Recurrence and survival in dogs with excised colorectal polyps: A retrospective study of 58 cases
Abstract
Background
Compared to humans, colorectal polyps are relatively rare in dogs. Epidemiological and prognostic data remain accordingly sparse, although they could help veterinary clinicians in the management of these cases.
Objectives
To report the epidemiological data of dogs with colorectal polyps and identify factors associated with recurrence and survival.
Animals
Fifty-eight client-owned dogs with colorectal polyps admitted to 7 veterinary hospitals (53 dogs from France, 5 dogs from Spain, and 4 dogs from Portugal) were included.
Methods
Retrospective multicentric cohort study. Medical records and long-term outcome of the dogs were reviewed. When available, histological samples were reassessed by 2 board-certified pathologists according to the revised Vienna classification (RVC).
Results
The West Highland White Terrier (WHWT) breed was significantly associated with the presence of colorectal polyps (OR: 20; 95% CI: 7.5-52; P < .001). The overall median time to recurrence was not reached after 2000 days. The overall estimated median survival time was 1640 days. WHWT breed and larger polyps were significantly associated with a shorter time of polyp recurrence after surgical removal (respectively, P = .05 and P = .01).
Conclusions and Clinical Importance
The probability of recurrence of colorectal polyps in dogs is low, but increased in WHWTs and larger polyps, which might benefit from routine screening after removal. No effective predictors of polyp recurrence and survival were identified using the RVC.
Abbreviations
-
- CI
-
- confidence interval
-
- NA
-
- not applicable
-
- NBI
-
- narrow-band imaging
-
- RVC
-
- revised Vienna classification
1 INTRODUCTION
In human medicine, a polyp is defined as a mucosal outgrowth visible to the naked eye and independent of its histological nature.1 A colorectal polyp therefore refers to a macroscopically visible mucosal protrusion in the colic or rectal lumen. This definition does not include poorly demarcated or “napkin-ring” masses.
In humans, several classifications are used to describe colorectal polyps based on morphology on endoscopy, narrow-band imaging or both modalities. Histologically, many classifications have been described. The revised Vienna classification (RVC) is the most widely used, and polyps are classified into 5 categories (no neoplasia, indefinite for neoplasia, mucosal low-grade neoplasia, mucosal high-grade neoplasia, and submucosal invasion of neoplasia).2 These classifications are routinely used to guide clinicians deciding whether a patient should be endoscopically monitored or undergo surgical resection.3-5
In dogs, morphological and narrow-band imaging classifications have not been used to categorize colorectal polyps, and they are mainly allocated into 3 histological categories: nonneoplastic (hyperplastic polyps), benign neoplasms (adenomas and carcinomas in situ), and malignant neoplasms (intramucosal or invasive adenocarcinomas).6-8 Only few descriptive studies on colorectal neoplasia in dogs have been conducted, with principal focusing on treatment procedures, immunohistochemistry, or genome analysis.9-18 One study investigated the use of endosonography in 25 dogs with polypoid lesions to assess their depth, but the technique missed submucosal invasion in 2 out of 4 cases.19 There is a significant association between adenocarcinomas with a nonpolypoid growth pattern and invasion/metastasis, compared to polypoid adenocarcinomas, but the authors do not differentiate between pedunculated and sessile polyps.20 Some studies identified some potential prognostic factors including the number of masses, the completeness of margins and the occurrence of complications during the excision procedure.21-24 In 78 cases of colorectal adenocarcinomas, dogs that were treated with excision or cryosurgery survived longer than those that only underwent biopsies.21 In 34 cases of adenomatous polyps and carcinoma in situ, dogs that had multiple masses or diffuse disease were at higher risk of recurrence and malignant transformation.22 In a third study that retrospectively included 93 dogs with benign or malignant epithelial rectal masses excised by submucosal resection via a transanal approach, recurrence was associated with complications of the procedure and incomplete margins, and death was more likely when recurrence occurred.23 Finally, in another retrospective study on 74 cases with rectal masses, hazard of death for dogs with incompletely excised masses was significantly greater than those with complete margins.24 The aims of this study were to (1) describe the demographic, clinical, endoscopic, and pathological data of dogs with colorectal polyps, and (2) identify factors, including epidemiological data, associated with the recurrence of colorectal polyps and survival.
