Pharmacokinetics of pimobendan after oral administration to dogs with myxomatous mitral valve disease
Abstract
Background
Pimobendan is an important therapy for dogs with myxomatous mitral valve disease (MMVD). The pharmacokinetics are reported in healthy dogs but not in dogs with heart disease.
Hypothesis/Objectives
To determine if dog characteristics such as age, breed, body condition score, ACVIM stage of heart disease or biochemical laboratory value alter the pharmacokinetics of orally administered pimobendan and its metabolite in a cohort of dogs with naturally occurring MMVD.
Animals
Fifty-seven client-owned dogs with MMVD ACVIM Stage B2, C, or D and administered pimobendan to steady state blood concentrations.
Methods
Prospective, observational study. Samples were collected using a sparse-sampling protocol at specific intervals after administration of pimobendan. Plasma pimobendan and the active metabolite (O-desmethyl-pimobendan, ODMP) concentrations were determined via high-pressure liquid chromatography and fluorescence detection. Data was analyzed via a population pharmacokinetic approach and nonlinear mixed effects modeling (NLME). Numerous covariates were examined in the NLME model.
Results
The absorption and elimination half-lives (t1/2) were approximately 1.4 and 1 hour for pimobendan and 1.4 and 1.3 hours for ODMP, respectively. Pharmacokinetic parameters were highly variable, especially the values for pimobendan absorption and elimination rate, and absorption rate of ODMP with coefficients of variation of 147.84%, 64.51% and 64.49%, respectively. No covariate evaluated was a significant source of variability.
Conclusions and Clinical Importance
The pharmacokinetic parameters were highly variable among this group of dogs with MMVD. The variability was not associated with the dog's age, body weight or condition score, stage of heart disease, dose, serum creatinine, or alkaline phosphatase.
Abbreviations
-
- AUC
-
- area-under-the-curve
-
- CKCS
-
- Cavalier King Charles Spaniels
-
- CL
-
- systemic clearance
-
- Cmax
-
- peak concentration
-
- HPLC
-
- high-pressure liquid chromatography
-
- MMVD
-
- myxomatous mitral valve degeneration/disease
-
- NLME
-
- nonlinear mixed-effects modeling
-
- ODMP
-
- O-desmethyl-pimobendan
-
- Tmax
-
- time to peak concentration
1 INTRODUCTION
Pimobendan is useful for treatment of congestive heart failure in dogs secondary to myxomatous mitral valve disease (MMVD)1-4 and in delaying onset of heart failure in dogs with preclinical heart disease.5-8 Knowledge of the pharmacokinetics of pimobendan in dogs is relatively limited and has primarily been evaluated in small single dose studies in healthy, typically research dogs.9-12 To the authors' knowledge, there has not been an investigation of pharmacokinetics of pimobendan in dogs with MMVD. An understanding of the effects of dog factors such as age, body weight, breed, and severity of heart disease on pimobendan disposition, and that of its active metabolite, is important for refining dosage recommendations and improving the medical management of heart disease.
The objective of this study was to determine if dog characteristics such as age, breed, body condition score, mg/kg dose, or stage of heart disease alter the pharmacokinetics of orally administered pimobendan. An additional objective of the study was to determine if the pharmacokinetics of pimobendan observed in dogs with heart disease is comparable to that in healthy dogs. Our hypothesis is that the pharmacokinetic properties of pimobendan and its metabolite, O-desmethyl-pimobendan (ODMP), are altered by medication dose, body condition, and ACVIM stage of MMVD.
