Adeno-associated virus-vectored erythropoietin gene therapy for anemia in cats with chronic kidney disease
Abstract
Background
A treatment of chronic kidney disease (CKD)-associated anemia in cats is needed. SB-001 is an adeno-associated virus-vectored (AAV)-based gene therapeutic agent that is administered intramuscularly, causing the expression of feline erythropoietin.
Hypothesis/Objective
We hypothesized that SB-001 injection would lead to a sustained increase in PCV in cats with CKD-associated anemia.
Animals
Twenty-three cats with International Renal Interest Society (IRIS) Stage 2 to 4 CKD-associated anemia were enrolled at 4 veterinary clinics.
Methods
In a prospective clinical trial, cats were treated with 1 of 3 regimens of SB-001 (Lo 1.2 × 109 genome copies [GCs] on Day 0; Lo ± Hi [supplemental 2nd dose of 3.65 × 109 GC on Day 42]; Hi 3.65 × 109 GC IM on Day 0) and followed for 70 days.
Results
A response to SB-001 at any time between Day 28 and Day 70 was seen in 86% (95% confidence interval 65, 97%) of all cats. There was a significant (P < .003) increase in PCV from Day 0 to Day 28 (mean increase 6 ± 6 percentage points [pp]; n = 21), Day 42 (8 ± 9 pp; n = 21), Day 56 (10 ± 11 pp; n = 17), and Day 70 (13 ± 14 pp, n = 14). Twelve cats were hypertensive at baseline, 4 of which developed encephalopathy during the study. An additional 6 cats became hypertensive during the study.
Conclusions and Clinical Importance
Results of this study suggest that SB-001 therapy represents a suitable single injection treatment that can address nonregenerative anemia in cats with CKD. It was generally well tolerated; however, hypertension and encephalopathy developed in some cats as previously described in association with erythropoiesis-stimulating agent therapy.
Abbreviations
-
- (r)AAV
-
- (recombinant) adeno-associated viral vector
-
- CKD
-
- chronic kidney disease
-
- DNA
-
- deoxyribonucleic acid
-
- EPO
-
- erythropoietin
-
- ESA
-
- erythropoiesis-stimulating agent
-
- GCs
-
- genome copies
-
- IRIS
-
- International Renal Interest Society
-
- PRCA
-
- pure red cell aplasia
-
- RAAS
-
- renin-angiotensin-aldosterone system
-
- r-HuEPO
-
- recombinant human erythropoietin
-
- TIBC
-
- total iron binding capacity
-
- TSAT
-
- transferrin saturation
1 INTRODUCTION
Chronic kidney disease (CKD) affects up to 50% of geriatric domestic cats, and causes death in up to 17%, making it the most common metabolic disease affecting this species.1-3 Anemia develops in up to 65% of cats with CKD and is associated with disease progression and death, likely through the induction of renal tissue hypoxia.1, 4, 5 Erythropoiesis-stimulating agents (ESAs), approved for humans, are used off-label to manage CKD-associated anemia in cats. Administration of epoetin alfa (ie, recombinant human erythropoietin, r-HuEPO) increases hematocrits in cats with CKD leading to improved appetite, energy, body condition, alertness, strength, and playfulness.6 Because HuEPO shares only 85% amino acid identity with feline erythropoietin (EPO), these beneficial effects are short-lived in some cats because of the production of anti-r-HuEPO antibodies and pure red cell aplasia (PRCA).6, 7 Recombinant feline EPO can increase hematocrit when administered to cats with CKD but it is not commercially available.8 Likewise, the use of darbepoetin alfa increases hematocrit and prolongs the survival of cats with CKD, even though only 56% of cats achieve the target PCV.9 There remains an unmet clinical need for a safe and effective feline-specific ESA.
Recombinant adeno-associated viral vectors (rAAVs) with tropism for muscle tissue successfully transfer deoxyribonucleic acid (DNA) into myocytes and allow for stable transgene expression after IM administration.10-12 Most AAV gene therapies are aimed at treating rare, usually monogenic, diseases by replacing the deficient or defective causative gene. However, there are opportunities to address acquired conditions such as CKD as the technology evolves. The development of many human gene therapies involves preclinical studies in cat and dog disease models, some of which are naturally occurring in certain species.13-15 Identification of effective AAV gene therapies for acquired conditions represents an opportunity to address unmet clinical needs in the domestic pet population.
Investigations involving nonhuman primates show that intramuscular administration of rAAV can confer long-term expression of rhesus EPO and enhance erythropoiesis.15 SB-001 is an AAV-based gene therapeutic agent that is administered intramuscularly, allowing for the expression of feline EPO. Studies in healthy cats indicated a single IM injection of SB-001 could support consistent long-term feline EPO expression (Figure S1). We conducted a trial to evaluate efficacy and optimal dose of SB-001 in cats with CKD-associated anemia. Our hypothesis was that SB-001 injection would lead to an increase in PCV in client-owned cats with naturally occurring CKD-associated anemia.