2 MATERIALS AND METHODS
2.1 Cases
We reviewed the medical records from 7 veterinary centers (Aquivet veterinary hospital [Eysines, France], Canis veterinary hospital [Palma de Mallorca, Spain], Frégis veterinary hospital [Arcueil, France], Oniris veterinary teaching hospital [Nantes, France], OnlyVet veterinary hospital [Saint-Priest, France], and VetAgroSup veterinary teaching hospital [Marcy l'Etoile, France]) for dogs diagnosed with colorectal polyps. Dogs were included if a macroscopically visible colic or rectal mucosal protrusion was identified, excised and submitted to histological examination. When available, histological preparations were reevaluated by 2 boarded certified pathologists. Preparations could be unavailable because of property rights or because they had been discarded for storage reasons. Cases were included between September 1, 2006, and December 31, 2020.
2.2 Data collection
An online questionnaire (Google Form, Googleplex, Mountain View, United States of America) for each included case was completed by an internal medicine board specialist. The collected information included details about age at diagnosis, sex, reproductive status, breed, size (small dogs were defined by a body weight of less than 15 kg and large dogs by a body weight of 15 kg or more), clinical signs at presentation and the duration before polyp removal, physical examination findings, method of diagnosis, number of polyps, macroscopic description of the polyp (diameter, pedunculated versus sessile, and smooth versus irregular), distance of the polyp to the anus, method of removal (pull-out method, endoscopic diathermy, or colectomy) and associated complications, imaging modalities, duration of follow-up, date to first recurrence of polyp, and date and cause of death if applicable.
The overall number of dogs and the number of dogs for each breed that attended the correspondent hospital in the same time period were also recorded at 4 French centers (Aquivet veterinary hospital, Oniris veterinary teaching hospital, OnlyVet veterinary hospital and VetAgroSup veterinary teaching hospital) to assess the overall prevalence of colorectal polyps and association with breeds. The 4 centers contributed by providing 45 cases out of the 58 included (77%). The software of the remaining 3 centers could not give these numbers.
2.3 Histological analysis
The specimens were processed according to standard procedures and sections stained with hematoxylin-eosin-safran (HES). All slides were reviewed by 2 board-certified pathologists (T. Larcher and E. Brisebard) in a blinded fashion according to the RVC. A descriptive sheet (Microsoft Excel 365, Microsoft Corporation, Redmond, United States of America) to collect histopathological data was designed based on a previous publication by Schlemper (Table 1) and used to assign the diagnosis to a category according to the RVC (Table 2).25 In case of disagreement between the 2 pathologists, specimens were re-evaluated to reach a final consensual diagnosis used to identify factors associated with the recurrence of colorectal polyps and survival. Margins were reported to be complete or incomplete, and submucosa was described as present or absent of each sample.
| Category | Histological finding |
|---|---|
| Invasion | 1. No invasion |
| 2. Pseudoinvasion (misplacement of glands into muscularis mucosae or submucosa) | |
| 3. Invasion into lamina propria | |
| 4. Invasion into muscularis mucosae | |
| 5. Invasion into submucosa | |
| Glandular structure | 6. Simple glands |
| 7. Slightly crowded but regular arrangement of glands | |
| 8. Irregular arrangement of glands | |
| 9. Variable size of glands | |
| 10. Variable shape of glands | |
| 11. Glands with complex budding or branching | |
| 12. Gland with gland and/or bridging or back-to-back (cribriform pattern) | |
| Nuclei | 13. Regular arrangement of basally oriented spindle-shaped nuclei |
| 14. Homogeneous chromatin | |
| 15. Mild or moderate hyperchromatism of nuclei | |
| 16. Marked hyperchromatism of nuclei | |
| 17. Mild or no stratification of nuclei | |
| 18. Marked stratification of nuclei | |
| 19. Variable size and/or enlarged nuclei | |
| 20. Increased nuclear-cytoplasmic ratio | |
| 21. Loss of nuclear polarity | |
| 22. Rounded nuclei | |
| 23. Vesicular nuclei | |
| 24. Enlarged prominent nucleoli | |
| 25. Frequent and/or atypical mitotic figures | |
| Cells | 26. Variable size and shape of epithelial cells |
| 27. Marked inflammatory infiltrate | |
| 28. Gradual transition of atypical to normal epithelium |
| Category | Diagnosis |
|---|---|
| 1 | No neoplasia |
| 2 | Indefinite for neoplasia |
| 3 | Mucosal low-grade neoplasia (low-grade adenoma/dysplasia) |
| 4 | Mucosal high-grade neoplasia |
| 4-1 | High-grade adenoma/dysplasia |
| 4-2 | Noninvasive carcinoma (carcinoma in situ) |
| 4-3 | Suspicious for invasive carcinoma |
| 4-4 | Intramucosal carcinoma |
| 5 | Submucosal invasion of neoplasia (carcinoma invading the submucosa or beyond) |
2.4 Statistical analysis
The normality of quantitative variables was assessed using Shapiro-Wilk testing. Normally distributed variables are presented as mean and SD; otherwise, results are presented as median and interquartile range. Categorical variables are presented as percentages. The odds ratios were used to quantify the association between each breed and presence (versus absence) of colorectal polyps. A Fisher's exact test with a Bonferroni-Dunn correction for multiple comparison was used to statistical significance.
