was associated with frequency of cardiac arrhythmias but not with outcome, and it did not differ from dogs with AKI of other etiologies (83%), suggesting an indirect complication of AKI rather than a direct organ manifestation of leptospirosis.83 Similarly, increased amylase and lipase activities have been reported in 40%-77% and 19%-100% of dogs with leptospirosis, respectively.66, 73, 77 These nonspecific changes may reflect pancreatitis, gastroenteritis, or decreased renal elimination. The more specific serum DGGR-lipase activity (which is increased in 29% of dogs with AKI) seems to be a feature of AKI rather than leptospirosis; it was not different between dogs with and without infectious causes of AKI.84
Urinalysis in dogs with leptospirosis typically shows isosthenuria, but hyposthenuria also has been reported.66, 67, 77, 79 Renal glucosuria (18%-83%) and cylindruria (8%-67%) are specific indicators of tubular damage.48, 66, 67, 71, 73-75, 77, 79 Proteinuria is reported in most dogs with leptospirosis (28%-81%) and its magnitude is usually mild to moderate (urinary protein-to-creatinine ratio [UPC] < 5), although UPCs of up to 20 have been reported.48, 77 Sodium dodecyl-sulfate polyacrylamide gel electrophoresis of urinary proteins suggests that the predominant mechanism of proteinuria in dogs with leptospirosis is defective tubular reabsorption of low molecular weight proteins (tubular proteinuria, 100%), accompanied by glomerular loss of high molecular weight proteins caused by altered permselectivity characteristics in 60%-90% of dogs (glomerular proteinuria).77, 85 Other changes observed on urinalysis include occasional mild pyuria (0%-100%), hematuria (17%-74%), and bilirubinuria (20%). Leptospires are not usually visible in the urine sediment using routine light microscopy, because the width of leptospires is below the resolution of light microscopy, but large numbers of small, faintly stained organisms were visualized in urine sediment from 1 dog.86
Evaluation of hemostasis in dogs with leptospirosis indicates hemostatic abnormalities in up to 83% and disseminated intravascular coagulation in 6%-44% of dogs with leptospirosis.48, 73-75, 77, 87 In addition to thrombocytopenia, the most common abnormalities were hyperfibrinogenemia (43%-75%), hypofibrinogenemia (20%), prolonged prothrombin time (6%-33%), prolonged activated partial thromboplastin time (6%-83%), low antithrombin concentration (94%), and increased D-dimer concentrations (39%-63%). Thromboelastometric evaluation showed hypercoagulable profiles in 14 dogs (40%) and hypocoagulable profiles in 7 affected dogs (20%).87
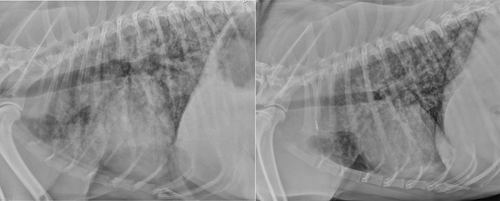
Radiographic changes indicative of the severe pulmonary form of leptospirosis with hemorrhages (LPHS) typically develop bilaterally in the caudodorsal lung fields as a mild interstitial pattern, progressing to a reticulonodular pattern, and to focal or generalized alveolar infiltrates (Figure 1).1, 48, 88, 89 Marked abnormalities can be seen on radiographs before the occurrence of recognizable respiratory impairment. Thoracic radiography is recommended in all dogs suspected to have leptospirosis, even in the absence of respiratory signs, because radiographic findings consistent with LPHS can aid in diagnosis of leptospirosis and should prompt judicious fluid therapy and close monitoring of respiratory function (LOA 10/10). Repeated thoracic computer tomographic evaluation has shown the highly dynamic nature of the pulmonary lesions over time and a tendency to underestimate the lesion type and their severity using radiography.90 Other radiographic changes include pleural effusion from fluid overload or a lobar alveolar pattern consistent with aspiration pneumonia.
Abdominal ultrasound examination often identifies abnormalities associated with the affected organ systems.71, 91-93 Reported kidney changes include renomegaly, cortical and sometimes medullary hyperechogenicity, decreased corticomedullary definition, mild pyelectasia, a medullary band of hyperechogenicity, and perirenal fluid accumulation. Changes of the hepatobiliary system include hepatomegaly, diffuse hepatic hypoechogenicity, and thickening of the gallbladder wall. Peritoneal effusion, enlargement and hypoechogenicity of the pancreas, thickening of the gastric and less commonly intestinal wall, intestinal intussusception, splenomegaly with mottled echotexture, and abdominal lymphadenomegaly also may be observed.
3 DIAGNOSIS
Leptospirosis should be considered a differential diagnosis in any dog evaluated for AKI, and especially when accompanied by hepatic dysfunction or evidence of pulmonary hemorrhage (see Case Definition). Although leptospirosis has been reported in dogs properly vaccinated using 4-serovar leptospirosis vaccines,94 other differential diagnoses should be considered more likely in adequately vaccinated dogs (LOA 11/11). Leptospirosis should be considered in cats with AKI, ≥1 additional clinicopathologic findings suggestive of a systemic infection, and no other explanation for their clinical signs (LOA 10/10).