2 MATERIALS AND METHODS
2.1 Recruitment
Four general and specialty veterinary clinics in the United States participated in the study between October 2019 and August 2021. The study was approved by the Institutional Animal Care and Use Committee at North Carolina State University (NCSU).
2.2 Adeno-associated virus vector
Codon-optimized feline EPO cDNA was cloned into an expression construct containing a chicken beta-actin promotor with a cytomegalovirus early enhancer, chimeric intron, and rabbit beta-globin polyadenylation sequence. The expression constructs were flanked by AAV2 inverted terminal repeats. Recombinant AAV serotype 1 virus vectors were generated via the triple transfection of HEK293 cells and iodixanol purification as previously described.16
2.3 Inclusion criteria
All cats enrolled were client owned and had International Renal Interest Society (IRIS) Stage 2, 3, or 4 CKD based on appropriate clinical findings (eg, small kidneys, polyuria, polydipsia), serum creatinine >1.6 mg/dL (http://www.iris-kidney.com/guidelines/staging.html), and a nonregenerative anemia with a PCV ≤25% consistent with anemia of CKD, which were confirmed during the screening visits. Chronic, concomitant diseases were stabilized before enrollment, in the opinion of the examining veterinarian, such that the cat was deemed likely to complete the study.
2.4 Exclusion criteria
Cats were excluded from study participation if they were: <2 years old, <2 kg, intended for breeding, pregnant, lactating, or had clinical pathology findings considered abnormal and clinically relevant, apart from evidence of CKD and previously identified stable chronic conditions. Specifically, cats were excluded if they had diagnosed or suspected acute kidney injury, clinically relevant or symptomatic cardiac disease, neoplasia (with the exception of suspected stable gastrointestinal lymphoma or inflammatory bowel disease), urinary tract infection, uncontrolled hyperthyroidism, uncontrolled diabetes mellitus, major surgery within the previous month or planned elective major surgery during the study period. Cats were not excluded if they had minor surgeries before Day 0 or during the study period if, in the opinion of the examining veterinarian, the surgery would not affect study assessments. Cats were also excluded if they had ever been treated with any ESA or had blood transfusions within 60 days before Day 0. Routine vaccinations had to be completed >7 days before Day 0.
2.5 Study design
The prospective, multisite, open-label study was planned to evaluate the efficacy and safety of SB-001 on CKD-associated anemia in client-owned cats and designed to support reasonable expectation of effectiveness for expanded conditional approval by the FDA. The numbers of animals were chosen to meet this requirement in a reasonable period of time.
There were 3 different treatment groups included in this study. Cats in the original dose cohort, Lo, were administered a single unit dose of 1.2 × 109 genome copies (GCs) of SB-001 IM on Day 0. Subsequently, the protocol was amended, and new cats were enrolled in a second cohort, Lo ± Hi. These cats were administered a single unit dose of 1.2 × 109 GC IM on Day 0 but also were administered a supplemental dose of 3.65 × 109 GC on Day 42 if the PCV did not increase into the reference range or by at least 10 percentage points (pp; eg, from 19% to 29%) by Day 42. After review of the data from Lo ± Hi, the protocol was amended, and new cats were enrolled in a third cohort, Hi. These cats were administered a single dose of 3.65 × 109 GC IM on Day 0 and supplemental dose of 3.65 × 109 GC if the PCV did not increase into the reference range or by at least 10 pp by Day 42.
2.6 Screening
The screening visit was conducted between Day −14 and Day −2. A complete history (including concomitant treatments and signalment [i.e., age, breed, sex]), physical examination, and laboratory analysis including a CBC, clinical chemistry, symmetric dimethylarginine, thyroxine, feline leukemia virus, feline immunodeficiency virus, total iron, total iron binding capacity (TIBC), urinalysis, and urine culture were completed on each cat. Cats were fasted for at least 6 hours before blood sampling. At least 5 blood pressure measurements were taken during the visit; the highest and lowest measurements were discarded, and the mean was calculated from the remaining measurements. Cats were not screened for neutralizing antibodies to the capsid because of the possible protective nature of IM administration and impracticality of this testing in veterinary clinics. The intention was to develop a product that could be used in the presence of existing antibodies; therefore, there was no reason to test for these antibodies before enrollment. Samples were collected for later analysis in case it was deemed necessary.
2.7 SB-001 administration and follow-up examination
SB-001 was administered on Day 0 via single deep IM injection in either the quadriceps or epaxial muscles, based upon clinician preference. Telephone assessments with the owner were made on Day 7 ± 1 (and Day 49 ± 1 for cases receiving the supplemental dose). If the owner reported concerns regarding the site of injection, the cat was returned to the clinic for evaluation if appropriate.
All subsequent visits were within ±3 days of the target date. Cats enrolled in Lo were scheduled for visits at Days 0, 14, 28, 56, and 70; whereas cats enrolled in Lo ± Hi or Hi were scheduled for additional visits at Days 84, 98, 112, and 126 if they received the supplemental dose. At each visit, a complete physical exam, blood pressure measurement (as described above), and CBC were completed. Documentation of all medications administered, and adverse events were documented throughout the study. Chemistry profiles were performed on Days 14, 28, 42, 70, 98, and 126, and urinalyses on Days 28, 70, and 126.