2 MATERIALS AND METHODS
2.1 Animals
Client-owned dogs were prospectively enrolled at the North Carolina State University Veterinary Hospital from June 2019 to April 2021. Inclusion criteria for enrollment were dogs diagnosed with naturally occurring MMVD who were receiving a consistent dose of the FDA-approved proprietary formulation of pimobendan (Vetmedin, Boehringer Ingelheim, Cologne, Germany) for at least 5 days. This product was used for the duration of the study. We targeted approximately 50 dogs for enrollment based on our experience with identifying significant covariates using nonlinear mixed effects pharmacokinetic modeling. The diagnosis for MMVD was based on echocardiography performed by a board-certified cardiologist or cardiology resident under cardiologist supervision. Dogs were not enrolled in the study if they had concurrent congenital heart disease or myocardial dysfunction (ejection fraction <50%); were receiving antacids or had chronic gastrointestinal disease, or right sided congestive heart failure (ascites) that could limit oral medication absorption; weighed less than 4 kg (due to the volume of blood required); had a temperament precluding serial blood draws or was in active congestive heart failure that required an increase in drug doses or a change in medications. This study was approved by the Institutional Animal Care and Use Committee. (Protocol # 19-013-O).
The dogs eligible for enrollment were ACVIM Stage B2, C, or D MMVD based on the 2009 ACVIM Consensus Guidelines for the diagnosis and treatment of MMVD.8 Due to the case management practices at the author's institution, dogs were assigned ACVIM Stage D if they were administered >0.6 mg/kg/d pimobendan and >4 mg/kg/d of furosemide concurrently, or were administered furosemide ≥6 mg/kg, or were receiving a ≥6 mg/kg/d furosemide equivalent dose of torsemide. To be eligible for the study, all medications (cardiac and noncardiac) were administered until steady state conditions were achieved, with no change in dosage for a minimum of 5 days before sampling. If the dog was administered a compounded version of pimobendan, they were transitioned to Vetmedin at least 5 days before sampling to ensure steady state conditions were attained.
2.2 Sampling
Food was withheld from the study dogs for a minimum of 8 hours before initial sample collection. A study investigator administered the pimobendan tablets simultaneously with other scheduled medications in the early morning. The tablets could be covered with no more than 1 teaspoon of a food vehicle if needed to facilitate medication administration. Dogs were fed the diet the owners provided 2 hours after the administration of pimobendan tablets, and the food consumption was recorded. Other prescribed medications were administered as per the client-specified treatment schedule. Water was provided at all times.
To minimize the frequency of blood sampling in the dogs with heart disease, a sparse-sampling strategy was used as in other studies performing pharmacokinetic analysis using population modeling.13-18 There were 10 designated sample points after administration of the pimobendan (zero minutes, 15 minutes, 30 minutes, 60 minutes, 90 minutes, 2 hours, 4 hours, 6 hours, 8 hours, and 12 hours). “Time zero” was considered any time between the dog arriving to the hospital and before the pimobendan was given. These time points were randomized for each dog so that no dog was sampled more than 3 times. Each enrolled dog received a study number which assigned the dog to randomized time points for collecting blood samples relative to pimobendan administration. Each dog had a total of 3 blood samples collected. At each sampling point, 2-3 mL of blood was collected from either the cephalic, saphenous or jugular vein and placed in a sodium heparin tube (BD Vacutainer sodium heparin tubes; Becton, Dickinson and Company, Franklin Lakes, NJ). Blood samples were centrifuged after collection, and the plasma was harvested and frozen. All samples were stored at −80°C until assayed.
2.3 Plasma and metabolite analysis
The plasma samples from the dogs were analyzed for both the parent drug, and the active metabolite, ODMP. Plasma pimobendan and ODMP were quantified with high-pressure liquid chromatography (HPLC) using a validated method developed in the author's (MGP) Clinical Pharmacology Laboratory. This laboratory uses the ICH validation guidelines for analytical methods (http://www.ich.org/products/guidelines/quality/quality-single/article/validation-of-analytical-procedures-text-and-methodology.html) and the guidelines published in Chapter <1225> of the United States Pharmacopeia (www.USP.org).
The samples were processed by thawing at room temperature, extraction of pimobendan and ODMP from 400 μL of plasma with solid phase extraction, and reconstituting with mobile phase. The samples were injected into an HPLC system that consisted of a quaternary solvent delivery system (flow rate, 0.5 mL/min), an autosampler (Agilent 1200 Series solvent delivery system, Agilent Technologies, Wilmington, DE), and detector (Agilent 1200 Series Detector, Agilent Technologies, Wilmington, DE). Chromatograms were integrated with a computer program (Agilent OpenLAB software, Agilent Technologies, Wilmington, DE). The column was a reverse-phase, 4.6 mm × 15 cm C8 column (Zorbax Rx-C1, MAC-MOD Analytical, Inc., Chadds Ford, PA) kept at a constant temperature of 40°C. Fresh mobile phase was prepared, filtered (0.45 μm), and degassed for each day's run.
