Differentiating airway inflammation in calves based on cluster analysis of bronchoalveolar lavage fluid cytology
Abstract
Background
Nonbronchoscopic bronchoalveolar lavage (nBAL) is routinely performed in calves, and airway cytology has great potential in airway disease diagnostics. A good reference framework for nBAL cytology is lacking.
Objectives
To distinguish different cytological profiles in nBAL from grouped housed calves using cluster analysis, and characterize these profiles on individual and herd levels.
Animals
Three hundred thirty-eight group-housed calves from 60 herds (mainly dairy and beef ).
Methods
Cross-sectional study. Differential counts of white blood cells were determined on nBAL fluid, followed by differentiation of cytological profiles by K-means-based cluster analysis. These profiles were characterized by reference values, decision tree analysis, and associations with clinical, ultrasonographic, bacteriological, and cytological features.
Results
A normal (55.9%), a neutrophilic (41.1%), and an eosinophilic profile (3.0%) were identified. The normal profile was characterized by reference values of 2.3% to 47.4% neutrophils, 35.1% to 95.1% macrophages, 0.4 to 22.9% lymphocytes, and 0.0% to 0.9% eosinophils. The neutrophilic profile was characterized by ≥44.5% neutrophils, <1.6% eosinophils, and <11.5% lymphocytes. This profile was associated with the isolation of Pasteurella multocida, the presence of neutrophils with toxic granulation, and the presence of phagocytosed bacteria in neutrophils. The eosinophilic profile was characterized by eosinophils ≥1.6% (neutrophilia present) or ≥2.4% (neutrophilia absent), and associated with the presence of mast cells. On herd level, the neutrophilic and eosinophilic profiles were present in 85.0% and 15.0% of the herds, respectively.
Conclusions and Clinical Importance
This study provides a first step in the development of cytological guidelines, aiding the assessment of airway health and inflammation in calves through nBAL fluid cytology.
Abbreviations
-
- BALf
-
- bronchoalveolar lavage fluid
-
- bBAL
-
- bronchoscopic bronchoalveolar lavage
-
- BRDC
-
- bovine respiratory disease complex
-
- COPD
-
- chronic obstructive pulmonary disease
-
- max
-
- maximum
-
- min
-
- minimum
-
- nBAL
-
- nonbronchoscopic bronchoalveolar lavage
-
- TNCC
-
- total nucleated cell count
-
- TUS
-
- thoracic ultrasound
1 INTRODUCTION
The bovine respiratory disease complex (BRDC) is a major cause of morbidity, mortality, production losses, and antimicrobial use in all cattle sectors.1-4 This complex is attributed to an interaction between viral and bacterial pathogens, environmental factors, and host immunity.5 Facing the emergence of antimicrobial resistance, targeted therapeutic, and preventive strategies depend on the identification of the involved pathogen in order to minimize antimicrobial use.6, 7 Therefore, the popularity of nonbronchoscopic bronchoalveolar lavage (nBAL) for pathogen identification increased in Western-European cattle practice because of its low cost, simplicity, and interpretability.8 Next to pathogen detection, the obtained bronchoalveolar lavage fluid (BALf) could also be used to assess airway inflammation through cytology. BALf cytology showed added value in the early distinguishment of different etiologies causing human9, 10 and equine11 pneumonia. Moreover, assessment of airway inflammation can evaluate the presence of noninfectious airway inflammation.12, 13 This phenomenon is well-recognized in human14 and equine15 medicine and, although poorly acknowledged, is also suspected to be present in calves.16, 17
Despite the potential of respiratory cytology, only a few researchers published normal cytological data on BALf in calves.18-21 Furthermore, there is a considerable degree of variation concerning the employed lavage techniques (eg, nBAL vs bronchoscopic bronchoalveolar lavage [bBAL], instillation volume used, etc.), which is known to influence the cytological findings. Challenges associated with identifying truly healthy calves may also contribute to this lack of an established reference framework. The use of clinical scoring is not well suited for this purpose because subclinical pneumonia is highly prevalent.16, 22 Thoracic ultrasound (TUS), although a well-established tool in the diagnosis of pneumonia,18, 23 cannot exclude the presence of bronchitis and upper airway inflammation. Complex diagnostics and targeted treatment of heterogeneous human respiratory diseases like asthma and chronic obstructive pulmonary disease (COPD) were improved by distinguishing subtypes using clustering methodologies. These methods group subjects based on the degree of similarity of particular characteristics such as cytological data of respiratory samples.24, 25 Since no reference values for calf nBAL cytology are established, a clustering algorithm could be used to identify a “truly healthy” reference population, hereby improving the interpretation of the cytological assessment of BALf.