On Days 0, 28, 56, and 70 (and Day 126 for cats receiving a supplemental dose), owners were also requested to complete a quality of life (QOL) assessment in which they were asked to score their cat's general health (ie, activity level, appetite, behavior, interactivity, playfulness, grooming) on Likert scales of 1 to 10. The average item-weighted impact score (AWIS) was calculated as the average value of the question and the impact score was the sum of the questions.
Total iron binding capacity was measured at screening, Day 28, and Day 70 for all cats and on Day 126 for cats receiving a supplemental dose in Lo ± Hi and Hi. Any cat with TIBC above or iron below the reference range on Day 0 or Day 28 or both was administered iron dextran every 3 to 4 weeks, dosed according to the attending clinician's discretion, and administered IM at a different site to SB-001; additional injections could be administered at the examining veterinarian's discretion. Transferrin saturation (TSAT) was calculated by total iron divided by TIBC multiplied by 100. Iron deficiency was defined by a TSAT < 20%.
Injection site evaluations were done by the examining veterinarian on Day 0 before treatment and on Days 14 and 70, and, if possible, at any unscheduled visit that resulted in withdrawal from the study. For cats administered a supplemental dose of SB-001, that injection site was evaluated before treatment and on Day 56 and Day 126.
Predetermined reasons for withdrawal after enrollment included: owner removal of consent; severe anemia (ie, PCV ≤ 14%) or polycythemia (ie, ≥65%), adverse events prohibiting further participation in the study; treatments potentially affecting study outcome (eg, ESA, blood transfusions); owner perceived lack of therapeutic effect; welfare reasons as decided by the veterinarian; or other reasons (eg, lost to follow-up). Cats received study-related veterinary care and medication free of charge.
2.8 Assessment of efficacy and safety
Increases in PCV from screening and Day 0 were analyzed to determine the efficacy of SB-001. The screening and Day 0 values were compared to Days 14, 28, 42, 56, and 70 for all cats. For cats administered a second dose on Day 42, the screening and Day 0 values were also compared to Days 84, 98, 112, and 126. A responder was defined as a cat that achieved a PCV in the reference range (ie, >28.2%; primary goal) or an increase in PCV of >5 pp from the PCV on Day 0 (secondary goal). A nonresponder did not achieve either of these goals.
2.9 Analytical methods
All clinical pathology samples (including PCV) were sent to a central commercial laboratory (IDEXX Laboratories, Inc) for analysis.
2.10 Pre-existing antibodies
Any animal that required the supplemental dose on Day 42, was evaluated post hoc for AAV1 neutralizing antibodies using stored samples collected during the screening process. The assay was performed by the University of Pennsylvania Gene therapy program as per confidential standard operating procedure.
2.11 Animal welfare
All owners signed an informed consent document detailing the known risks involved in the study. Cats remained in the care of their owners. They were checked regularly by the examining veterinarian. The owner, the examining veterinarian, or an investigator was able to withdraw the cat from the study at any point if it required any medical procedure or therapy not authorized by the study protocol or for any welfare concern. The protocol required that any cat with severe anemia (ie, PCV ≤ 14%) or polycythemia (ie, ≥65%) be medically managed based on current standard of care. Adverse events defined as any undesirable reaction or adverse effect observed after the administration of SB-001 were recorded and appropriate medical therapy was provided.
2.12 Data analysis
All tests of significance, unless otherwise stated, were performed at alpha = .05, 2-sided. All data calculations were generated using SAS version 9.4 (SAS Institute, Cary, North Carolina). The primary effectiveness variable to evaluate the improvement of anemia was PCV values. All other variables were considered secondary.
For the primary variable, PCV (%), the Day 0 values were compared to Days 14, 28, 42, 56, 70, 84, 98, 112, and 126. Descriptive statistics were calculated for each study day and for the changes from Day 0 to each subsequent study day. For each cohort, possible differences between the changes from Day 0 were assessed by repeated measures analysis of variance modeling. The models included study day as a fixed effect and site as a random effect. The normality of the residuals from each model was investigated using the Shapiro-Wilk test. If the P value was ≤.01, then the values were ranked before generating the model.
Changes in QOL variables from Day 0 were calculated for each cat. Descriptive statistics were evaluated for each visit and for the change from Day 0 to Day 28, Day 56, and Day 70 (and Day 126 if applicable). A change in QOL (ie, AWIS and impact scores) was evaluated between responders and nonresponders using analysis of variance with response as a fixed effect. These questions were also evaluated at each time point compared to Day 0 as to whether they were improved (+1), unchanged (0), or decreased (−1) and the cases were given a sum total score at each time point.