The samples were quantified using a calibration curve that consisted of fortified blank dog plasma with 7 calibration standards ranging from 1 to 1000 ng/mL and included a blank (0 ng/mL). The limit of quantitation for the assay was 1 ng/mL. Fresh calibration standards and quality control samples were prepared daily for each day's run.
2.4 Drug and pharmacokinetic analysis
The analysis was performed using a population pharmacokinetic analysis and a sparse sampling design. These samples were analyzed using nonlinear mixed-effects modeling (NLME) and Phoenix software (Phoenix, NLME, Certara, St. Louis, MO).
Various models were tested with different error structures to determine the best fit base model. Final model selection was based on goodness of fit plots, diagnostic plots of residuals, scatter plots of predicted vs observed values, and statistical significance between models using the minimum value of the objective function (MOF).
Because there was substantial variation among dogs, addition of covariates was explored in the analysis to determine if there were any other factors that could explain the source of variability. Covariates were examined for their effect on random source (between subject) variability (η). In this analysis, covariates were added 1 at a time and the improvement (reduction) in the minimum value of the objective function was observed to determine if the effect was statistically significant. The minimum value of the objective function is proportional to minus twice the log-likelihood (−2LL) of the data. Therefore, a likelihood ratio allows for the comparison of 2 hierarchical models. The test between 2 models uses a Chi square distribution with v-degrees of freedom for each covariate tested. In our analysis, we used a reduction in the minimum value of the objective function of 6.64 (value found in standard Chi square tables) to determine if a covariate was significant. The 3 primary parameters—Ka (absorption rate), Ke (elimination rate), and V (volume of distribution, which is really V/F) were examined. Covariates explored were the class of heart failure (B2, C, and D) and body condition score used in a categorical analysis. Dog age, body weight (kg), pimobendan dose (mg/kg), serum creatinine concentration, and alkaline phosphatase activity were examined as continuous covariates. One dose was an extreme outlier (Dog # 54, dose, 1.3 mg/kg) and eliminated from the analysis. The most represented breed was Cavalier King Charles Spaniel (CKCS); therefore, this was examined as a categorical covariate as CKCS vs all others.
3 RESULTS
3.1 Study cohort characteristics
A total of 57 dogs were enrolled, including 35 ACVIM stage B2 dogs, 14 stage C dogs, and 8 stage D dogs. Twenty-five female and 32 male dogs were included. The median age was 11 years (range, 6-15 years). The median body weight was 8.5 kg (range, 4.35-27.1 kg). The median body condition score was 6/9 (range, 4-8/9). The most common breed represented was the CKCS (n = 14). No other breed was represented by more than 3 dogs in the study sample. The median dose of pimobendan for the entire study sample was 0.59 mg/kg/d (range, 0.41-2.68 mg/kg/d). The doses for pimobendan and furosemide were higher with increasing severity of MMVD. More details regarding dog characteristics and relevant concurrent medications are presented in Table 1.
| Parameter (units) | Stage B2 | Stage C | Stage D |
|---|---|---|---|
| Number of dogs | 35 | 14 | 8 |
| BCS (x/9) | 6 (4-8) | 6 (4-8) | 7 (4-7) |
| Body weight (kg, range) | 8.3 (4.43-21.2) | 10.85 (4.97-18.3) | 8.3 (7.7-19) |
| Pimobendan dose (mg/kg/day) | 0.55 (0.37-0.93) | 0.70 (0.50-1.56) | 1.44 (1.04-2.68) |
| Furosemide dose (mg/kg/day) | – | 3.94 | 6.25 |
| ACE-inhibitor (yes/no) | 31/4 | 12/2 | 8/0 |
- Note: For the variables listed, the median value is presented with the range provided in parentheses.