We allocated endoscopic diathermy and pull-out methods for polyp removal into the same group mucosectomy.
Because we did not have margins available in all cases, we considered categories 4.2 and above of the RVC (noninvasive carcinoma, suspicious for invasive carcinoma, intramucosal carcinoma, or submucosal invasion of neoplasia) as worse diagnosis likely to benefit from colectomy and therefore considered separately from categories 4.1 and below in statistical analysis for the associations with time to recurrence of polyps or death.
T0 was the time of first diagnosis of colorectal polyps; Trecurrence was the time of the first recurrence, if applicable; and Tdeath was the time of (all-cause) death, if applicable. The remission time was the time between Trecurrence and T0, and the survival time was the time between Tdeath and T0. In the survival analysis where the outcome was recurrence of polyps, recurrence-free dogs were censored at the time of the last follow-up appointment or at Tdeath if they died before the study endpoint (December 31, 2020). In the survival analysis where the outcome was all-cause death, surviving dogs were censored at the time of the last follow-up appointment.
The following explanatory variables were considered for the 2 survival analyses: age at T0, sex, reproductive status, size of the dog (small versus large dogs), breed (West Highland White Terrier versus non-West Highland White Terrier), basis of the polyp (pedunculated or sessile), largest diameter of the polyp, method of polyp removal (mucosectomy versus colectomy), the histological diagnosis (category 4.2 or above versus category 4.1 or below of the RVC) and the completeness of the margins (complete versus incomplete). We tested these variables for their association with time to recurrence or time to all-cause death using Kaplan-Meier curves and univariable Cox proportional hazard regression models. The variables were included into a multivariate Cox proportional model if the P-value in univariate analysis was ≤.2 by using a backward stepwise selection procedure based on the Akaike information criterion. Interaction terms between all the variables were tested, and they were included in the model if they were found to be significant. The assumption of proportionality of hazards was investigated using the scaled Schoenfeld residuals method. The following variables were not considered for survival analysis because they could participate to the outcome by influencing other already considered variables: duration before polyp removal, distance to the anus. Significant complication associated with the method of polyp removal was not reported and could not be used as a potential prognostic factor. Adjunctive medical treatments were not included either in this analysis because of their high diversity in terms of nature of drugs/diets, duration and combination of treatments.
Statistical analyses were performed using R (R Core Team [2021]. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/). P-values <.05 indicated statistically significant differences.
3 RESULTS
A total of 58 dogs were included in the study (49 dogs from France, 5 dogs from Spain, and 4 dogs from Portugal). Paraffin-embedded blocks for histology were available for reassessment for 31 dogs. For only 1 dog with a recurrence of colorectal polyp, both histological preparations (at first diagnosis and at recurrence) were available.
3.1 Epidemiological findings
The mean age of the dogs was 7.8 years (3.3). The reproductive status was known in 56 dogs. The group included 27 intact males (48%), 13 spayed females (23%), 12 castrated males (21%), and 4 intact females (7%). They were composed of 34/58 small dogs (59%) and 24 large dogs (41%). The odds ratio results for the identification of breeds associated with the presence of colorectal polyps are summarized in Table 3. After Bonferroni-Dunn correction, West Highland White Terriers were significantly overrepresented among dogs with colorectal polyps compared to dogs without colorectal polyps (OR: 20.0; 95% confidence interval [CI]: 7.5-52.1; corrected P < .001).