For overall survival, the median time to death was calculated using the Kaplan-Meier method and survival curves were calculated by cohort, IRIS stage, all cats, each responder definition, and by IRIS stage within each cohort and all cats. For each analysis, possible differences in the median time to death were assessed by the log-rank test.
For the response definition, the number and percentage of responders/nonresponders and the 95% confidence interval were calculated by cohort and overall. Descriptive statistics were prepared for responders/nonresponders for body condition score at Day 0, serum iron status at Days 0, 28, 42, and 70 during the study and the number of medications. Possible differences between responders and nonresponders were assessed by analysis of variance.
3 RESULTS
3.1 Demographics
Thirty-two cats were screened for the study; 9 (25%) did not meet the inclusion criteria for the following reasons: higher than allowed PCV (n = 4); feline immunodeficiency virus positive (n = 1); cardiac failure (n = 1); blood transfusion <60 days before Day 0 (n = 1); euthanasia before Day 0 (n = 1); or death by natural causes (n = 1). A total of 23 cats met the criteria for inclusion based on protocol compliance at 4 different sites: NC State Veterinary Hospital, Raleigh, North Carolina (n = 10); Friendship Hospital for Animals, Washington, DC (n = 7); VCA Sacramento Veterinary Referral Center, Sacramento, California (n = 4); and Bulger Veterinary Hospital, Lawrence, Massachusetts (n = 2). The 23 cats enrolled in the study were mostly domestic shorthair cats, with a larger percentage of males than females (Table 1). The mean age of enrolled cats was 13.8 ± 4.57 years. There were 2 CKD Stage 2 cats, 12 CKD Stage 3 cats, and 9 CKD Stage 4 cats.
| Characteristic | Lo (N = 6) | Lo ± Hi (N = 9) | Lo (N = 8) | |
|---|---|---|---|---|
| Breed | Abyssinian | 0 | 1 (11%) | 0 |
| American Shorthair (pedigree) | 0 | 1 (11%) | 0 | |
| Domestic Medium Hair (non-pedigree) | 0 | 0 | 1 (13%) | |
| Domestic Shorthair (non-pedigree) | 5 (83%) | 5 (56%) | 5 (63%) | |
| Himalayan | 0 | 1 (11.1%) | 0 | |
| Mixed Breed | 0 | 0 | 2 (25%) | |
| Ragdoll | 0 | 1 (11%) | 0 | |
| Russian Blue | 1 (17%) | 0 | 0 | |
| Approximate age (years) | n | 6 | 9 | 8 |
| Mean (SD) | 13.1 (5.03) | 13.7 (4.78) | 14.6 (4.50) | |
| Median | 14.4 | 12.7 | 15.4 | |
| Min, Max | 5.92, 18.0 | 6.00, 19.8 | 5.58, 20.0 | |
| Sex/status | Female spayed | 3 (50%) | 3 (33%) | 1 (13%) |
| Male castrated | 3 (50%) | 6 (67%) | 7 (88%) | |
| Creatinine (mg/dL) | n | 6 | 9 | 8 |
| Mean (SD) | 4.30 (0.74) | 5.30 (1.45) | 3.55 (1.27) | |
| Median | 4.50 | 5.70 | 3.35 | |
| Min, Max | 3.20, 5.30 | 3.10, 6.90 | 1.80, 6.00 | |
| IRIS stage | 2 | 0 | 0 | 2 (25.0%) |
| 3 | 4 (67%) | 3 (33%) | 5 (63%) | |
| 4 | 2 (33%) | 6 (67%) | 1 (13%) |
- Abbreviation: IRIS, International Renal Interest Society.
Cats were enrolled by order of presentation into the following treatment groups: Cats in Lo (n = 6) received a single dose on Day 0. Nine cats were enrolled in Lo ± Hi; 3 responded to the single dose on Day 0 and did not require the supplemental dose, whereas 5 cats received both the Day 0 and Day 42 dose. Eight cats were enrolled in Hi; 1 received the supplemental dose on Day 42 but was euthanized because of uremic crisis before it was reevaluated.
3.2 Change in PCV
For all cats combined, there was a significant (P < .04) increase in PCV from Day 0 to Day 28 (mean increase 5.67 ± 6.36 pp, n = 21), Day 42 (mean 7.57 ± 8.87 pp, n = 21), Day 56 (mean 9.59 ± 11.0 pp, n = 17), and Day 70 (mean 13.1 ± 13.5 pp, n = 14; Figure 1).