- Abbreviations: ACE, angiotensin converting enzyme; BCS, body condition score.
3.2 Pharmacokinetic analysis
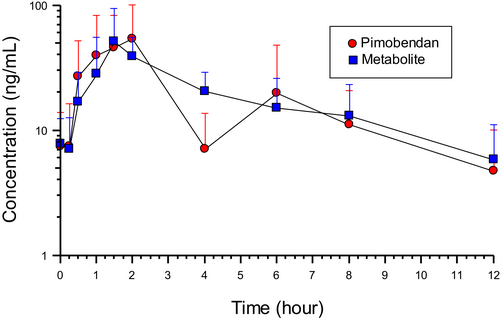
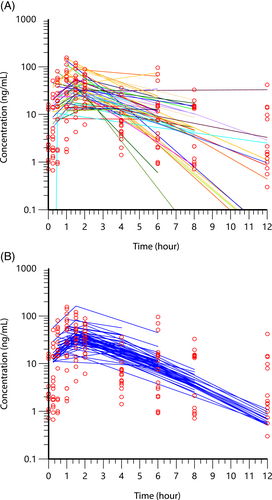
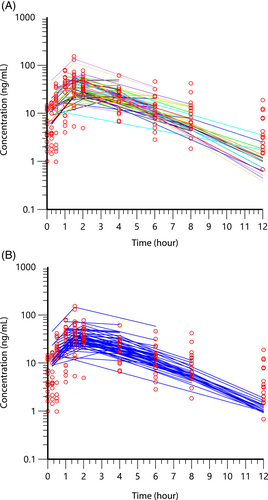
Time zero had 11 samples while the remaining time points had a median of 16 (range, 15-19) samples. The plasma concentrations representing mean concentrations from the study are shown in Figure 1. The population pharmacokinetic modeling results from the NLME are shown in Figures 2 and 3. In Figures 2 and 3, the left-side panel is the spaghetti plots of each individual dog, with open points representing each sample. The right-side panel is the fitted curve for the population after accounting for the between subject variation. The pharmacokinetic values from the analysis of pimobendan and ODMP are shown in Table 2 and Figures 1-3.
| Pimobendan | ODMP metabolite | ||||
|---|---|---|---|---|---|
| Parameter | Unit | Value | CV% | Value | CV% |
| θKa | 1/h | 0.503 | 147.84 | 0.502 | 64.49 |
| θV/F | L/kg | 2.52 | 26.20 | 3.23 | 13.11 |
| θKe | 1/h | 0.71 | 64.51 | 0.52 | 16.64 |
| Tmax | hours | 1.66 | 1.96 | ||
| AUC | ng*h/mL | 197.11 | 210.98 | ||
| Cmax | ng/mL | 42.96 | 39.65 | ||
| CL/F | L/kg/h | 1.80 | 1.68 | ||
| Ka t1/2 | hours | 1.38 | 1.38 | ||
| Ke t1/2 | hours | 0.97 | 1.33 | ||
- Note: Pharmacokinetic parameters were determined by nonlinear mixed effects modeling (NLME). Typical values for the population (θ, theta) are depicted, as well as the following parameters: V/F, volume of distribution; Ke, elimination rate constant, and respective half-life (t1/2); Ka, absorption rate, and respective half-life (t1/2); AUC, area under the drug concentration curve; Cmax, peak plasma concentration; CL/F, systemic clearance. Note that because the administration was not intravenous, the V and CL are expressed as per fraction absorbed (V/F and CL/F, respectively).
A high degree of variability was observed in this group of dogs as noted in the Figures 2 and 3 spaghetti plots on the left side of the panel, and parameters in Table 2. This variability was higher for pimobendan than its metabolite, ODMP. The coefficient of variation for pimobendan and ODMP for all dogs was 147.8%, 26.2% and 64.5%; and 64.5%, 13.1% and 16.6% for absorption rate, volume of distribution and elimination rate constant, respectively. Sources of variability examined were dog age, weight, dose, body condition score, stage of heart disease (B2, C, and D; B2 and combined C, D), serum creatinine concentration, serum alkaline phosphatase activity, and breed (CKCS vs other breeds). We entered these as covariates in the analysis, but the contribution to the random source variability (η) did not meet the threshold for a statistically significant effect to explain the variability of the data. No differences were detected in absorption, elimination, and volume of distribution in the 3 ACVIM stages of MMVD as well as a comparison of preclinical MMVD (stage B2) and dogs with congestive heart failure (combined ACVIM stage C & D). The stage of MMVD disease did not account for the variability in these parameters.