| Breed | Number of dogs of the breed that developed colorectal polyp | Number of dogs of the breed that did not develop colorectal polyp | Number of dogs of other breeds that developed colorectal polyp | Number of dogs of other breeds that did not develop colorectal polyp | Odds ratio | Upper 95% CI | Lower 95% CI | Adjusted P value |
|---|---|---|---|---|---|---|---|---|
| Fauve de Bretagne Basset | 1 | 122 | 27 | 144 994 | 44 | 327 | 5.9 | .6 |
| Pointer | 1 | 240 | 27 | 144 876 | 22 | 165 | 3.0 | 1 |
| West Highland White Terrier | 5 | 1578 | 23 | 143 538 | 20 | 52 | 7.5 | <.001 |
| Samoyed | 1 | 286 | 27 | 144 830 | 19 | 138 | 2.5 | 1 |
| Shetland Sheepdog | 1 | 478 | 27 | 144 638 | 11 | 83 | 1.5 | 1 |
| English Springer Spaniel | 1 | 545 | 27 | 144 571 | 9.8 | 72 | 1.3 | 1 |
| Doberman Pinscher | 1 | 683 | 27 | 144 433 | 7.8 | 58 | 1.1 | 1 |
| German Shorthaired Pointer | 1 | 695 | 27 | 144 421 | 7.7 | 57 | 1.0 | 1 |
| Beauceron | 2 | 1834 | 26 | 143 282 | 6.0 | 25 | 1.4 | 1 |
| Fox Terrier | 1 | 886 | 27 | 144 230 | 6.0 | 44 | 0.82 | 1 |
| Bull Terrier | 1 | 1125 | 27 | 143 991 | 4.7 | 35 | 0.64 | 1 |
| Boxer | 3 | 3727 | 25 | 141 389 | 4.6 | 15 | 1.4 | .85 |
| Brittany | 2 | 2466 | 26 | 142 650 | 4.4 | 19 | 1.1 | 1 |
| Cane Corso | 1 | 1257 | 27 | 143 859 | 4.2 | 31 | 0.58 | 1 |
| Lhasa Apso | 1 | 1293 | 27 | 143 823 | 4.1 | 30 | 0.56 | 1 |
| Golden Retriever | 3 | 5029 | 25 | 140 087 | 3.3 | 11 | 1.0 | 1 |
| French Bulldog | 4 | 7243 | 24 | 137 873 | 3.2 | 9.1 | 1.1 | 1 |
| Beagle | 1 | 2150 | 27 | 142 966 | 2.5 | 18 | 0.33 | 1 |
| English Setter | 1 | 2106 | 27 | 143 010 | 2.5 | 19 | 0.34 | 1 |
| Australian shepherd | 1 | 2217 | 27 | 142 899 | 2.4 | 18 | 0.32 | 1 |
| English Cocker Spaniel | 1 | 2279 | 27 | 142 837 | 2.3 | 17 | 0.32 | 1 |
| Poodle | 1 | 3017 | 27 | 142 099 | 1.7 | 13 | 0.24 | 1 |
| Jack Russell Terrier | 1 | 3574 | 27 | 141 542 | 1.5 | 11 | 0.20 | 1 |
| German Shepherd | 1 | 4892 | 27 | 140 224 | 1.1 | 7.8 | 0.14 | 1 |
| Labrador | 1 | 8306 | 27 | 136 810 | 0.61 | 4.5 | 0.083 | 1 |
| Mixed | 7 |
Twelve dogs suffered from comorbidities: leishmaniasis was reported in 3/58 dogs (5%), atopy and myxomatous mitral valve disease in 2/58 (3%) dogs each, steroid-responsive chronic enteropathy, urethral incompetence, chronic kidney disease, exocrine pancreatic insufficiency, and recurrent anal sacs impaction in 1/58 (2%) dogs each.
3.2 Clinical findings
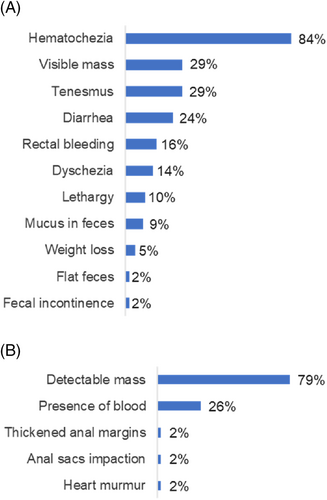
Owners' concerns and clinical findings at physical examination are summarized in Figure 1. Hematochezia was described in 49/58 dogs (84%), and polyps were detectable (visible or palpated) at physical examination in 46/58 dogs (79%).
3.3 Imaging findings
Only 46/58 (79%) files contained information on the possible performance of imaging studies. Among these, imaging investigation was performed in 34/46 dogs (74%) and included abdominal ultrasonography, thoracic radiographs and abdominal and thoracic computed tomography in 33/34 (97%), 9/34 (26%) and 1/34 dogs (2.9%), respectively.
On the single computed tomography study that was performed, no sign for submucosal invasion nor local, regional or distant metastasis was found.
Polyp was visible on ultrasound examination in only 1/34 dogs (5.9%). Invasion of the submucosa was observed in 1 (2.9%) which proved to be a carcinoma on histological analysis.