Specific diagnosis of leptospirosis is based on clinical suspicion together with the results of serologic tests and NAATs. Because all available diagnostic tests have limitations, application of a combination of serologic assays and organism detection tests is recommended to optimize diagnosis of leptospirosis (LOA 12/12).
The reference standard test for diagnosis of leptospirosis remains acute and convalescent serologic testing using the MAT. The report to the clinician lists the serovars tested and the serum titer at which 50% of organisms agglutinate as observed using darkfield microscopy. Veterinary diagnostic laboratories typically include a limited number (6 to 7) serovars. In contrast, panels used in reference laboratories for humans may include >30 different serovars, and it has been recommended that laboratories include both recent and locally circulating serovars.95 Assays that include a large number of serovars are more sensitive, but more laborious to perform. The incubation period for leptospirosis is approximately 2 to 14 days, and in the first week of illness, titers are negative or low because of insufficient time for production of detectable antibody. Antimicrobial treatment early in the course of illness may suppress antibody production.95 Thus, the sensitivity of a single acute MAT titer >1:800 in dogs has been estimated at only 50%.96, 97 Conversely, unless very high (>1:3200), a single positive MAT titer lacks specificity and high titers in the first few days of illness should raise suspicion for recent previous vaccination, recent subclinical exposure, or longer duration of illness caused by Leptospira spp. infection than recognized by the owner. Such a situation should prompt consideration of alternative causes of illness. Titers can persist for at least 1 year after natural infection, and in 1 study, generally decreased by 4 months after vaccination.98 Post-vaccinal titers may persist for longer and be maintained at high levels (≥1:1600), including titers to non-vaccinal serovars, if ongoing exposure to field strains occurs.98 Diagnosis of leptospirosis based on a single positive MAT test result is not recommended, and leptospirosis should not be ruled out based on a single negative test, especially when MAT includes a limited number of serovars (eg, 6 to 7 serovars; LOA 12/12). Although seroconversion (≥4-fold change in titer) can occur as early as 3 to 5 days after dogs are presented to a veterinarian, an interval of 7 to 14 days is recommended between acute and convalescent phase samples to identify seroconversion (LOA 12/12).
Serological results should not be used to make firm conclusions about the infecting serovar because of paradoxical serologic cross-reactivity, especially early in the course of illness, and the potential for absence of the infecting serovar in the panel.14, 41, 95, 99 Interlaboratory variation in MAT results and challenges associated with maintenance of cultures over time further complicate this issue. Inclusion of more serovars in a panel is recommended to increase the sensitivity of the assay, not to aid in identification of the infecting serovar. To promote quality assurance for MAT testing, practitioners should submit samples to laboratories that participate in the International Leptospirosis Society's Leptospira proficiency testing platform (LOA 11/11).100
Point-of-care serologic tests based on lateral flow technology are available for rapid detection of antibodies to pathogenic leptospires in dogs.101 Studies from different geographic regions have yielded variable sensitivities and specificities for these assays in a clinical setting (Table 2), and positive and negative predictive values also will vary based on the estimated prevalence of Leptospira exposure in the tested dog population. As with MAT, these assays may be negative because of the lag in antibody production, or positive because of recent subclinical exposure or vaccination. The SNAP Lepto (IDEXX Laboratories, Inc, Portland, Maine) assay detects antibodies to the Leptospira membrane protein LipL32.102 An 83.2% agreement between this assay and MAT was observed when MAT titers were ≥1:800; specificity was 96%.102 Similar to the MAT, the assay detects vaccinal antibodies up to 1 year post-vaccination.102 The WITNESS Lepto Rapid Test (Zoetis, Parsipanny, New Jersey) is a point-of-care assay that detects IgM antibodies to whole cell extract from L. kirschneri serovar Grippotyphosa and L. interrogans serovar Bratislava.103, 104 Detection of vaccinal antibodies was noted in 24% of vaccinated dogs at 12 weeks post-vaccination.104 To date, studies of the clinical specificity of this assay suggest that a positive IgM result in an unvaccinated dog with signs consistent with leptospirosis strongly supports a diagnosis of leptospirosis (specificity > 97%; Table 2).104-106 However, because the sensitivity and specificity of these assays may vary regionally depending on circulating strains, more validation studies are needed from different geographic regions in dogs with leptospirosis before point-of-care antibody detection immunoassays can be recommended over MAT for diagnosis of leptospirosis. If antibodies are detected, quantification of the antibody titer using MAT is recommended as part of acute and convalescent phase titer evaluation to document recent exposure. A negative result with any point-of-care antibody detection assay does not rule out leptospirosis, and should be followed with a second antibody test, 7 to 14 days later, to document seroconversion (LOA 12/12).