4 DISCUSSION
While there are studies evaluating the pharmacokinetics of pimobendan in a small number of normal dogs (and cats).9-12 This study examined pimobendan pharmacokinetic values after oral administration to dogs with naturally occurring MMVD using sparse sampling protocol. Previous population pharmacokinetic studies have been helpful to investigate drug pharmacokinetics in clinically affected animals and exotic species15-19 as these study animals cannot be sampled as often as a research colony of dogs. In our study, each dog had blood samples collected at three of the 10 time points. To compensate for the lack of time points for each animal, the investigators enrolled a relatively high number of animals (n = 57) compared to the studies using a standard pharmacokinetic design. The relatively high number of dogs allowed for a median of 16 samples at each time point in the study which is a relatively large number of samples as compared to previously referenced population pharmacokinetic studies. Previous published pimobendan pharmacokinetic studies9-12 have used more intense sampling techniques in a small number of healthy animals.
The population estimates obtained in this study using all 57 clinical dogs with MMVD given a median dose of 0.36 mg/kg were an elimination t1/2 (hours) of 0.97 and 1.33; Cmax (ng/mL) of 42.96 and 39.65; and AUC (ng*h/mL) of 197.11 and 210.98 for pimobendan and its metabolite, respectively. The pharmacokinetic parameters in the only other published study11 evaluating a similar pimobendan formulation are mostly comparable to this study except for the elimination half-life. In that study of 8 healthy dogs comparing oral vs rectal administration of pimobendan, pimobendan administered orally at a dose of 0.5 mg/kg had an elimination t1/2 (hours) of 1.8 ± 0.8 and 5.0 ± 2.7; Cmax (ng/mL) of 49.1 ± 28.7 and 30.9 ± 10.4; and AUC (ng*h/mL) of 148.4 ± 71.6 and 167.8 ± 36.2. In the manufacturer's package insert,19 the stated elimination half-lives of pimobendan and ODPM after a single dose of 0.25 mg/kg were approximately 0.5 and 2 hours which are similar to those of the present study.
These discrepancies highlight the most important finding in our study which is the high individual variability with regards to pharmacokinetic parameters. The coefficient of variation for pimobendan and ODMP for all dogs was 147.8%, 26.2% and 64.5%; and 64.5%, 13.1% and 16.6% for the typical population values of absorption rate, volume of distribution and elimination rate constant, respectively as noted in Table 2. In pharmacokinetic studies, greater than 40% coefficient of variability is considered highly variable, whereas <40% is low to moderate variability.13 The variables the authors hypothesized would alter the pharmacokinetics of pimobendan and ODMP included body condition score, dose of pimobendan (mg/kg), and stage of MMVD. However, when these variables were analyzed as a source of variability, there was no statistical significance. Because of the high individual variability in absorption and elimination combined with sparce sampling (3 samples per dog) in a relatively low number of dogs (57 dogs), we may have failed to identify a significant variable that altered the pharmacokinetic parameters (type II error). Dogs were on incrementally higher pimobendan doses as the stage of disease progressed as represented in Table 1. There was no significant difference in the pharmacokinetics when the dose or the ACVIM Stage were analyzed as the covariate. The dose of pimobendan is often escalated in clinical practice in dogs with worsening MMVD due to perceived positive response and the positive results of an experimental study in dogs with acute mitral valve regurgitation that demonstrated decreased left atrial pressure in a dose-dependent manner.20 Additionally, pimobendan resulted in reduction in reoccurrence of pulmonary edema in dogs in a dose-dependent manner.2 Our investigation did not assess clinical response, and therefore, we cannot report the clinical effects of high doses in this study. There is not a significant difference in glomerular filtration rate and cardiac size and function in dogs with nonazotemic, preclinical MMVD when given standard-dose (0.2-0.3 mg/kg q12h) and high-dose pimobendan (0.5-0.6 mg/kg q12h).21 Our results showed that the concentrations in plasma were highly variable, and dose was not a significant factor in the absorption or elimination of pimobendan or its metabolite.