A primary, single lung mass was found in 1 dog (2.9%) with benign polyp. A diagnosis of a lung carcinoma was finally reached based on radiographic aspect (lobar opacification of the left caudal lung lobe), cytological examination (consistent with anaplastic carcinoma), and follow-up (died 910 days after polyp removal).
Equivocal images for distant metastasis were found in 4/34 dogs (12%) using abdominal ultrasonography. One dog had colic lymphadenopathy (hypoechoic and enlarged lymph nodes). The dog was diagnosed with carcinoma invading the submucosa on histopathology and died of pulmonary metastasis 1 year after polyp removal. A second dog had nonspecific hypoechoic liver nodules and was diagnosed with carcinoma invading the submucosa on histopathology. It died because of mandibular osteosarcoma 990 days after polyp removal. The third dog had hypoechoic and enlarged caudal colic, ileocolic and mesenteric lymph nodes. Polyp was not available for histological reassessment but primary diagnosis was adenoma. The dog was not followed up. The fourth dog had hypoechoic splenic nodules; polyp was not available for histological reassessment but primary diagnosis was adenocarcinoma; the rectal polyp recurred 90 days after surgical removal and the dog was then lost at follow-up.
3.4 Polyp description and colonoscopic findings
A colonoscopy was performed in 47/58 dogs (81%) and a single polyp was found in 44/47 dogs (94%) and 2 polyps in 3/47 dogs (6%). Macroscopic evidence of associated colitis (mucosal thickening, irregularity, hyperemia, ulceration, or a combination of these findings) was documented in 21/47 dogs (45%).
The median diameter of polyps was 10 mm (6.0-20.0 mm), and the median distance of the polyps to the anus was 3.0 cm (1.0-5.0 cm). The basis of the polyps was pedunculated in 29/49 dogs (59%) and sessile in 20/49 dogs (41%). The macroscopic aspect of the polyps was smooth in 48/58 dogs (83%) and irregular in 10/58 dogs (17%). Similar findings (basis and macroscopic appearance) were reported in dogs with 2 polyps.
3.5 Method of polyp removal and adjunctive medical therapy
The polyps were removed using the pull-out method in 45/58 dogs (78%), endoscopic diathermy resection in 7/58 dogs (12%), and colectomy in 6/58 dogs (10%).
Adjunctive medical treatments included metronidazole (21/58 dogs, duration range: 5-60 days, dose range: 12.5-20 mg/kg BID), amoxicillin/clavulanate (18/58 dogs, duration range: 5-21 days, dose range: 12.5-20 mg/kg BID), lactulose (8/58 dogs, duration range: 2-30 days, dose range: 0.2 mL/kg BID-0.3 mL/kg TID), tramadol (7/58 dogs, duration range: 5-10 days, dose range: 3-4 mg/kg BID), prednisolone (5/58 dogs, duration range: 10-20 days, initial dose range: 0.5-1 mg/kg/day, then tapered), highly-digestible diet (5/58 dogs, duration range: 7-30 days), firocoxib (4/58 dogs, duration range: 10-60 days, dose: 5 mg/kg SID), meloxicam (2/58 dogs, duration: 5 days, dose: 0.1 mg/kg SID), cefalexin (2/58 dogs, duration range: 10-15 days, dose: 15 mg/kg BID), robenacoxib (1/58 dogs, duration: 3 days, dose: 1 mg/kg SID), piroxicam (1/58 dogs, duration: 30 days, dose: 0.3 mg/kg SID), cimicoxib (1/58 dogs, duration: 21 days, dose: 2.5 mg/kg SID), mesalamine (1/58 dogs, duration: 10 days, dose: 10 mg/kg BID), tylosin (1/58 dogs, duration: 10 days, dose: 10 mg/kg TID) and/or hydrolyzed diet (1/58 dogs, duration: 60 days, dose).
3.6 Histologic findings
Margins were available for assessment in 25/32 samples (78%). Submucosa was present in 25/32 (78%) samples. Based on the final histological report, polyps were classified as mucosal low-grade neoplasia (category 3) in 3/32 samples (9%), high-grade adenoma/dysplasia (category 4-1) in 2/32 samples (6%), noninvasive carcinoma (category 4-2) in 4/32 samples (13%), carcinoma with suspected invasion (category 4-3) in 4/32 samples (13%), intramucosal carcinoma (category 4-4) in 12/32 samples (38%), and neoplasia with submucosal invasion (category 5) in 7/32 samples (22%). For the dog with samples from time of first presentation and from the recurrence, polyps were classified as category 5 and 4-4, respectively.
3.7 Outcome
Median follow-up duration was 142.5 (30-530) days.