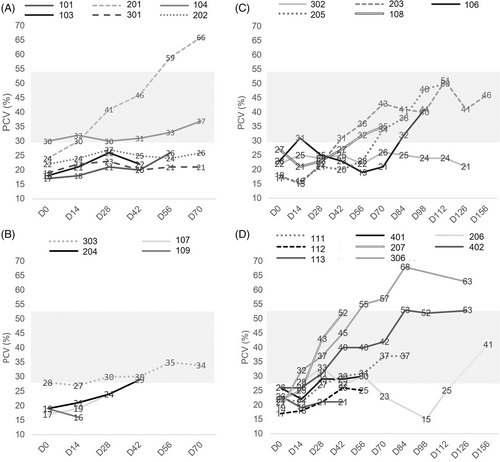
The cohort of Lo cats (n = 6) exhibited a 6.33 pp increase in PCV (P = .04) by Day 28 (Table 2).
| Lo | Lo ± Hi | Hi | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | N | Mean (SD) | N | Mean (SD) | ||||
| Day 0 | 6 | 21.7% (4.84) | 7 | 22.0% (4.32) | 8 | 22.0% (3.12) | |||
| Visit | N | Mean change in PCV (SD) | P valuea | N | Mean change in PCV (SD) | P valuea | N | Mean change in PCV (SD) | P valuea |
| Day 14 | 6 | 2.83 pp (1.72) | <.001 (r) | 7 | 0.29 pp (4.54) | .87 | 8 | 1.88 pp (5.77) | .41 |
| Day 28 | 6 | 6.33 pp (5.82) | <.001 (r) | 7 | 2.14 pp (2.97) | .79 | 8 | 8.25 pp (7.96) | .05 |
| Day 42 | 6 | 5.67 pp (8.09) | <.001 (r) | 7 | 4.43 pp (5.06) | .43 | 8 | 11.8 pp (11.09) | .01 |
| Day 56 | 5 | 10.2 pp (14.17) | .002 (r) | 6 | 5.33 pp (7.47) | .24 | 6 | 13.3 pp (11.55) | .008 |
| Day 70 | 4 | 13.8 pp (18.95) | .02 (r) | 4 | 9.67 pp (9.73) | .04 | 6 | 17.8 pp (14.93) | .004 |
- Note: r indicates values were ranked prior to generating analysis of variance (ANOVA).
- a P values generated for each cohort by repeated measures analysis of variance with study day as a fixed effect and site as a random effect.
Individual cats in Lo ± Hi (n = 9) demonstrated increases in PCV but the mean PCV of the cohort was not significantly different from baseline at any timepoint. When data from cats administered a supplemental dose at Day 42 was evaluated independently, the PCV did not increase significantly from baseline or from Day 42 (mean increase of 6.60 ± 6.61 pp from Day 42 to Day 70, n = 5, P = .1). Attrition of this study sample might have impacted the statistical analysis: 6 cats alive at Day 56, 5 alive at Day 70, 4 at Day 84, 3 at Days 98 and 112, and 2 at Day 126.
A significant increase in PCV from Day 0 was noted in the cohort of Hi cats (n = 8) at Day 28 (P = .05), Day 42 (P = .01), and Day 70 (P = .004) (Table 2).
3.3 Response
For all cats, the response rate to injection of SB-001 (Figure 2) was 86% (n = 19/22; 95% CI, 65%, 97%) defined as achieving the primary or secondary goal at any time from Day 28 to Day 70. Only 4 cats failed to achieve any response, 1 of which was euthanized on Day 14 and not included in the response analysis. For cats exiting the study early, data from the last visit were carried forward regardless of the day of exit.
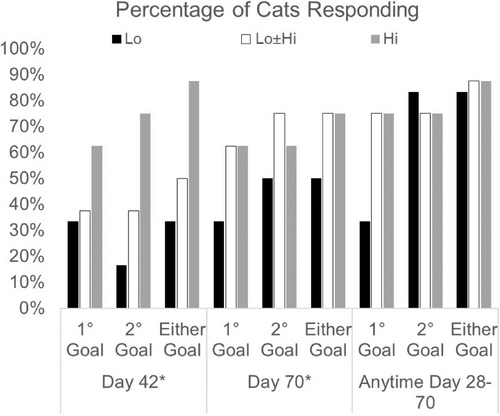
Cats reaching the primary goal at Day 42 had a higher mean body condition score at Day 0 (4.5 vs 2.75 out of 9, P = .02).
3.4 Response by cohort
Five of 6 cats in Lo achieved either the primary (n = 2) or secondary (n = 5 including the 2 meeting the primary goal) goals at any time between Day 28 and Day 70. Two cats from Lo achieved the primary goal by Day 28 and maintained this magnitude of response until the end of the study. In 1 of the 3 cats achieving only the secondary goal, the response was noted at presentation for euthanasia and might have represented hemoconcentration. In the other 2 cats, the secondary goal was not sustained beyond Day 28. One of the Lo cats failed to respond at any time point.
Six of 8 cats in Lo ± Hi achieved the primary goal and maintained it until either the end of the study (n = 3) or death (n = 3). In 1 additional cat, the secondary goal was met at presentation for euthanasia and might have represented hemoconcentration. A response was not achieved in 2 Lo ± Hi cats. Four out of 5 cats receiving a supplemental dose achieved the primary goal and 1 failed to respond. One cat was euthanized on Day 14 and was not included in the analysis of response.