Therefore, the first objective of this study was to identify differential cytological profiles of BALf in calves through cluster analysis and the associations of these profiles with clinical signs, TUS, bacteriology, and other cytological factors. The second objective was to establish reference values for the identified normal cytological profile and to create a decision tree that would allow the classification of future samples. The last objective of the study was to visualize the presence of these profiles at the herd level.
2 MATERIALS AND METHODS
2.1 Study design and data
For this study, a dataset from a previous cross-sectional study on the association of barn climate parameters and airway cytology was used.17 This dataset consisted of 439 approximately 1-week to 4-month-old dairy, beef, and veal calves. Data collection occurred between January and April 2017 on 62 commercial herds (23 dairy, 23 beef, 14 mixed, and 2 veal) in West and Eastern Flanders (Belgium). The methods regarding the collection of these data were approved by the local ethical committee under experimental license number EC2016-89 and are described in detail in the original study, but will be briefly summarized in the following section.17 Farms eligible for inclusion needed to be without an epidemic episode of respiratory disease and were selected with the help of different local veterinary practices and by their willingness to cooperate.
2.2 Examination and sampling
On each farm, 8 to 10 calves were randomly selected and sampled. Examination and sampling consisted of an extensive clinical exam, including the determination of both the Wisconsin26 and Davis respiratory score,27 TUS,18 and nBAL.28 A linear probe of 7.5 MHz, a scanning depth of 8 cm, and a maximal gain was used to perform TUS. The right and left lungs were visualized from the 10th to the 1st intercostal space and the 10th to the 2nd intercostal space, respectively. The presence of lesions and their depth as well as the presence of comet tails was recorded. Comet tails were recorded as multiple when at least 8 comet tails were present. For the nBAL procedure, an instillation volume of 0.6 mL/kg body weight was used in one aliquot. The percentage of saline retrieved and the presence of macroscopically visible blood contamination were noted.
2.3 Cytology and bacteriology of BALf
The cytological assessment consisted of a differential count and a manual determination of the total nucleated cell counts by a sole experienced operator. Differential counts were performed on a Diff-Quick (Merck KGaA, Darmstadt, Germany) stained cytospin preparation by counting 400 nucleated cells. Differential counts were calculated from the number of macrophages, neutrophils, lymphocytes, and eosinophils, excluding epithelial cells and mast cells. Epithelial cells were represented as a percentage of all the nuclear cells as an approximation of the degree of the bronchial component in BALf. Mast cells were evaluated as present or absent. The identification of Pasteurellaceae was determined by Matrix Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry (MALDI-TOF MS) after 24 hours incubation (37°C, CO2 5.0%) on Columbia blood agar enriched with 5% sheep blood (Oxoid, Hampshire, UK). The cultures were determined as negative, pure culture, dominant, mixed, or polymicrobial based on previously published criteria.29 For the detection of Mycoplasma bovis, BALf was cultured on a previously described selective indicative agar, and interpreted as positive or negative after incubation for 5 days (37°C, CO2 5.0%).8, 30
2.4 Statistical analysis
2.4.1 Clustering
All statistical analyses were performed in R (version 4.1.1)31 and graphics were made with the “ggplot2” package32 and Illustrator CS6 (version 16.0.0, Adobe, San Jose, California). The percentage of neutrophils, macrophages, lymphocytes, and eosinophils was scaled as preparation for clustering. Cluster tendency was determined with Hopkins statistics by use of the “factoextra” package.33 A value >0.7 of this statistic indicates a clustered dataset, whereas values around 0.5 indicate a random dataset.34 Clustering for 2 to 9 clusters was achieved by using a modified K-means algorithm. This algorithm implements bootstrap analysis and the use of data depth in the initialization process (The BRIk Algorithm35). Different validation indexes have been developed to choose an appropriate number of clusters. However, these indexes describe specific characteristics and might be inappropriate to use as a single index. Hence, an aggregation index of different internal quality indexes and a visual evaluation of average silhouette width plots were used. The following indexes were made with the “fpc” package37: within-cluster widest gap, separation index, average silhouette width, Pearson gamma, withinss, and parsimony. With these indexes, an aggregation index was made similar to the methods described by Henning.36 The cluster analysis was repeated for the most ideal number of clusters. Further cluster validation consisted of stability analysis performed with the “clv” package38 following the methods described by Ben-Hur and Guyon39 with 50 repetitions. The impact of blood-contaminated samples on cluster analysis was determined by repeating the cluster analysis on the dataset without the blood-contaminated samples and comparing this with the original cluster analysis. Since only a negligible effect was observed, these samples were maintained in the final cluster analysis. After cluster analysis, clusters were named as different cytological profiles based on the relative abundance of the studied cell types.