3.7.1 Recurrence
Surveillance for recurrence was done using clinical signs in 29/58 dogs (50%), clinical signs and rectal examination in 19/58 dogs (33%), clinical signs, rectal examination, and ultrasound examination in 4/58 dogs (7%) and clinical signs and colonoscopy in 6/58 dogs (10%). Polyp recurrence was documented once in 8 dogs and twice in 1 dog over the study period. The estimated median recurrence time was not reached after 2000 days. The estimated recurrence probability 4 years after the first removal of the polyp was 0.21 (95% CI: 0.07-0.34). The results of the Cox proportional hazard regression model to identify variables associated with recurrence are presented in Table 4. Recurrence was significantly associated in the final multivariate model with diameter of the polyp (P = .01) and being a West Highland White Terrier (P = .05). Because none of the dogs in the group of category 4.1 or less of the histological diagnosis variable had recurrence of the polyp, an error resulted when attempting the P-value calculation using the Wald test. We therefore used Kaplan-Meier curves for comparison of the histological diagnosis (category 4.1 or less versus category 4.2 or above). The association between categories of histological diagnosis and time to recurrence was not significant (P logrank = .35; median survival time in group category 4.2 or above: NA, 95% IC: NA-NA).
| Explanatory variable (reference) | Univariable model hazard ratio (95% CI) | Univariable model P-value | Multivariable model hazard ratio (95% CI) | Multivariable model P-value |
|---|---|---|---|---|
| Age (continuous) | 1.0 (0.9-1.3) | .7 | ||
| Sex (male) | 1.4 (0.3-6.9) | .66 | ||
| Reproductive status (neutered) | 0.9 (0.2-3.8) | .88 | ||
| West Highland White Terrier (yes) | 5.6 (1.3-24.0) | .02 | 7.9 (1.02-60.9) | .05 |
| Basis of the polyp (sessile) | 4.0 (0.8-21.0) | .1 | ||
| Largest diameter of polyp (continuous) | 1.04 (1.008-1.07) | .01 | 1.04 (1.009-1.08) | .01 |
| Method of polyp removal (pull-out or endoscopic diathermy) | 0.5 (0.06-4.2) | .51 | ||
| Completeness of margins (complete) | 1.003 (0.10-9.7) | 1 |
- Note: Variables with P ≤ .2 in the univariable model were included in the initial multivariate model. An error occurred in calculation of the hazard ratio and associated P-value using the Wald test for the histological diagnosis as a potential explanatory variable because no event occurred in the group of category 4.2 or lower.
3.7.2 Survival
Fourteen dogs were dead at the time of data collection. The estimated median survival time was 1640 days (Figure 2). The cause of death was related to the polyp in 4/14 dogs (29%), with suture dehiscence after colectomy at recurrence in 1 dog and distant metastasis in 3 dogs. Four out of 14 dogs (29%) died of unknown cause while in 6/14 dogs (43%) the cause of death was unrelated to the polyp (1 dog in each of the following categories: congestive heart failure, car accident, presumed abdominal mesothelioma, mandibular osteosarcoma, hemangiosarcoma, and status epilepticus).
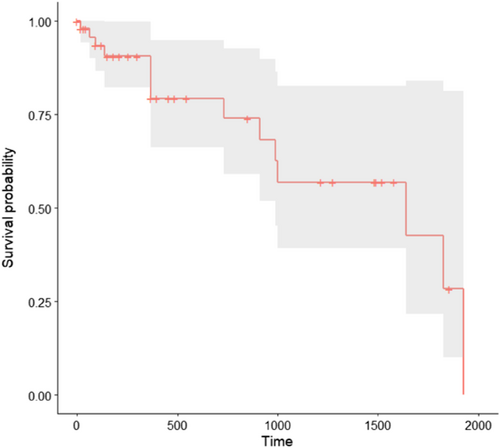
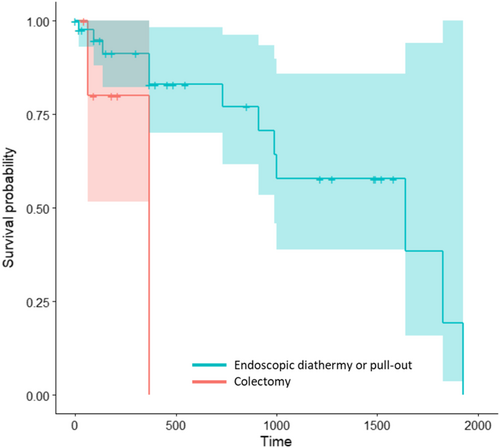
The results from the Cox proportional hazard regression model for identifying risk factors of death are presented in Table 5. Because none of the dogs in the group of category 4.1 or less of the histological diagnosis variable and none of the dogs in the group of incomplete margins of the sample died, an error resulted when attempting the P-value calculation using the Wald test. Among the other variables, the method of polyp removal was the only 1 to be eligible for multivariate analysis using the Wald test. We therefore used Kaplan-Meier curves for comparison of the histological diagnosis (category 4.1 or less versus category 4.2 or above), the margins of the sample (complete versus incomplete) and the method of polyp removal (endoscopic diathermy or pull-out versus colectomy). Time to death was not significantly different between categories of histological diagnosis or between categories of completeness of margins of samples (histological diagnosis: P logrank = .2, median survival time in group category 4.2 or above: 990 days, 95% IC: 990-NA; margins: P logrank = .05; median survival time in group complete margins: 990 days, 95% IC: 990-NA). Dogs that experienced colectomy had a higher probability of death during follow-up than those that underwent endoscopic diathermy or pull-out (P = .05, Figure 3). Two dogs died in the colectomy group: the first dog died only 2 months after the polyp removal because of a car accident. The second dog died 1 year after polyp removal because of distant metastasis of invasive carcinoma.