An overall response rate of 7/8 (88%, 95% CI, 47%, 100%) was seen in Hi, with 6 cats meeting the primary goal and 1 cat meeting only the secondary goal at any time from Day 28 to Day 70. In 5 of these cats, the primary goal was achieved by Day 28 and sustained until the end of the study (n = 3) or death (n = 2). In addition, 1 cat achieved and sustained the primary goal and 1 cat achieved and sustained the secondary goal by Day 42 while 1 cat failed to respond.
Stable inflammatory bowel disease or GI lymphoma was present in 2 Lo (1 responder, 1 nonresponder), and 2 Lo ± Hi (both responders) cats.
3.5 Quality of life
The AWIS and the impact score were higher on Day 28 for responders (Table 3). Most responders had an increased sum total score (Table 4). Many owners struggled with completing the questionnaires, especially as the study progressed. The responses to the questionnaires became more difficult to evaluate as the animals developed progressive CKD and comorbidities. It was not uncommon for 1 or more clinical variables to improve even when the cat's overall condition and QOL deteriorated.
| Response | N | Mean (SD) | Min | Median | Max | P value | |
|---|---|---|---|---|---|---|---|
| AWIS | Respondera | 12 | 0.61 (1.06) | −1.08 | 0.56 | 2.81 | .01 |
| Nonresponder | 10 | −0.95 (1.55) | −3.89 | −0.52 | 0.98 | ||
| Impact score | Responder | 12 | 7.25 (13.19) | −14.00 | 7.00 | 6.50 | .01 |
| Nonresponder | 10 | −9.20 (15.65) | −37.00 | −6.50 | 13.00 |
- Abbreviation: AWIS, average-weighted impact score.
- a Defined as an increase in PCV to within the reference range (>28.2%).
| Visit day | Owner reported change at visit | Responder | ||
|---|---|---|---|---|
| Any time, Days 28-70 | Day 42 | Day 70 | ||
| D28 | Cats with QOL Data | (n = 19) | (n = 13) | (n = 15) |
| Animals with sum ≥1 | 12 (63%) | 8 (62%) | 11 (73%) | |
| D56 | Cats with QOL data | (n = 14) | (n = 9) | (n = 11) |
| Animals with sum ≥1 | 11 (79%) | 6 (67%) | 9 (82%) | |
| D70 | Cats with QOL data | (n = 12) | (n = 8) | (n = 9) |
| Animals with sum ≥1 | 9 (75%) | 6 (75%) | 7 (78%) | |
- Note: A responder was defined as a cat that achieved either the primary goal (PCV >28.2%) or secondary goal (PCV increase of >5%).
3.6 Serum iron status
Iron status was not different between responders and nonresponders at Day 70. Iron deficiency (TSAT < 20%) at screening, Day 28, and Day 70, was noted in 2, 8, and 7 responders, and 1, 3, and 1 nonresponders respectively.
3.7 Pre-existing antibodies
The 5 Lo ± Hi cats that received the supplemental dose were analyzed post hoc and negative for AAV1 neutralizing antibodies at screening. No other cats were evaluated for neutralizing antibodies.
3.8 Medications potentially affecting feline EPO response
Medications associated with hyporesponsiveness to ESAs were administered to both responders and nonresponders (defined at Day 70) during the study period. Specifically, renin-angiotensin-aldosterone system (RAAS) inhibitors (enalapril maleate, telmisartan) were administered to 2 responders and 3 nonresponders. Aluminum hydroxide was administered to 7 responders and 5 nonresponders.
3.9 Adverse events
Most adverse clinical signs noted in the study population could be attributed to the CKD or comorbidities. The events often reflected the target population (ie, elderly cats with CKD). Comorbidities were identified in responders and nonresponders; however, nonresponders received significantly more concurrent medications (excluding iron) than responders at Day 42 (P = .01, by primary goal; P = .04, by secondary goal) and on Day 70 (P = .01 by primary goal) suggesting they might have had more severe disease.
Polycythemia developed in 3 cats (1 in Lo, 2 in Hi): 2 cats developed a PCV of 66% and 57%, respectively on Day 70, and 1 additional cat peaked at 53% on Day 85. None of these cats required treatment for hypertension. The cats with polycythemia were followed after the study end, to Days 82, 125, and 135 with final PCVs of 67%, 63%, and 53%, respectively. None of these cats required therapeutic intervention for polycythemia at any time.
Moderate-to-severe hypertension (ie, systolic blood pressure ≥160 mm Hg) was observed in 12 of 23 cats at Day 0. Antihypertensive medications were administered to 4 of these cats before enrollment, initiated in an additional 6 of these cats and reinstated in 1 cat who had previously been treated but the treatment had been discontinued giving a total of 10 cats treated for hypertension by or after Day 0. An additional 6 cats developed hypertension during the study; only 5 cats remained normotensive. Hypertension with signs of encephalopathy, including seizures, was noted in 4 cats (3 responders by primary goal, 1 responder by secondary goal). The 4 cats that developed signs of encephalopathy were hypertensive at Day 0 (Day 0 mm Hg: 178, 184, 187, and 184, respectively). Once hypertension was confirmed and situational hypertension excluded, all hypertensive cats in the study were treated with either amlodipine or telmisartan (agent and dose determined by clinician discretion); except for 1 cat that could not be orally medicated. Most cats remained in the same or moved to a lesser IRIS hypertension substage during the study, including the cats that developed signs of encephalopathy. Exacerbation of hypertension did not appear to directly correlate with the dose of SB-001 or the increase in PCV.