| Location | Point-of-care test | Sensitivity | Specificity | Reference |
|---|---|---|---|---|
| Northeastern US | IDEXX SNAP Lepto | 15/22 (68%) | 111/131 (85%) | 107 |
| Germany | Zoetis WITNESS | 28/37 (76%) | 59/60 (98.3%) | 104 |
| Italy | Zoetis WITNESS | 30/42 (71%) | 36/36 (100%) | 105 |
| IDEXX SNAP Lepto | 33/42 (79%) | 26/36 (72%) | ||
| Switzerland | Zoetis WITNESS | 31/41 (76%) | 28/28 (100%) | 106 |
Nucleic acid amplification tests can detect Leptospira DNA in blood or urine early in the course of disease, before a serologic response occurs.108 To optimize sensitivity of NAATs, specimens should be collected before administration of antibiotics. Also, both blood and urine should be submitted (LOA 12/12). In humans, the leptospiremic phase of infection is very brief. When evaluated using blood, 1 assay had a sensitivity of 86% during the first 6 days of illness, which decreased to 34% after 7 days of illness.109 In dogs with leptospirosis, the sensitivity of a different PCR assay was 9/42 (21%) when applied to blood or urine or both.105 In another study, the sensitivity of PCR on blood and urine in dogs was 25% and 69%, respectively; specificity was 100% for both sample types.110 Some assays do not detect P2 species.108 Negative urine NAAT results also may result from intermittent urinary shedding or the presence of inhibitors of nucleic acid amplification. Heparin can interfere with molecular assays, so blood should be collected into EDTA tubes. The stability of DNA may be adversely affected by storage; for submissions to the Centers for Disease Control and Prevention (CDC), urine specimens should be tested within 3 days of storage at 4°C, blood within 14 days at 4°C, and both urine and blood samples within 28 days of storage at −20°C, with a maximum of 3 freeze-thaw cycles.111 Proficiency testing programs for laboratories that perform Leptospira PCR are available in some countries.112 Provided laboratory quality control is appropriate, a positive NAAT test result on blood in conjunction with consistent clinical signs is diagnostic for leptospirosis (see Case Definition). A negative result on blood or urine should not rule out leptospirosis. Because the DNA of pathogenic leptospires can be found in the urine of up to 20% of apparently healthy dogs,113-116 a positive NAAT test on urine must be interpreted in conjunction with clinical signs and the results of other diagnostic tests (LOA 12/12). Vaccination with inactivated Leptospira vaccines should not lead to positive PCR assay results.117
Other organism detection tests include darkfield microscopy and culture. Darkfield microscopy and culture have low sensitivity, and require technical expertise for proper interpretation, and thus are not recommended for routine diagnosis, although culture and serotyping may be valuable to inform prevention strategies in outbreak investigations (LOA 12/12). Culture requires inoculation of special media, ideally at point-of-care, and laboratory expertise is required for subsequent isolation and identification of Leptospira spp. Although the sensitivity of culture has been considered low and prolonged incubation times have been required, improvements in media and patient-side media inoculation have been associated with increased yields after incubation times as short as 24 to 72 hours.118 In the future, whole genome sequencing methods such as selective whole genome amplification may allow for diagnosis and sequence typing in outbreak investigations, without the need for culture. However, until vaccines are available that provide immunity that is not serovar-specific, culture and serotyping is required to inform vaccine selection.
Because of the characteristic patterns of laboratory abnormalities in leptospirosis, the disease lends itself well to diagnosis using machine learning. An algorithm for early diagnosis of leptospirosis that took into account CBC, serum biochemistry and urinalysis findings had a sensitivity of 100% and a specificity of 91% when applied to the hospital population at the University of California-Davis; specificity increased to 93% when a MAT titer obtained at patient intake was included in the model.97 Efforts to refine such algorithms to enhance the early recognition of leptospirosis are encouraged.
3.1 Case definition for leptospirosis in dogs
The following clinical and laboratory criteria have been developed as a case definition for leptospirosis in dogs (based on the CDC case definition for leptospirosis in humans119). A probable case meets the clinical criteria AND has ≥1 supportive laboratory criteria. A confirmed case meets the clinical criteria AND has ≥1 confirmatory laboratory criteria (LOA 12/12).
3.1.1 CLINICAL CRITERIA
-
Onset of systemic illness (nonspecific fever, lethargy, polyuria, polydipsia, anorexia, or some combination of these signs) within the past 2 weeks, with or without other clinical signs suggestive of leptospirosis:
- Gastrointestinal (vomiting, diarrhea, abdominal pain)
- Pulmonary (tachypnea, cough, hemoptysis)
- Ocular (uveitis, conjunctivitis, scleral injection, punctate retinal hemorrhages)
- Clinical suspicion for AKI (oliguria/anuria)
- Icterus
- Hemorrhage (ecchymoses, petechiae, epistaxis, hematuria, melena, hematemesis)
-
Two or more of the following clinicopathologic abnormalities:
- Neutrophilic leukocytosis, with or without a left shift
- Thrombocytopenia
- Biochemical evidence of AKI (eg, isosthenuria together with increased serum creatinine or symmetric dimethyl arginine [SDMA] concentrations or both)
- Biochemical evidence of cholestatic hepatopathy
- Biochemical evidence of pancreatitis (increased serum pancreatic lipase or DDGR-lipase activity)
- Increased CK activity
- Glucosuria despite normoglycemia
- Active urine sediment (pyuria or granular casts)
- Radiographic findings consistent with pulmonary hemorrhage syndrome
- Abdominal ultrasonographic findings consistent with leptospirosis (findings supportive of pancreatitis, hyperechoic renal cortices, perirenal fluid)
- ECG-documented cardiac arrhythmias or increased serum troponin concentration
3.1.2 SPECIFIC LABORATORY CRITERIA
-
Supportive:
- Leptospira MAT titer ≥800 in ≥1 serum specimens
- Detection of IgM antibodies against Leptospira in an acute phase serum specimen
- Detection of pathogenic leptospires in urine using a NAAT
- Visualization of spirochetes in a blood or urine specimen using darkfield microscopy by a Leptospira reference laboratory
-
Confirmatory:
- Fourfold or higher increase in Leptospira agglutination titer at a single laboratory between acute- and convalescent-phase serum specimens
- Detection of pathogenic leptospires in blood using a NAAT
- Isolation of Leptospira from a clinical specimen by a Leptospira reference laboratory
4 TREATMENT AND PROGNOSIS
Treatment of leptospirosis in dogs includes specific antimicrobial therapy and care of the individual organ systems affected to allow for repair, functional recovery, and survival. Depending on the grade of organ damage and dysfunction, interventions range from simple monitoring to supportive care, and in severe cases to temporary organ replacement (eg, dialysis) as a bridge to recovery.