Bloodwork variables were also investigated as potential sources of variation. A full chemistry panel allowed for routine review of renal and electrolyte values as well as protein concentration and alkaline phosphatase. Pimobendan and ODMP are approximately 90% protein bound.19 Because protein binding can affect hepatic drug clearance, serum albumin concentration was analyzed as a possible covariate, but this factor was not significantly different in this sample of dogs. The variation in serum alkaline phosphatase among dogs was also examined as a source of pharmacokinetic variability since pimobendan is metabolized by the liver to ODMP. However, Alkaline Phosphatase value also did not explain the variation in pharmacokinetics of the pimobendan metabolite, ODMP, in this study population.
According to the U.S. patent, pimobendan requires a specific pH, and excipient (citric acid) for adequate solubility and subsequent absorption.22 Therefore, the manufacturer originally recommended administration on an empty stomach and the correct proportion of excipients: pimobendan ratio. The newer pimobendan product insert states that food decreases the bioavailability of the aqueous solution but the effect on the tablet form is unknown.23 We used the approved FDA formulation of pimobendan and avoided compounded forms to ensure that a consistent formulation was administered. The dogs in this study were administered pimobendan after feed withholding to mitigate variable rates of absorption. Despite this strategy, the gastric pH even in fasted animals can range widely from 1 to 6.24 Some studies suggest stress, such as being in the hospital, can delay gastric emptying as much as 30% to 45%.24 Therefore, we delayed feeding until 2 hours after the pimobendan administration. This time was selected so the food would have a minimal effect on gastric pH even if gastric emptying was delayed from being hospitalized. Many dogs are likely to receive pimobendan with food in their home environment.
Another limitation of the study was the relatively fewer dogs with MMVD stage C and D as compared to stage B2. In hopes of overcoming this limitation, we performed analysis on the combined heart failure dogs (ACVIM stage C and D) as compared to the preclinical dogs (ACVIM stage B2). However, this analysis failed to show any relationship to the pharmacokinetic variables. Other variables such as possible genetic differences in metabolic pathways, or more sensitive markers of organ function could have provided some explanation for the high variability. Due to the high amount of variability seen in this clinical study, prohibitively large number of animals would have been needed to find a source of significant variability. Alternatively, it is unknown as to whether significance of covariates would have been identified if each dog had every time point sampled which is a limitation of the study. However, in intense sampling of client-owned dogs with advanced MMVD is generally not feasible. Furthermore, this study only assessed a single dose of pimobendan. Therefore, it is unknown what the potential daily variation in pharmacokinetics are for this drug in clinical patients.
In conclusion, the absorption, elimination of pimobendan and its metabolite, ODMP, were highly variable in this cohort of dogs with various stages of naturally occurring MMVD. The source of the variability was not identified.
ACKNOWLEDGMENT
Funding provided by the American College of Veterinary Internal Medicine (ACVIM) Cardiology Resident Research Grant (Pacemaker Fund). This project was presented in abstract form as a poster presentation at the 2021 ACVIM Forum Virtual. The authors acknowledge Delta Dise for her assistance in performing the drug concentration measurements; Allison Klein, Petra Vasilik, Bevin Annis, and Brooke Bridges for collecting samples and dog care as well the families who enrolled their pets in the study.
CONFLICT OF INTEREST DECLARATION
Teresa C. DeFrancesco participated in a video used in the launch of Vetmedin CA-1 in May 2022 for which she was paid an honorarium. Her participation in the video was after the completion (enrollment and data analysis) of this study. The results of this study were presented in June 2021. No other authors declare a conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the IACUC at North Carolina State University (protocol # 19-013-O).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