2.4.2 Associations of cytological profiles with clinical signs, TUS, bacteriology, and cytological features: categorical data
Associations between the obtained cytological profile affiliation and animal characteristics, clinical, sonographical, bacteriological, and cytological data were examined with multilevel logistic regression for categorical data. Exploration of categorical data was done through the examination of crosstabs. If in a parameter zero values were observed within a certain profile, this profile was omitted in further regression analysis. Then, multilevel logistic regression was performed with the herd as a random factor, using the “lme4” package.40 After the regression, a Holm post hoc analysis was performed using the “multcomp” package.41 This was done for the following clinical and sonographic features: breed (Holstein Friesian vs Belgian blue, Holstein Friesian vs other breeds, Belgian blue vs other breeds), weight category (<100 kg vs ≥100 kg), age category (1 week till 2 months vs 2 months till 4 months), head tilt (absent vs present), nasal discharge (absent vs present), ocular discharge (absent vs present), spontaneous cough (absent vs present), tracheal cough reflex (negative vs positive), and lung auscultation (presence of wheezes, crackles or pleural friction sounds vs normal breathing sounds), Wisconsin score (≥5 vs <5), Davis score (≥5 vs <5), sonographic consolidation (consolidation ≥1 cm vs consolidation <1 cm, consolidation ≥3 cm vs consolidation <3 cm) and comet tail artifacts (multiple comet tails vs few or no comet tails). The clinical and sonographic diagnosis was combined to create 4 categories: upper respiratory tract infection (URTI; Wisconsin score ≥ 5 and consolidation <1 cm), subclinical pneumonia (Wisconsin score < 5 and consolidation ≥1 cm), clinical pneumonia (Wisconsin score ≥ 5 and consolidation ≥1 cm), and healthy (Wisconsin score < 5 and consolidation <1 cm). Then logistic regression was applied as previously described. Lastly, the following characteristics of the nBAL were also examined with the same procedure: volume retrieved (<20% vs 20%-40%, 20%-40% vs >40%, <20% vs >40%), macroscopic blood contamination of the BALf (absent vs present), presence of Pasteurella multocida (absent vs present in pure or dominant culture), presence of Histophilus somni (absent vs present in pure or dominant culture), presence of Mannheimia haemolytica (absent vs present in pure or dominant culture), presence of Mycoplasma bovis (absent vs present), neutrophils with toxic granulation (absent vs present), phagocytized bacteria by neutrophils (absent vs present), multinucleated macrophages, phagocytized cells by macrophages (absent vs present), squamous epithelial cells (absent vs present), extracellular bacteria (absent vs present), and the presence of mast cells (absent vs present).
2.4.3 Associations of cytological profiles with clinical signs, TUS, bacteriology, and cytological features: continuous factors
Multilevel linear regression with the herd as a random factor was performed on continuous parameters: neutrophils, macrophages, lymphocytes, eosinophils, breathing frequency, body temperature, total nucleated cell count (TNCC), and the percentage of epithelial cells. Exploration of the normality of the data was done through histograms and Q-Q plots. If necessary, a transformation was applied before regression. A transformation was performed on following parameters: neutrophils (√x), lymphocytes [log10(x + 1)], breathing frequency [log10(x)], TNCC [log10(x)], and the percentage of epithelial cells (x0.25). After performing the regression, a Holm post hoc analysis was performed and the residuals were checked on normality using histogram, Q-Q plots, and grouped boxplots. The percentage of eosinophils presented as zero-inflated left skewed data, which was not possible to transform into normal data. An alternative method such as multiple-step regression42 was not reliable because of the low number of samples containing these cell types. Following, statistical analysis was not performed for this parameter.