No adverse events were noted in association with SB-001 injection. Injections were well tolerated; cats did not need additional sedation for the injection.
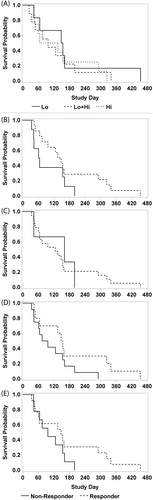
3.10 Survival
Median days alive for cats completing Day 28 was 130.5 (range, 34-454; n = 22). Overall median survival time for all cats was 126 days (n = 23). There was no difference between median survival of cats responding by primary goal at any time from Day 28 to Day 70 (141.5 days, n = 14) vs nonresponders (63 days, n = 8; P = .12). Median survival times were not different between the 3 cohorts (Figure 3) or between cats of different IRIS CKD stage.
4 DISCUSSION
In this prospective, multicenter efficacy and safety study, we report that intramuscular administration of SB-001 successfully induced erythropoiesis (as manifested by an increase in PCV) in most elderly cats with CKD. This rAAV-based gene therapy delivers a single-stranded DNA feline EPO transgene cassette to muscle cells to support EPO production.
The 2 primary functions of EPO are to maintain steady state RBC volume and rapidly replace that volume after blood loss. In health, EPO is synthesized primarily in the kidneys in response to a fall in tissue oxygen pressure.17 The liver and brain are minor, secondary sites of EPO production; EPO mRNA can also be found in the spleen, lung, and testis. These sources of EPO do not effectively compensate for the loss of renal EPO production in CKD. Cats with CKD do not synthesize adequate amounts of EPO, leading to normocytic, normochromic, and nonregenerative anemia. Anemia-associated hypoxia represents a risk factor for progressive CKD. Erythropoietin expression, and potentially regulation, differs between renal, hepatic, and skeletal muscle cells.17, 18
The initial dose administered to cats enrolled early in the study led to only a modest increase in PCV in all but 1 cat. As a result, the next cohort of cats enrolled in the study were administered the same dose on Day 0 with the option for a supplemental and larger dose of SB-001 administered on Day 42 if they had not responded by that day. Although the response of the cohort was not significant, two thirds of these cats demonstrated positive responses, with more robust responses seen in those cats administered the supplemental dose. The next cohort was administered the larger dose on Day 0. A significant increase in PCV was seen in this cohort, with three-quarters achieving the primary goal. The dose of 3.65 × 109 GC used in the Hi group cats is recommended for future larger studies because of this appropriate clinical response within a clinically relevant timeframe to assist in management of anemia.
The QOL assessments were oftentimes difficult to interpret and owner compliance was poor. The scales are designed to predict declining QOL and assist the owners in euthanasia, rather than to detect improving QOL. Nonetheless, changes in scores suggested responders had an improved QOL.
Because SB-001 is incorporated into the muscle cells, EPO production is expected to continue until cell death.19 For all cohorts, responses were sustained until the end of the study or death. Longer duration studies are needed to determine whether cats with CKD that receive SB-001 in muscle maintain PCV within the reference range over an extended period.
There might be an advantage to SB-001 in that it delivers consistent, closer to physiologic doses of feline-specific EPO compared to higher concentrations from an injectable ESA that undergo clearance over time. This might prevent the adverse events associated with high doses of ESA (eg, increased cardiovascular events and death in ESA-resistant humans).20
Expression levels of AAV delivered feline EPO could not be measured in these cats because the currently available assay is not sensitive enough to detect the very low levels expressed. Variability of EPO expression in individual cats might have contributed to hyporesponsiveness in some cats.
A potential disadvantage of this therapy is that it cannot be discontinued; clinicians will have to weigh their choices. None of the cats in this study became symptomatic for polycythemia; however, cats could undergo phlebotomy if this adverse event developed.
There are several reasons why humans might be resistant or hyporesponsive to an ESA. The most common reasons include absolute or relative iron deficiency and inflammation, whereas hyperparathyroidism, RAAS inhibitors, aluminum toxicosis, nutrient deficiencies (eg, cobalamin, folate, vitamin C, carnitine), comorbid hematologic disorders are less common reasons; PRCA induced by anti-EPO antibodies is rare.21-23 In this study, nonresponders were more likely to be administered a higher number of medications compared to responders. The number was too small to determine if there was medication-induced hyporesponsivity or if more severe comorbidities that required treatment with medications disrupted ESA action. Body condition score at enrollment was greater in responders vs nonresponders. Cats with a lower body condition score might have had comorbidities, such as inflammation or nutrient deficiencies, contributing to ESA hyporesponsiveness. Muscle mass was assessed during physical examination of all cats, muscle condition score was not assigned across sites limiting our ability to objectively assess correlations between response and muscle mass. However, it is possible that reduced muscle mass allowed for fewer myocytes being available to produce EPO.
