Pharmacokinetics and pharmacodynamics of intranasal and intramuscular administration of naloxone in working dogs administered fentanyl
Ciara A. Barr and Joanne Haughan contributed equally to this study.
Abstract
Background
Working dogs exposed to narcotics might require reversal in the field.
Objective
To explore the pharmacokinetic and pharmacodynamic effects of naloxone administered intramuscularly (IM) or intranasally (IN) to reverse fentanyl sedation in working dogs.
Animals
Ten healthy, working dogs aged 1.7 ± 1 year and weighing 26 ± 3 kg.
Methods
In this randomized, controlled cross-over study dogs received either 4 mg of naloxone IN or IM 10 minutes after fentanyl (0.3 mg IV) administration. Sedation was assessed at baseline and 5 minutes after fentanyl administration, then at 5, 10, 15, 20, 25, 30, 60 and 120 minutes after reversal with naloxone. Blood samples for naloxone detection were obtained at 0, 5, 10, 30, 60 and 120 minutes. Pharmacokinetic parameters and sedation scores were compared between IM and IN naloxone groups.
Results
There was a significant increase in sedation score from baseline (0.25 [−4 to 1] IM; 0 [−2 to 1] IN) after fentanyl administration (11 [5-12] IM; 9.25 [4-11] IN), followed by a significant reduction at 5 (0.5 [−0.5 to 1.5] IM; 1.25 [−1.5 to 4.5] IN) through 120 minutes (−0.5 [−2 to 1] IM; 0 [−4.5 to 1] IN) after reversal with naloxone. Route of administration had no significant effect on sedation score. Maximum plasma concentration was significantly lower after IN administration (11.7 [2.8-18.8] ng/mL IN, 36.7 [22.1-56.4] ng/mL IM, P < .001) but time to reach maximum plasma concentration was not significantly different from IM administration.
Conclusion and Clinical Importance
Although IM administration resulted in higher naloxone plasma concentrations compared to IN, reversal of sedation was achieved via both routes after administration of therapeutic doses of fentanyl.
Abbreviations
-
-
- area under the plasma concentration versus time curve from zero to infinity
-
-
- % of extrapolated after the last time point to infinity
-
-
- area under the first moment curve extrapolated to infinity
-
- Clast
-
- last measured concentration
-
- Cmax
-
- maximum measured plasma concentration
-
- F%
-
- relative bioavailability
-
- IN
-
- intranasal
-
- Ka
-
- absorption rate constant
-
- Kel
-
- elimination rate constant
-
- LLOQ
-
- lower limit of quantification
-
- L/min
-
- liters per minute
-
- LOD
-
- limit of detection
-
- MRM
-
- multiple reaction monitoring
-
- MRT
-
- mean residence time
-
- QC
-
- quality control
-
- SPE
-
- solid phase extraction
-
- ,
-
- half-lives
-
- Tmax
-
- time at maximum measured plasma concentration
-
- Tlast
-
- time of last measurable observed concentration
-
- λz
-
- rate constant associated with the terminal slope
-
-
- terminal half-life
1 INTRODUCTION
Opioids are potent analgesic agents, which have been increasingly implicated in a rise in overdose deaths.1 This rise in opioid deaths has led to a public health crisis, with an increase in risk of accidental exposure to first responders and working dogs. While there are anecdotal reports across multiple states of police dogs being exposed to fentanyl during drug raids,2 there is currently no national reporting database, thus true numbers are difficult to assess.
The paucity of literature on working dog exposure to fentanyl might also be because of the lower sensitivity of dogs to fentanyl as compared to humans. The dose of fentanyl required to sedate a dog is approximately 10 times the dose that will sedate a human3 and the lethal dose is higher.4, 5 Fentanyl is well tolerated causing minimal changes in cardiopulmonary values in dogs.6 The most common adverse effect of fentanyl administration to dogs is a decrease in heart rate6, 7 with other side effects associated with opioids such as overt sedation, mild respiratory depression and dysphoria being less common than with morphine.8 Given this range of effects fentanyl exposure might not immediately be recognized by dog handlers thus putting both dogs and handlers at risk of further exposure to fentanyl that might be present on the dog or in the environment.