4.1 Antimicrobial therapy
Based on the marked morbidity and potential mortality of the disease, and the risk of zoonotic transmission, as recommended for humans by the CDC,120 dogs with suspected or probable leptospirosis should be treated with appropriate antimicrobials (LOA 12/12), despite lack of evidence for a clear benefit for affected humans.121 The optimal antimicrobial treatment for leptospirosis is unknown. However, the sequential combination of initial treatment with an IV penicillin derivative to suppress bacteremia followed by PO doxycycline to avoid intra-renal persistence has been the traditional strategy in both humans and dogs with leptospirosis.1, 50 The justification for an initial penicillin-based therapy is the poor tolerance to PO doxycycline in animals with leptospirosis in which the clinical picture is dominated by gastrointestinal signs. Other antimicrobials with activity against leptospires (reviewed in the 2010 Consensus Statement)1 are either less efficacious in clearing organisms from kidney tissue in rodent models, or are critical antimicrobials reserved in some countries for treatment of resistant bacterial infections in humans. Based on these data, dogs with leptospirosis should be treated with doxycycline at a dosage of 5 mg/kg q12h PO for 2 weeks. Treatment should not be delayed pending results of diagnostic testing for leptospirosis. The optimal duration of antimicrobial treatment requires further investigation. If vomiting or other adverse reactions preclude doxycycline administration, dogs with leptospirosis should be treated initially with ampicillin (20-30 mg/kg IV q6-8h), amoxicillin (20-30 mg/kg IV q6-8h), or penicillin G (25 000-40 000 U/kg IV q6-8h; LOA 11/11). For penicillins, the panel recommends doubling the administration interval in dogs with AKI International Renal Interest Society (IRIS) Grade 4 and higher (serum creatinine concentration >440 μmol/L or >5 mg/dL; LOA 10/11 [1 reviewer recommended that interval adjustments be made based on kidney function regardless of AKI grade]).122
4.2 Supportive care
Treatment of organ dysfunction from leptospirosis is consistent with recommendations for other etiologies, prioritizing appropriate fluid, electrolyte, acid-base, and blood pressure management and support of gastrointestinal disturbances using antiemetics and gastroprotectants, as described in the 2010 Consensus Statement.1 Early and proactive nutritional support, individualized pain management, supplemental oxygen therapy, mechanical ventilation, and short-term kidney replacement therapy in severe cases are additional management requirements in selective patients. Attention should be taken to avoid iatrogenic fluid overload, which exacerbates organ dysfunction and leads to additional complications.123 Pulmonary manifestations in dogs with leptospirosis further limit tolerance to iatrogenic fluid excess.89 Diuretics may increase urine production in AKI but are ineffective and even detrimental for prevention and treatment of AKI.124 Their use should be limited to support volume management and increase potassium excretion in oliguric patients. Dogs with non-oliguric kidney failure may be markedly polyuric; some patients may require fluid rates >20 mL/kg/h to prevent hypovolemia. Blood pressure should be monitored at least twice daily in the initial phase of the disease and persistent hypertension not responsive to appropriate analgesia and volume correction should be treated using amlodipine (0.25-0.75 mg/kg/d, PO). Opioids are usually appropriate for pain control; non-steroidal anti-inflammatory drugs are not recommended. The use of diets for patients with kidney disease may not provide the protein needs of animals recovering from leptospirosis-induced AKI, and the fat content of kidney diets may predispose to or exacerbate concurrent pancreatitis. Although the ideal food composition has not been defined, dogs with suspected or confirmed leptospirosis should be fed a highly digestible, normal-to-high protein diet with sufficient energy content to support gastrointestinal recovery and minimize catabolism as early as possible (LOA 10/10). The use of naso-esophageal, esophageal, or esophago-jejunal feeding tubes facilitates efficient and early nutritional support with minimal risk of complications.
In dogs with hepatic dysfunction, consideration could be given to management of oxidative injury and inflammation using S-adenosyl methionine, silymarin, or vitamin E. However, there are no reported trials of these agents in patients with leptospirosis, and choleretics such as ursodeoxycholic acid have the potential for harm given current understanding of the disease's pathogenesis. More evidence is needed before supplements such as S-adenosyl methionine, silymarin, vitamin E, or ursodeoxycholic acid can be recommended for treatment of leptospirosis (LOA 11/11).