| Explanatory variable (reference) | Univariable model hazard ratio (95% CI) | Univariable model P-value | Multivariable model hazard ratio (95% CI) | Multivariable model P-value |
|---|---|---|---|---|
| Age (continuous) | 1.1 (0.9-1.3) | .31 | ||
| Sex (male) | 1.6 (0.5-6.0) | .46 | ||
| Reproductive status (neutered) | 1.5 (0.5-4.5) | .53 | ||
| West Highland White Terrier (yes) | 0.7 (0.09-5.9) | .75 | ||
| Basis of the polyp (sessile) | 1.6 (0.4-6.1) | .51 | ||
| Diameter of (largest) polyp (continuous) | 1.02 (0.99-1.05) | .15 | ||
| Method of polyp removal (pull-out or endoscopic diathermy) | 0.19 (0.04-1.1) | .06 | 0.19 (0.04-1.1) | .06 |
- Note: Variables with P ≤ .2 in univariable model were included in initial multivariate model. An error occurred in calculation of the hazard ratio and associated P-value using the Wald test for the histological diagnosis and completeness of margins as potential explanatory variables because no event occurred in the group of category 4.2 or lower and in the group of incomplete margins.
4 DISCUSSION
We found that West Highland White Terriers were overrepresented among dogs with colorectal polyps, which was already suggested in a previous study, although this finding was solely based on the percentage of dogs of the breed among the diseased cohort.17 Unlike miniature Dachshunds or Jack Russell Terriers in Japan, Dachshunds and Jack Russell Terriers of our cohort were not found to be overrepresented.20, 26, 27
Hematochezia, rectal bleeding or both abnormalities were consistently reported in this study, a similar finding to that reported in a study on colorectal adenocarcinomas in dogs.19 However, diarrhea was only present in one quarter of the dogs. Hematochezia/rectal bleeding without diarrhea should thus increase suspicion of colorectal polyps and motivate further investigation.
The median time to recurrence was not reached after 2000 days, and the recurrence probability at 4 years was 0.21 (95% CI: 0.07-0.34) in this study. These results are in contrast with those of a previous study, in which recurrence occurred in 12/22 (55%) carcinomas in situ with a median disease-free interval of 80 days.22 Differences in the methods of polyp removal could explain these discordant results. Recurrence might have been underestimated in our study because half of dogs were monitored for recurrence using only clinical signs.