Because of the risk of fentanyl exposure both in humans and working dogs, first responders typically carry the opioid antagonist, naloxone (NARCAN, Adapt Pharma, Inc., Radnor, PA).9-11 Naloxone is available for intranasal or intramuscular administration12 in humans. While naloxone is not approved for use in dogs, handlers working with their veterinarian might be trained to administer naloxone via either the IN or IM route. While a recent study suggests naloxone is readily absorbed from the intranasal route,13 unfortunately, there are no studies examining the pharmacokinetic and pharmacodynamic effects of intranasal administration of naloxone in dogs receiving opioids thus it remains necessary to determine whether this route would be effective in dogs exposed to potent opioids.
As working dogs are at risk for exposure to fentanyl, this study aimed to explore the pharmacokinetic and pharmacodynamic effects of naloxone administered IM or IN to reverse fentanyl sedation in working dogs. We hypothesized that there would be a higher Cmax and lower sedation scores in dogs administered IM naloxone as compared to IN.
2 MATERIALS AND METHODS
2.1 Design
Randomized, blinded, cross over design with a 1-week wash-out period.
2.2 Animals
The protocol was approved by the Institutional Animal Care and Use Committee for University owned dogs (Protocol #806379) and for the privately-owned dog (Protocol #806413).
Ten working dogs (age 1.7 ± 1 year, weight 26 ± 3 kg) comprised of German Shepherds (n = 5), Dutch Shepherds (n = 2), Belgian Malinois (n = 1), and Labrador Retrievers (n = 2) were used for the study. Five dogs were female and 5 were male. All dogs were assessed to be healthy based on physical examination by a veterinarian.
2.3 Treatments
All dogs were administered 1 mg/kg of maropitant citrate orally at 8 pm the evening before the study. Pretreatment with maropitant was utilized to prevent vomiting and thereby decrease risk of aspiration as the working dogs were routinely fed treats while the IV catheter was placed to decrease stress and increase compliance during the study protocol. Aside from the treats administered for catheter placement, dogs were not fed after 10 pm the evening before the study but had free access to water.
An 18-gauge catheter was placed in the saphenous vein to allow for administration of fentanyl and blood sampling for pharmacokinetic analysis of naloxone plasma levels. Dogs were administered 0.3 mg (0.05 mg/mL) of fentanyl citrate IV. Heart rates, blood pressure and respiratory rates were monitored before sedation, 5 minutes after sedation and 5 minutes after reversal, using an oscillometeric blood pressure monitor (petMAP, graphic II, Ramsey Medical Inc, Tampa, FL) for heart rate and blood pressure. Heart rate was confirmed via auscultation and pulse palpation and respiratory rate was obtained by observing the chest wall excursion. Dogs that were deemed to be hypopneic with respiratory rates below 13 breaths per minute14 had supplemental oxygen supplied via loose fitting facemask at 5 L/min during this time period in accordance with our hospital sedation guidelines.
All dogs were randomly assigned to receive either 4 mg of naloxone spray (NARCAN) intranasally in the right nostril or 4 mg of intramuscular naloxone (1 mg/mL) in the left lumbar epaxial muscle 10 minutes after fentanyl administration.
2.4 Sedation scoring
After placement of an intravenous catheter, a baseline sedation score was obtained utilizing a previously described subjective sedation scoring system with slight modification (see Appendix S1).15 All observations were made while the dogs were loosely held on a leash by a known handler. Five minutes after fentanyl administration, a sedation score was obtained. Sedation scores were then obtained every 5 minutes for the first 30 minutes after reversal, and then again at 60 and 120 minutes after reversal. All observations were made by a single observer (CB) who was masked to the route of the naloxone administration. At each time point a video was obtained and reviewed by a second, masked observer that was not present during the execution of the study (KV). This second observer reviewed the clips in a random order so was unaware of when the dogs received fentanyl or naloxone.
2.5 Sample collection and measurement of naloxone concentrations
Blood was collected after fentanyl administration but before naloxone administration, and then at 5, 10, 30, 60 and 120 minutes after naloxone administration. Samples were obtained from the catheter by withdrawing 1-2 mL of blood, discarding it, drawing 3 mL of blood, and placing it in a 5 mL vacutainer with lithium heparin (56 USP units; Becton Dickinson, Franklin Lakes, NJ). If blood was unable to be sampled from the preplaced catheter, it was obtained by direct puncture of the cephalic vein. Samples were centrifuged (Fisher Healthcare Laboratory Centrifuge Model 614B; Thermo Fisher Scientific, Waltham, MA) for 10 min at 3150 rpm. Plasma was transferred to a cryovial and stored at 4°C until all samples were collected, then frozen at −80°C until analysis.