The therapeutic approach to the hemostatic disorders in dogs with leptospirosis varies based on the suspected dominant mechanism of the coagulopathy in an individual patient based on coagulation testing. Treatments may include replacement of consumed clotting factors with transfusions of fresh frozen plasma, which may require plasma exchange to prevent hypervolemia. A proactive approach should be considered in animals with leptospirosis, especially in the presence of hepatopathy, uremic thrombocytopathy, gastrointestinal ulceration, pulmonary hemorrhage, and systemic hypertension, when bleeding complications can become rapidly fatal. The use of desmopressin to facilitate von Willebrand factor release from the endothelium has shown benefit in humans with uremic thrombocytopathy, although it has the potential for adverse effects such as hyponatremia and arterial thromboembolic events.125 Neither desmopressin nor pulse dexamethasone treatment showed benefit for treatment of humans with LPHS in a randomized clinical trial.126
4.3 Extracorporeal kidney support therapy
Leptospirosis is 1 of the most frequent etiologic indications for extracorporeal kidney support therapy (EKST), having a high potential for successful outcome.127 The indications for hemodialysis are prioritized to resolve hyperkalemia, severe azotemia, fluid imbalance, metabolic acidosis, and the cumulative systemic comorbidities attending progressive AKI. Early hemodialysis has been associated with increased survival and shorter hospital stays in humans with leptospirosis.128 Early dialytic intervention is recommended to prevent the morbidity of AKI rather than as a delayed salvage for failed conventional management. Early referral to centers providing EKST should be considered for dogs in IRIS AKI Grade 4, when serum creatinine concentration exceeds 5 mg/dL (440 μmol/L; LOA 11/11). Extracorporeal kidney support therapy extends the window for recovery by restoring fluid, electrolyte and acid-base balances, the opportunity for nutritional support, and establishing an acceptable quality of life during the critical phase of kidney failure. More than 1 to 2 weeks of dialytic support rarely are required. A study of 36 dogs with leptospirosis reported >80% recovery in dogs with severe AKI treated with EKST after failing prior conservative medical management.67 Both intermittent hemodialysis and continuous hemodialysis platforms have been used successfully in dogs with leptospirosis67, 129 With the incorporation of a hemoperfusion cartridge in the extracorporeal circuit, cytokines, and even pathogens or their products may become therapeutic targets.130 Alternatively, plasma separated from the circulating blood by continuous flow centrifugation or by membrane filtration can be treated using adsorptive techniques or replaced with fresh frozen plasma from healthy donors (therapeutic plasma exchange) to modulate the exaggerated immune response of the animal to the pathogen.131 This approach has been applied successfully to human patients132 and results are promising in dogs with severe leptospirosis.133
4.4 Monitoring response to treatment
At a minimum, consideration should be given to performing a serum biochemistry panel in dogs with acute leptospirosis every 24 hours during hospitalization to monitor kidney function, bilirubin concentration, liver enzyme activities, serum protein concentrations, C-reactive protein, and electrolyte and acid-base status. Consideration also should be given to monitoring packed cell volume every 24 hours, and the CBC every 48 hours during hospitalization (LOA 10/10). Urine production can be measured using a closed urine collection system or, to decrease the risk of nosocomial urinary tract infection, frequent monitoring of body weight (eg, every 3 hours for anuric or severely polyuric dogs). Serial physical examinations with monitoring of body weight, blood pressure, respiratory rate, lung sounds, and possibly thoracic imaging are indicated to assess for early signs of overhydration. Initially, urine output should be monitored or estimated at least every several hours. Administered fluid volumes should be adjusted accordingly to prevent overhydration. Referral to a 24-hour care facility is recommended if adequate monitoring is not available within the practice (LOA 11/11). For dogs requiring urinary catheterization, indwelling, rather than intermittent, urinary catheterization is recommended to decrease the risk of exposure of attending staff to infective urine. Once azotemia resolves or stabilizes and the patient can maintain hydration and vascular volume, IV fluid therapy should be gradually tapered and then discontinued. After at least 7 days from the onset of illness, consideration should be given to obtaining convalescent MAT titers before discharge from hospital to increase the likelihood of follow-up testing.