Clinical samples were not analyzed for transgene expression or neutralizing anti-fEPO antibodies. The currently available assay for feline EPO lacks sensitivity to detect the expressed protein in healthy cats administered higher doses of SB-001 despite obvious response to therapy (data not shown). It is unlikely that the assay could detect the feline EPO in clinical cats treated at lower doses. There was no need to analyze any samples for neutralizing antibodies to feline EPO as this would have manifested as PRCA and no cat developed PRCA, which would have manifested as a rapid and severe drop in PCV.21 Because SB-001 encodes wild-type feline EPO, the risk of antibody generation to the transgene product is reduced.
Comorbidities were subjectively more severe in some cats that failed to respond to SB-001. Renin-angiotensin-aldosterone system inhibitors and aluminum hydroxide were administered to both nonresponders and responders; the numbers were too small to perform statistical analysis.
The use of ESAs is associated with several complications, some of which were seen in the study cats. Iron deficiency is anticipated after ESA administration because of the increased demand for iron during the erythropoietic response.6, 8, 9 Iron deficiency developed during treatment in both responders and nonresponders. Polycythemia developed in 2 cats but did not require treatment.
The natural means of regulating EPO response within the bone marrow is unclear; it remains to be determined if downregulation of EPO receptors leading to normalization of RBC mass would eventually occur in cats developing polycythemia after SB-001 injection. The higher magnitude of response to lower doses in CKD cats compared to the lower response to higher doses in normal cats might suggest that EPO receptors are upregulated in cats with EPO deficiency.
Worsening hypertension with concurrent evidence of target organ damage to the central nervous system was noted in 4 cats of this study. These cats were hypertensive at the start of the study and managed based on clinician discretion; uncontrolled hypertension was not an exclusion criteria for this study. Of the 28 normal nonanemic cats treated with SB-001 to date, none developed hypertension despite some developing polycythemia (data on file). Regardless of cause, prompt treatment of hypertension should be emphasized in further clinical studies. It might be necessary to modify existing guidelines for management of hypertension in cats with CKD that will be treated with an ESA to reduce the period of observation intended for the exclusion of situational hypertension. Seizure activity, with or without systemic hypertension, has been reported in association with administration of r-HuEPO (2 of 11 cats), recombinant feline EPO (1 of 26 cats), or darbepoetin (4 of 25 cats) for CKD-associated anemia and occurs in 1% to 2% of people treated with r-HuEPO or darbepoetin.6, 8, 9, 24
Survival for cats in this study was longer than expected, although the study was not designed to assess survival of cats with CKD-associated anemia. The median survival for cats in a previous study was only 25 days from the point of intervention for their anemia, whereas median survival time for all cats in the study reported herein was 126 days.25 Furthermore, the overall survival for all cats in the previous study was much longer (771 days) than that of cats with anemia emphasizing that cats with CKD-associated anemia have worse outcomes. It remains to be determined if earlier intervention in the management of anemia would lead to longer survival in cats with CKD.
In summary, we present data that indicate AAV-based SB-001 gene therapy represents a suitable treatment for non-regenerative anemia in elderly cats with CKD. In this study, the risk-benefit ratio was favorable. Additional studies at the final selected dose, with higher numbers of animals, will be required to determine full efficacy and safety.
ACKNOWLEDGMENT
No funding was received for this study. We acknowledge the following individuals for their contributions to this study: Clinical Investigators: Dr Kathryn Meurs, Dr Annalisa Prahl; Study Coordinators: Carrie Emke, Brianna Johnson, Miranda Worthington, Kevin Huynh, Amanda Lavoie, for providing care to study cats; and Statistician: Margie Bell for data analysis and preparation of the study report.
CONFLICT OF INTEREST DECLARATION
Shelly Vaden serves as Associate Editor for the Journal of Veterinary Internal Medicine. She was not involved in the review of this manuscript. Anne Traas, Jacky May, Lauren Olenick and Matt Wilson are employees and shareholders of Scout Bio, Inc, the company that is developing SB-001. Jim Wilson and Christian Hinderer are also shareholders in Scout Bio. Beth Oman is a paid consultant for Scout Bio. James M. Wilson is a paid advisor to and holds equity in iECURE, Scout Bio, Passage Bio, and the Center for Breakthrough Medicines (CBM). James M. Wilson also holds equity in the former G2 Bio asset companies. James M. Wilson has sponsored research agreements with Amicus Therapeutics, CBM, Elaaj Bio, FA212, former G2 Bio asset companies, iECURE, Passage Bio, and Scout Bio, which are licensees of Penn technology. Christian J. Hinderer holds equity in Scout Bio and a former G2 Bio asset company. James M. Wilson and Christian J. Hinderer are inventors on patents that have been licensed to various biopharmaceutical companies and for which they might receive payments.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC at North Carolina State University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