Naloxone plasma concentration was measured using liquid chromatography coupled to mass spectrometry (LC-MS/MS) after solid-phase extraction. An LC-MS/MS assay was developed for the analysis of dog plasma samples. The developed assay was validated using blank, heparinized working dog plasma (obtained from the same working dogs used for the study) to prepare calibration curves and quality control samples. Plasma was spiked at 400 ng/mL with naloxone (1000 μg/mL Naloxone; Cerilliant, Round Rock, TX) and serially diluted with plasma to produce the following concentrations: 100, 25, 6.25, 1.56, 0.391, 0.098 ng/mL which served as calibrators. Isotopically-labeled naloxone (1000 μg/mL Naloxone; Cerilliant, Round Rock, TX) was spiked into each sample to produce a final concentration of 5 ng/mL in each sample. The assay was validated according to the FDA guidelines regarding bioanalytical method development.16 Calibration curves were generated in duplicate and analyzed in triplicate and a total of 6 sets of calibration curves were prepared over nonconsecutive days (5 interday and 1 intraday). Quality control (QC) samples were prepared at 50, 5 and 0.5 ng/mL. QC samples were used to determine intra- and interday variability. Quantification of the QC samples was accomplished by running a calibration curve on each day. A linear least squares analysis with a 1/y weighting scheme was used to calculate the calibration parameters. The precision (%CV) was calculated using the formula: %CV = (SD/mean) × 100% and the accuracy (%error) was calculated using the formula: % error = ([calculated concentration − actual concentration]/actual concentration) × 100%. Precision and accuracy were below 15% for all validation samples and QCs as recommended by the FDA guidelines regarding bioanalytical method development.16
Before processing, animal samples were stored at −80°C. Samples were thawed and 200 μL transferred to clean micro centrifuge tubes. Isotopically-labeled naloxone (1000 μg/mL Naloxone; Cerilliant Round Rock, TX) was spiked into each sample to produce a final concentration of 2 ng/mL in each sample. A calibration curve was prepared each day that samples were processed. All calibrators, QCs and samples were extracted by solid-phase extraction (SPE) using Oasis 1 cc HLB cartridges with 30 mg sorbent (Waters Corp, Milford, MA). The SPE procedure was as follows: (1) Wash with 2 mL methanol containing 1% formic acid; (2) Wash with 2 mL water containing 20 mM ammonium formate; (3) Load 100 μL sample; (4) Wash with 2 mL water containing 20 mM ammonium formate; (5) Elute with methanol. The eluent for all calibrators, QCs and samples was evaporated under a dry nitrogen stream at 40°C. Samples were reconstituted in 90 μL of 10% methanol in 0.1% formic acid in water. Extraction was performed in duplicate and the replicates were analyzed via LC-MS/MS in triplicate.
Liquid chromatography was performed using a liquid chromatograph (Agilent 1290 Infinity Liquid Chromatograph, Agilent Technologies, Santa Clara, CA). Separation was performed on a 2.7 μm, 2.1 mm × 50 mm column (Halo C18 Column; Advanced Materials Technology, Wilmington, DE) with a chromatographic ramp with mobile phase B = 0.2% formic acid in methanol and mobile phase A = 0.2% formic acid, consisting of the following schedule: 0 minute ➔ 3 minutes (2% mobile phase A ➔ 95% mobile phase A), 3 minutes ➔ 4 minutes (95% mobile phase A), 4.0 minutes ➔ 4.1 minutes (95% mobile phase A ➔ 2% mobile phase A), 4.1 ➔ 7 minutes (2% mobile phase A). The flow rate was 500 μL/min and an injection volume of 5 μL was used. A retention time of 0.9 minutes was observed.