Moreover, 2 important findings of our study were that being a West Highland White Terrier dogs and having a larger polyp were associated with a higher risk of recurrence at any time after removal. In a study on hereditary polyposis of Japanese Jack Russell Terriers, a high percentage of recurrence (7/11 dogs that underwent total surgical resection between 4 months and 2 years) was also reported.27 In studies focusing on inflammatory colorectal polyps of Japanese Miniature Dachshunds either treated by immunosuppression or excised with or without additional immunosuppression, recurrence likewise seemed to be more frequent than the overall probability that we found.26, 28 Thus, West Highland White Terriers at least from France, and Miniature Dachshunds and Jack Russell Terriers at least from Japan might benefit from endoscopic surveillance after treatment of colorectal polyps. Similarly, dogs with larger polyps might be good candidates for endoscopic monitoring for recurrence. In people with colorectal polyps treated with endoscopic mucosal resection (with or without thermal ablation of the mucosal defect) or endoscopic submucosal resection, some but not all studies suggested that larger resected polyps also were a significant risk factor for recurrence and might warrant increased surveillance.29, 30
The estimated median survival time in this study was about 5 years, and the cause of death in most dogs was unlikely to be related to the polyp. The results of univariable Cox proportional hazard regression models only identified the method of removal eligible for multivariable analysis. We could not assess the effect of the method of polyp removal in a multivariable model; the log rank test comparing the 2 groups of method of polyp removal suggested that this factor was significant but among the 2 dogs that died in the colectomy group, 1 had a car accident 2 months after removal of the polyp and the other 1 died of distant metastasis. One might believe that presumably more invasive tumors would have been more likely managed by colectomy (based on the aspect of the polyp or ultrasound appearance), but our data remain insufficient to test that hypothesis because of the retrospective nature of our study. We could not use the univariable model based on the histological diagnosis for interpretation, as no dog died in the group of categories 4.1 or lower. Although the median survival time in the group of category 4.2 or above was 990 days, the log rank test results failed to show any significant difference between the histological diagnosis categories using Kaplan-Meier analysis. This result might be the consequence of a lack of statistical power. In a previous study on 74 dogs with rectal masses undergoing pull-through surgery, the median survival time in dogs with respectively benign and malignant tumors was 1558 and 726 days, but the log rank test also did not show a significant difference between the 2 groups.24 To have 80% chance to show a significant difference (α = .05) of survival between dogs with benign masses (category 4.1 or lower) and dogs with malignant masses (category 4.2 or upper), estimating that the median survival time in the benign group would be 1500 days and the median survival time in the malignant group would be 750 days, and expecting that the drop-out rate would be 10%, the maximum follow-up time for 1 individual would be 3 years, and the maximum time to recruit all the necessary individuals would be 1 year, 115 dogs should be included in each group of a future prospective study.
Some limitations of our study need to be underlined. First, the small number of histological samples still available for standardized reassessment resulted in suboptimal statistical power; we think that we would collectively benefit from wider studies aiming to test the histological diagnosis of colorectal polyps for an effect in the recurrence, the survival or both in the dog. Second, because of the small number of dogs included, no death occurred in 1 histological group, which precluded searching for survival factors using a multivariable model. Also, most of the dogs had pull-out used for surgery and only 6 dogs were subsequently not in the mucosectomy group, making this study underpowered to test the effect of the method of polyp removal on recurrence and survival. We again expect that this issue could be addressed in studies including a larger number of dogs. Finally, we failed to find any significant association between the histological diagnosis and the recurrence or survival probabilities. Our findings are insufficient as a basis for advising pathologists and clinicians to use the RVC in its current edition in guiding the management and surveillance of colorectal polyps in dogs. Separating apart dogs with cancer invasion of the submucosa and dogs with cancer restricted to the mucosa in analysis might yield some distinctions or allow uncovering significant associations; in this study, we could not discriminate these dogs in this manner because the margins were not complete in all samples. Inclusion of the submucosa is thus highly recommended in case of biopsy or surgical resection for a further histopathological analysis. In humans, the RVC recommends management of category 5 colorectal polyps using urgent surgical resection as the risk for metastasis is high, whereas cancer restricted to the mucosa can be treated endoscopically or by local surgery.2 Biopsies of underlying submucosa after endoscopic resection of colorectal polyps in dogs might therefore help clinicians define the benefit of additional surgical excision; further research is needed to evaluate this conjecture. In human medicine, several other morphological and histological classifications are currently used in addition to RVC to help clinicians decide whether a patient should be endoscopically monitored or undergo surgical resection after endoscopic polypectomy. These tools include Narrow-Band Imaging International Colorectal Endoscopic (NICE), Japanese Narrow-Band Imaging Expert Team (JNET), Kudo Pit Pattern, Paris, Pragmatic Laterally Spreading Tumor (LST), Nonlifting Sign, Kikuchi and Kitajima, and Haggitt classifications.31, 32 For example, both pedunculated and sessile polyps with type 3 NICE classification (brown or black color, areas of disrupted or missing vessels, and amorphous or absence of pattern) should be considered to have deep submucosal invasion. The use of such a criterion is strongly recommended in human consensus statements, emphasizing the need for the refinement of endoscopic (including narrow-band imaging), morphological, and histological observations of colorectal polyps in veterinary medicine.
In conclusion, our main finding in this study was that West Highland White Terriers breed was associated with a shorter time to recurrence after the removal of colorectal polyps. We found an overall low probability of recurrence and an overall high median survival time in our study cohort. No significant differences between histological categories using the RVC were found but discriminating cancers based on whether there is invasion of the submucosa or not might lead to different results.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