Dogs should be re-examined no later than 1 week after discharge, at which time a CBC, serum biochemistry panel, urine specific gravity should be performed. If not obtained at discharge, convalescent MAT titers should be considered at this time. Dogs recovering from severe forms of AKI often show various degrees of permanent loss of kidney function and should be monitored and managed appropriately for their IRIS Stage of chronic kidney disease.134
Treatment typically is associated with gradual return of serum urea, SDMA, and creatinine concentrations to reference ranges within 10 to 14 days. Regeneration of kidney tissue may continue for weeks to months, and residual damage may remain long-term. The serum bilirubin concentration decreases more slowly than activities of serum ALT and ALP. Platelet counts often improve within 1 week of initiating antimicrobial treatment. C-reactive protein concentrations decrease within 4 to 10 days of antimicrobial treatment; serial monitoring is recommended in animals with slow recoveries or ongoing comorbidities. A delay in normalization of C-reactive protein concentration may indicate secondary inflammatory or infectious complications and justifies re-evaluation with additional diagnostic testing.72
5 PROGNOSIS
Negative prognostic factors in dogs include hyperbilirubinemia and hypocoagulability.135 Negative prognostic findings in humans include oliguria, a combination of hyperbilirubinemia and high serum creatinine concentration, respiratory complications, hypocoagulability, and severe thrombocytopenia; icterus alone may not be a negative prognostic finding.136, 137 In 254 dogs with acute leptospirosis, a serum bilirubin concentration of at least 10 μmol/L (0.6 mg/dL) was strongly associated (odds ratio [OR], 16.4; P < .001) with death or euthanasia.49 Leptospiral pulmonary hemorrhage syndrome has been associated with mortality of up to 70%48, 49 although mild forms exist that can resolve with antimicrobial treatment. Normalization of C-reactive protein with treatment parallels a favorable clinical course of the disease.72 A clinical scoring system has been developed and validated for dogs with AKI managed with EKST; higher scores are associated with poorer outcomes.138, 139 For dogs treated using EKST (and not for other dogs with leptospirosis), this model could be used cautiously as a tool for therapeutic decision making. Of several models developed (A through D), Segev's model C, using a cut-off score of 19.9, has demonstrated the optimal combination of sensitivity and specificity, correctly predicting survival outcomes in 80% to 87% of dogs (Table 3).138-140 A diagnosis of leptospirosis was reported in 21%-31% of dogs in these studies. Model C includes a weighting factor for leptospirosis (−8.47), which improves likelihood of survival. This model was applied to a cohort of 40 dogs with AKI treated in Italy with EKST, 5 of which had leptospirosis.140 Negative prognostic factors in this study were anuria, respiratory complications, disseminated intravascular coagulation, pancreatitis, and IRIS grade of AKI.
| Variable | Range (score)a | ||
|---|---|---|---|
| Body weight (kg) | >36 (+1.00) | 27.2-36.0 (+1.61) | ≤27.1 (+2.73) |
| Red blood cells (×106 cells/μL) | >4.93 (+1.00) | 3.54-4.93 (+1.51) | ≤3.53 (+3.61) |
| Lymphocyte count (cells/μL) | >1000 (+1.00) | 510-999 (+1.69) | ≤509 (+3.44) |
| Creatinine (mg/dL) | ≤13.2 (+1.00) | >13.2 (+2.26) | |
| Phosphorus (mg/dL) | ≤18.2 (+1.00) | >18.2 (+3.13) | |
| Ionized calcium (mmol/L) | >1.1 (+1.00) | 0.87-1.1 (+1.99) | <0.86 (+4.16) |
| Anion gap (mmol/L) | ≤18.2 (+1.00) | >18.2 (+2.74) | |
| Albumin (g/dL) | >1.9 (+1.00) | ≤1.9 (>2.52) | |
| Alanine aminotransferase (U/L) | <210 (+1.00) | ≥210 (−2.43) | |
| Urine production (mL/kg/h) | >1.31 (+1.00) | 0.1-1.31 (+1.44) | 0 (+5.55) |
| Respiratory system involvement | No (+1.00) | Yes (+2.48) | |
| Neurological involvement | No (+1.00) | Yes (+3.76) | |
| Disseminated intravascular coagulation | No (+1.00) | Yes (+2.3) | |
| Etiology (for dogs with known leptospirosis and EG status) | Not leptospirosis or EG toxicity (+1.00) | Leptospirosis (−8.46) | EG toxicity (+2.3) |
- a Scores for each variable are added (or subtracted as designated) to calculate the final predictive score. Scores > 19.9 suggest severe disease and are correlated with poor outcome. Because predictions can be inaccurate when applied to individual dogs, they should be used cautiously as a guide for therapeutic decision-making but should not be used to discourage owners from pursuing treatment.
6 PREVENTION
Currently, bacterin vaccines containing serovars Icterohaemorrhagiae, Canicola, Grippotyphosa, and Pomona are available in North America for prevention of leptospirosis in dogs. Quadrivalent vaccines containing serovars Icterohaemorrhagiae, Canicola, Grippotyphosa, and Australis are available in Europe. Monovalent and bivalent vaccines for serogroups Icterohaemorrhagiae or Canicola or both remain available in many geographic locations including North America, Europe, South Africa, and Australia, but may not be adequate to protect against the serovars commonly found in those regions. Trivalent vaccines also are available in some regions, such as Europe and South America. Current vaccines can prevent disease resulting from experimental challenge and can decrease shedding of vaccinal serovars. A recent systematic review and meta-analysis of experimental trials of 21 commercially available vaccines found >80% protection against clinical disease and kidney carrier status in dogs (studies of vaccines designed for dogs in hamsters, guinea pigs, and other mammalian species were excluded).37 However, trials typically involved use of high doses of leptospires administered intraperitoneally, conditions which do not mimic natural exposure. Vaccines protect for at least 12 months,141-143 with several manufacturers providing product guarantee for 15 months after proper administration. Currently available bacterins elicit serogroup-specific immunity, although partial immunity to heterologous serogroups has been documented in some studies.1, 144, 145 Leptospirosis in dogs that have been fully vaccinated with 4-serovar vaccines has been documented,6, 94 consistent with <100% efficacy of bacterins, but infections appear to be uncommon. Nevertheless, published data is limited regarding the incidence of naturally occurring leptospirosis (clinical or asymptomatic shedding) in such dogs. This situation may partly relate to the difficulty in definitively diagnosing leptospirosis in fully vaccinated dogs.