Tandem Mass Spectrometry was accomplished using a 6500 QTrap Triple Quadrupole Mass Spectrometer (Sciex, Ottawa, Ontario). It was operated in electrospray mode using multiple reaction monitoring (MRM). The ion source temperature was 700°C. Capillary voltage was +5500 V, curtain gas was 30 and the collision assisted dissociation gas was medium. Ion source gas 1 and 2 were 50 and 70. Declustering potential was 50 V and entrance potential was 10 V. For naloxone, the quantifier ion transition was 328.2 to 212.1 Da with collision energy of 61 eV and collision exit potential of 11 V while the qualifier ion transition was 328.2 to 253.0 Da with collision energy of 35 eV and collision exit potential of 8 V. For naloxone-D5, the quantifier ion transition was 333.2 to 212.1 Da with collision energy of 62 eV and collision exit potential of 12 V while the qualifier ion transition was 333.2 to 258.0 Da with collision energy of 36 eV and collision exit potential of 9 V. Peak areas were integrated using Analyst software.
The limit of detection (LOD) of the assay, determined by the calibration curve was 0.098 ng/mL. The lower limit of quantification (LLOQ) was determined across all analytical runs as the lowest concentration where precision, accuracy, and CV% met the FDA guidelines for bioanalytical method development16 at 0.488 ng/mL.
2.6 Statistical analysis
After testing data for normality using the Shapiro Wilk test, the Wilcoxon-signed rank test was used to compare sedation scores after administration of fentanyl to baseline, and 5, 10, 15, 20, 25, 30, 60 and 120 minutes after reversal with naloxone. Additionally, sedation scores at each time point were compared between IN and IM administration groups. Maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) were derived directly from the measured plasma concentration versus time data. Differences between Cmax and Tmax for the IN and IM administration routes were analyzed by pairwise comparison of the marginal means using a mixed effects model with the fixed effect set on administration route and the random effect set on the level of the individual animal. To correct for small departures from normality robust estimation of the variation was used. All statistical analysis was performed using statistical software (Stata version 17, Stata Corp, College Station, TX). A P value <.05 was considered significant.
3 RESULTS
All dogs tolerated fentanyl administration without vomiting or regurgitating. The mean ± SD delivered fentanyl dose was 0.01 ± 0.001 mg/kg for both the IN and IM naloxone administration groups. After fentanyl administration and before reversal with naloxone, 2 dogs were hypopneic (12 breaths per minute). Although oxygen supplementation was not indicated because of hypoxemia and arterial carbon dioxide levels were not measured to determine the level of hypoventilation, these 2 dogs received preemptive oxygen supplementation in accordance with our hospital's sedation guidelines. Data from 1 dog (animal 3) were removed from the IN dataset as it inadvertently received half of the IN naloxone dose. The mean ± SD delivered naloxone dose was 0.16 ± 0.02 mg/kg (range, 0.13-0.19 mg/kg) for the IM administration and 0.15 ± 0.02 mg/kg (range, 0.13-0.19 mg/kg) for the IN administration. Eight dogs panted after administration of naloxone via either route. Three dogs were bradycardic with heart rates below 60 beats per minute after fentanyl administration before to naloxone reversal. No hypotension was noted in any dog. No adverse effects were noted after naloxone administration.
Sedation scores over time for the IN and IM administration groups are summarized in Figure 1. There was a significant increase in sedation score from baseline after fentanyl administration in both the IM and IN administration groups. Sedation scores were statistically significantly reduced at all time points after naloxone administration in both groups as compared to 5 minutes after fentanyl administration (P < .05). No statistically significant differences in sedation scores between the IN and IM groups were noted at any time point.