Leptospirosis vaccines require 2 initial injections spaced 4 weeks apart, which can be started in puppies at 12 weeks of age or later. Leptospirosis vaccines have similar immunological adverse effects as do other parentally administered vaccines.146, 147 Patient factors such as breed and size can influence the risk of vaccine-associated adverse events, regardless of the antigen source. Research approximately 2 decades ago indicated that some vaccines for dogs that included a Leptospira component contained high concentrations of bovine serum albumin, which could account for post-vaccinal IgE-based adverse events.148 More recent research indicates protein content, concentrations, and severe adverse event rates are not higher for leptospirosis vaccines than distemper-parvovirus or rabies vaccines.147, 149 Vaccines should be administered annually to all dogs starting at 12 weeks of age, regardless of breed, because leptospirosis is a zoonotic disease, can be severe or fatal despite treatment, and exposure can occur regardless of age, geography, or lifestyle. Because subclinical shedding has been documented in shelter dogs,113-115 initial and booster vaccinations, spaced 3 weeks apart, should be administered to shelter dogs at intake. Leptospirosis vaccination should be required for dogs by boarding or daycare facilities, because outbreaks have occurred in association with such facilities.26-28 In the absence of culture and serotyping information to determine the serovars causing disease in dogs in a region, vaccines containing the broadest array of serovars available should be used. Results of MAT-based serosurveys should not be used to guide vaccine selection when multiple vaccine types are available. There is no published evidence that seasonal timing of vaccination is important (LOA 12/12).
No leptospiral vaccines are currently approved for cats, nor is the use of biologicals for dogs currently recommended for cats.
Evidence of recurrent leptospirosis in dogs after proper treatment is lacking. Nevertheless, vaccination is recommended as soon as possible after recovery from leptospirosis because (1) such dogs are at risk of ongoing exposure to the same or other serogroups, and (2) it is unknown whether or not life-long immunity results from natural infection (LOA 12/12). More studies are required to establish the true duration of immunity and degree of cross protection among specific serovars after natural infection in dogs.
Other methods of prevention include decreased access to potential sources of infection, such as marshy areas and standing water, and minimizing contact with wildlife and domestic animal reservoir hosts using fencing and rodent control.
7 PUBLIC HEALTH IMPLICATIONS
Leptospirosis in humans most often is subclinical or presents as an influenza-like illness that occurs after an incubation period of 3 to 30 days.150 Transplacental infection during pregnancy can cause abortion or stillbirth. The most severe manifestations of leptospirosis in humans are hepatic and kidney failure (Weil's disease) or LPHS.150 Readers are referred to the CDC website for more information about the disease in humans.151
Globally, leptospirosis in humans is most prevalent in regions with high humidity, rainfall, and flooding.150 Globally, rodents are the main source of infection, although exposure to spirochetes shed by other wildlife species and production animal reservoir hosts also contributes to the disease in humans. Free-roaming dogs may represent a reservoir of infection for humans,152 although when both free-roaming dogs and rodents are present, isolates may be shared between rodents and dogs, with rodents being the main contributor to infection of humans.40 Contact with adopted wild and pet rodents also has resulted in disease in humans.153-155
In developed countries, most exposure occurs because of recreational activities that involve water, or occupations that result in exposure to production animal reservoir hosts, wildlife, or contaminated water sources.150 Increased rodent exposure resulting from homelessness, such as occurred after the COVID-19 pandemic or flooding associated with climate change, has the potential to contribute to emergence of leptospirosis in humans in developed countries.156 In 2021, increased numbers of cases of leptospirosis in humans in New York City compared to previous years were thought to be the effect of decreased rodent control during the SARS-CoV-2 pandemic.157
Transmission from sick animals with leptospirosis (ie, incidental hosts) to other animals is rarely reported.158 The few reports159-162 suggesting transmission of pathogenic leptospires from pet dogs to humans have not been substantiated using molecular methods, although transmission from a sick dog to a child in the household recently was documented, likely because of handling of contaminated urine.163
Although the potential exists for zoonotic transmission of pathogenic leptospires from sick dogs with leptospirosis to humans, the risk of such transmission appears to be low, especially when basic precautions are taken (LOA 12/12). In an investigation by the CDC, no evidence of zoonotic transmission was identified during an outbreak of leptospirosis in dogs in Arizona with high-risk exposures for humans.164 Serosurveys of veterinarians, veterinary students, and rehabilitation workers handling wildlife potentially infected with leptospires found no seroreactivity in veterinarians and veterinary students; only 2 of 213 rehabilitation workers tested positive, with titers of 1:200 to a single serovar.165-167
7.1 Veterinary healthcare workers
The risk of zoonotic transmission of leptospires in a small animal clinic can be minimized when veterinary staff have a high index of suspicion for leptospirosis in dogs with consistent clinical signs and appropriate handling precautions are implemented. Anecdotal evidence suggests it is difficult to detect leptospires using NAATs in the urine of dogs receiving penicillin or doxycycline treatment, so appropriate antimicrobial treatment also should decrease the possibility of zoonotic transmission.168 Positive PCR results detected in animals receiving antimicrobials also may represent non-viable organisms. All dogs with AKI should be suspected to have leptospirosis until an alternate diagnosis has been made, or if no other diagnosis is made, until 48 hours of appropriate antimicrobial treatment has been administered. However, given that apparently healthy dogs can shed pathogenic leptospires,114 standard hygiene precautions that decrease transmission of zoonotic pathogens in veterinary clinic environments, as defined by the National Association of State Public Health Veterinarians,169 are recommended regardless of the presence or absence of illness (LOA 12/12).
During the first 48 hours of treatment, movement of suspected or confirmed cases around the hospital should be minimized, and areas of contact disinfected promptly. Warning labels should be placed on cages, and pregnant humans should avoid contact with these patients. Because leptospires are not easily transmitted between dogs (and between dogs and people), hospitalization in an isolation ward is not required, and it has the potential to negatively impact the level of care required for many critically ill dogs with leptospirosis (LOA 10/10). If possible, patients should be placed in floor-level cages and housed away from high traffic areas. Care should be taken to avoid needle-stick injuries. Gloves, a waterproof disposable gown, and full-face protection (such as using a face shield) should be worn if aerosolization of urine is possible. Pressure washing of runs should be avoided. If a urinary catheter is not in place, dogs should be walked frequently enough that urination does not occur within the hospital. Patients should be taken to urinate in a restricted area, preferentially an area without moisture or water accumulation and with good drainage and exposure to ultraviolet light. Urine spills should be immediately cleaned and disinfected. Hand hygiene should be performed before and after handling each patient after glove removal, and cages cleaned and disinfected daily. Normal laundering of bedding will inactivate leptospires, but individuals handling soiled bedding should wear protective clothing. Disposable bedding should be placed in biohazard bags and handled appropriately.
Although all routine hospital disinfectants are active against leptospires, large volumes of urine in collection vessels can be inactivated by 1:1 dilution with 10% bleach (5000 ppm sodium hypochlorite), made fresh on the day of use. In dogs with indwelling urinary catheters, disinfectant should be injected directly into the collection bag before disposal (after removal from the patient).
All personnel who may have direct or indirect contact with a dog suspected to have leptospirosis should be informed of the risk. These include radiology personnel and laboratory personnel handling blood, urine, or tissue specimens from patients. Local public health agencies or the CDC can be contacted for guidance if additional questions arise regarding the public health risks and zoonotic transmission of leptospirosis or if an exposure occurs. Further information can be found on the CDC webpage on Leptospirosis for Healthcare Workers.170
7.2 Precautions in the home environment
Because leptospiruria usually does not commence until 7 to 10 days after infection, dogs in the first few days of illness (before veterinary care is sought) may not represent a clinically relevant source of zoonotic infection. Treated dogs returned to the home environment also represent a low risk to household members. Nevertheless, until 48 hours of treatment with doxycycline has been completed, owners should avoid contact with their dog's urine and wear gloves and eye protection when cleaning up urine. Veterinarians should educate owners of affected animals about the zoonotic risk of leptospirosis, and recommend they contact their medical practitioner if they have questions about the disease in humans (LOA 12/12). Owners should be informed that their dog likely contracted leptospirosis through ongoing direct or indirect contact with rodents, wildlife, or farm animals, and that they also may be at risk from such sources. Owners can be directed to the CDC's webpage on Leptospirosis in Pets for additional information.171 Routine vaccination of pet dogs is recommended to decrease the risk of zoonotic transmission of the disease, either by direct or indirect transmission.40
Subclinical seroconversion has been documented in some dogs living in the same household with dogs with leptospirosis, possibly because of common exposure. Because of the zoonotic potential of leptospirosis, after risk assessment, practitioners should consider prophylactic treatment of other dogs in the household that may have been coincidentally exposed, ideally with monitoring of acute and convalescent phase antibody titers (LOA 12/12). The recommended treatment is doxycycline, 5 mg/kg q12h PO for 14 days. More evidence is needed before prophylactic treatment can be routinely recommended for other apparently healthy animals in an exposure situation, such as cats, but a One Health approach to outbreak investigations is encouraged (LOA 12/12). The extent of urinary shedding in such exposed pets, if it occurs at all, requires further study.
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge Renee L. Galloway, BS, MPH, Microbiologist, Centers for Disease Control and Prevention, Atlanta, GA, USA; David A. Haake, MD, Health Sciences Clinical Professor, David Geffen School of Medicine, University of California-Los Angeles, CA, USA; Michael R. Lappin, DVM, PhD, DACVIM(SAIM), Professor, Colorado State University, CO, USA; Jarlath Nally, PhD, National Animal Disease Center, Agriculture Research Service, USDA, Ames, IA, USA; Gilad Segev, DVM, DACVIM-CA, Koret School of Veterinary Medicine, The Hebrew University of Jerusalem, Rehovot, Israel; and Jason Stull, VMD, MPVM, PhD, DACVPM, The Ohio State University, Columbus, OH, USA and the University of Prince Edward Island, Charlottetown, Canada.
CONFLICT OF INTEREST DECLARATION
Thierry Francey received financial support from MSD Animal Health to hire support staff for data collection toward retrospective analysis of canine leptospirosis (2017-2020). Jane Sykes received speaking honoraria and research support from Elanco, Boehringer Ingelheim, Merck, Zoetis, and IDEXX. No other authors declare a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