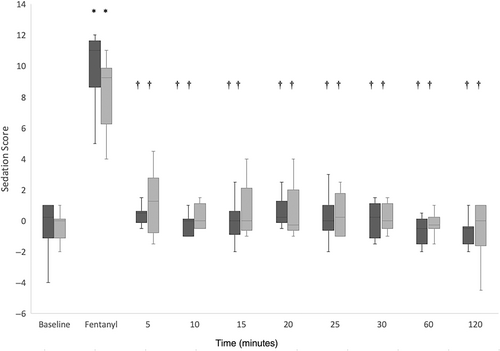
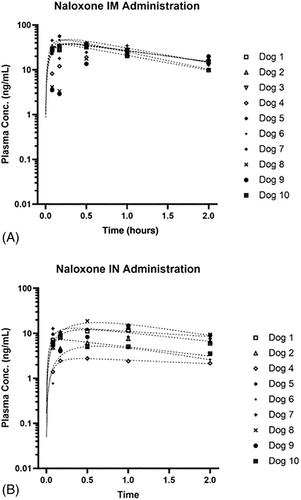
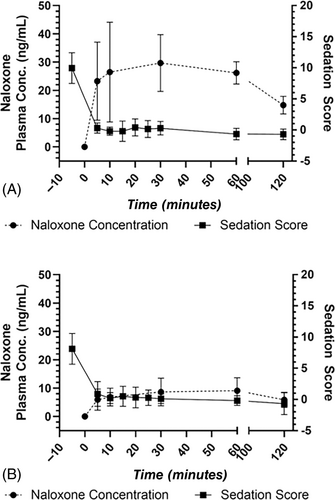
Figure 2 shows plasma concentration over time curves after administration of 4 mg naloxone to 10 dogs via the IM route (Figure 2A) and to 9 dogs via the IN route (Figure 2B). Mean ± SD plasma concentrations are listed in Table 1. Median (range) Cmax after IM administration of 4 mg naloxone was 36.7 (22.1-56.4) ng/mL. Median (range) Cmax after IN administration of 4 mg naloxone was 11.7 (2.8-18.8) ng/mL, which was statistically significantly lower compared to IM administration (P = <.001). Median (range) Tmax after IM administration of 4 mg naloxone was 0.50 (0.08-1.00) hours. Median (range) Tmax after IN administration of 4 mg naloxone was also 0.50 (0.08-1.00) hours. Tmax was not significantly different between groups (P = .591). Although IM administration resulted in much higher naloxone plasma concentrations compared to IN, similar reversal of sedation was achieved via both routes (Figure 3).
| IM (n = 10) | IN (n = 9) | |
|---|---|---|
| Time (h) | Mean conc. (ng/mL) | Mean conc. (ng/mL) |
| 0.00 | ND | ND |
| 0.08 | 23.25 ± 13.81 | 5.93 ± 3.71 |
| 0.17 | 26.45 ± 17.64 | 6.85 ± 3.11 |
| 0.50 | 29.66 ± 10.44 | 8.65 ± 4.88 |
| 1.00 | 26.18 ± 3.98 | 9.08 ± 4.53 |
| 2.00 | 14.79 ± 3.11 | 5.90 ± 2.66 |
- Abbreviation: ND, not detected.
4 DISCUSSION
Intranasal and intramuscular naloxone both reversed the sedative effects of fentanyl as demonstrated by statistically significant decreases in sedation scores after administration of naloxone by either route. The sedation scores increased significantly from baseline 5 minutes after administration of fentanyl which is consistent with the onset time of 2-3 minutes.17 Fentanyl, similar to other μ agonists, causes sedation in dogs via activation of the μ opioid receptor. The level of sedation in dogs receiving fentanyl in this study was more profound as compared to a study using the same sedation scoring scale in dogs receiving hydromorphone.15 This might be because of the dogs in this study receiving fentanyl at approximately double an equipotent dose18 intravenously as compared to dogs receiving hydromorphone intramuscularly (0.01 mg/kg vs 0.1 mg/kg). Fentanyl has previously been noted to cause mild restlessness at doses of 5 mcg/kg and below with increasing doses causing increasing levels of sedation.19 Fentanyl at the therapeutic dose of 0.01 mg/kg has previously been shown to cause decreased arousal for up to approximately 80 minutes.18 Naloxone reverses fentanyl's bradycardic effects as well as sedation in beagles receiving an overdose of transdermal fentanyl.20, 21 Naloxone reverses opioid induced sedation in dogs receiving oxymorphone, resulting in arousal in all dogs.22 These findings are consistent with our results indicating that naloxone reversed fentanyl sedation in all dogs regardless of route.
The IM Cmax achieved in this study was 36.7 ng/mL. A study in beagle dogs,23 in which naloxone was administered alone without any prior sedative at a much lower dose (0.04 mg/kg) intramuscularly into the epaxial muscle, resulted in a lower Cmax of 23.19 ng/mL. The aforementioned study also reported a shorter Tmax (0.17 hour) compared to this study (0.5 hour). Factors that may explain differences in Tmax between this and other studies include dog breed and sex differences as well as co-administration of fentanyl resulting in possible drug-drug interactions. However, this was not investigated as it was not the objective of this study.
The IN Cmax of 11.7 ng/mL and Tmax of 0.5 hour reported in this study was comparable to a previous study conducted in mixed-breed dogs in which a similar IN naloxone dose (0.17 ± 0.02 mg/kg) was administered without sedation and resulted in a Cmax of 9.3 ng/mL and Tmax of approximately 0.4 hour.13 Tmax was not statistically different between the IN and IM routes suggesting absorption of naloxone was rapid via both routes.
Cmax was statistically significantly higher after IM administration of naloxone compared to IN administration. However, without conducting the appropriate pharmacokinetic studies, the reason for this difference cannot be determined. Bioavailability has been determined at 34% for IN administration of naloxone to dogs compared to IV administration13 but to the authors knowledge has not been determined for IM administration of naloxone to dogs. Despite the differences in pharmacokinetic parameters, similar levels of sedation reversal were achieved with both IM and IN administration of a 4 mg dose of naloxone to dogs. Whether differences in sedation reversal between the 2 routes will be apparent when other doses of fentanyl and naloxone are administered remains to be determined in future studies.
While to our knowledge, the minimal plasma naloxone concentration necessary to reverse fentanyl sedation has not been documented in dogs, a previous study examining naloxone reversal of oxymorphone sedation found a minimal dose of 0.01 mg/kg naloxone IV to be sufficient22 which is much lower than the dose used in this study (0.15 and 0.16 mg/kg for IM and IN naloxone, respectively). While a lower dose is appropriate in clinical settings24 the higher dose of naloxone appeared well tolerated in dogs with no adverse effects noted. The plasma level required to reverse the sedation caused by 0.01 mg/kg of fentanyl is most likely significantly lower than the Cmax as all dogs were clinically no longer sedated (see Supplemental videos S1-S3), as evidenced by the sedation scores within 5 minutes of receiving naloxone while the average Tmax was reached after 17 minutes. Furthermore, no dogs showed any evidence of renarcotization even after the naloxone plasma concentration declined.
The main aim of this study was to determine whether there was any difference in the ability of IM or IN naloxone to reverse fentanyl sedation in working dogs. Given this aim, standardized amounts of fentanyl and naloxone were administered to each dog regardless of weight. This was to simulate the environment in which these dogs would be working. Officers have access to intranasal naloxone in the form of a 4 mg naloxone (NARCAN) nasal spray in case of human exposure. This formulation does not allow for titration of dose therefore, in order to compare the effects of IN to IM naloxone the same amount was used IM. This variation in dosing across weights could have increased variation in plasma naloxone concentrations and the resulting sedation scores. However, given that all dogs were administered the same amount of fentanyl (0.3 mg), it is expected that the pharmacodynamic effects of fentanyl and naloxone would be consistent as each dog received consistent amounts of the μ agonist and antagonist. Furthermore, the cross-over design allowed for direct comparison of the IN and IM effects of naloxone in each individual dog.
A further limitation of this study was that a relatively short-acting opioid was used and thus this study did not address the potential for renarcotization with longer acting opioids. This study did not include a control group that received fentanyl without naloxone. As the half-life of fentanyl is between 0.75 and 6 hours7 with clinical effects subsiding within 2 hours, the rapid reversal seen within 5 minutes of naloxone administration and 15 minutes of fentanyl administration suggests that this was because of naloxone's antagonistic effects rather than declining effects of fentanyl. The risk for renarcotization with longer acting opioids has been documented previously when naloxone has been used for oxymorphone reversal.22 This study chose fentanyl as it has become increasingly used in illicit drug activity and thus poses a high risk of exposure for working dogs.
ACKNOWLEDGMENT
Funding provided by the Department of Homeland Security, HSHQDC-17-P-00112. Pharmacokinetic data were presented in abstract form at the International Veterinary Emergency and Critical Care Symposium, September 14-18, 2018, New Orleans, LA.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC for University of Pennsylvania owned dogs (Protocol #806379) and for the privately-owned dog (Protocol #806413).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