2.4.4 Reference values determination and decision tree analysis
Reference values were established with the freeware “Reference Value Advisor”43 on samples that were clustered in the “normal profile” Since the distribution of the percentage of lymphocytes and eosinophils was severely skewed, a nonparametric method was used. Following cluster analysis, a regression and classification decision tree was made with the “rpart”44 package for establishing cut-off values to determine the cytological profile affiliation. Herein, the root node containing all data is continuously divided into leaf nodes in such a way that the homogeneity of these nodes is maximized for a specific characteristic45 (eg, the cytological profile). For this purpose, the Gini index, a measure of the impurity of the leaf nodes is minimalized. Division of leaf nodes was stopped based on an optimized complexity parameter, a minimum of 5 samples in the final node, and a minimum of 10 samples in the leaf nodes
2.5 Cytological profiles on herd level
The within-herd prevalence of pneumonia (consolidation ≥1 cm), the presence of a major respiratory pathogen (P. multocida, M. haemolytica, H. somni, or M. bovis), and their 95% confidence interval (Wilson interval with continuity correction) were calculated and used in graphical representations.
3 RESULTS
3.1 Herd and animals
From the original dataset of 439 calves, 101 observations were excluded because of missing values in the differential count data. The remaining 338 samples originated from 60 Belgian herds (23 dairy, 22 beef, 14 mixed, and 1 veal). The minor part (4.1%, 14/338) of the calves was less than 4 weeks old; 37.6% (127/338) were aged between 4 and 8 weeks, and 58.3% (197/338) were between 8 and 16 weeks. The frequency of breeds was as follows: 48.2% (163/338) Holstein Friesian calves, 46.2% (156/338) Belgian blue calves, and 5.6% (19/338) calves from other breeds or cross-breeds. Wisconsin scoring resulted in 20.8% (70/337) calves with BRDC, whereas Davis scoring resulted in 22.0% (74/337). On TUS, a consolidation ≥1 cm and ≥3 cm was found in 43.5% (147/338) and 29.5% (100/338) of the calves, respectively. For nBAL, an average fluid return rate and a SD of 35.0% ± 10.0% of the instilled volume was noted. Macroscopic contamination of BALf with blood was seen in 11.6% (35/338) of samples. A relevant bacterial pathogen (P. multocida, M. haemolytica, H. somni, or M. bovis) was found in 51.2% (173/338) of the cases. The median differential counts and the interquartile ranges were 51.6% ± 22.9% macrophages, 40.9% ± 24.2% neutrophils, 17.0% ± 17.29% lymphocytes, and 0.0% ± 0.97% eosinophils.
The mean herd prevalence and SD of healthy animals diagnosed by a negative Wisconsin score and no lesion of ≥1 cm on TUS was 51.7% ± 29.2% (min-max: 0.0%-100.0%). For URTI, subclinical and clinical pneumonia the observed prevalence was 7.4% ± 11.7% (min-max: 0.0%-50.0%), 29.8% ± 24.4% (min-max: 0.0%-100.0%), 11.0% ± 16.5% (min-max: 0.00%-66.0%), respectively. The prevalence of specific pathogens at the herd level is represented in Figure 1. Because of the aforementioned missing values, 2 to 9 calves per herd were available. From 21.7% (13/60) of the herds, data of less than 5 calves was applicable.
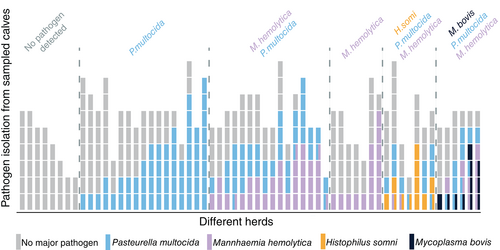
3.2 Cluster analysis
Clustering the differential count data was considered meaningful as the Hopkins value was 0.897. Following aggregation of different internal quality indices, a number of 3 clusters was found most appropriate. The average silhouette width was 0.42 and the estimated stability was 0.88. Because one of the clusters contained a significantly higher number of neutrophils (P = <.001), this cluster was called the “neutrophilic profile.” Another cluster had a strikingly higher eosinophil count than the other clusters, and was therefore named the “eosinophilic profile.” The remaining cluster had the highest percentage of macrophages (P = <.001). Since an abundance of macrophages is described as normal in BALf of calves,18-21 and the composition of this cluster resembles normal values of other species,46, 47 this cluster was considered the “normal profile.” Lymphocytes were significantly higher in the normal profile compared to the eosinophilic profile (P = .007) and the neutrophilic profile (P = <.001). A total of 55.9% (189/338) of the samples were classified as normal profile, 41.1% (139/338) as neutrophilic profile, and 3.0% (10/338) as eosinophilic profile. The results of the clustering algorithm are shown in Figure 2. The median and interquartile range of the different cell types in each cytological profile are given in Table 1.
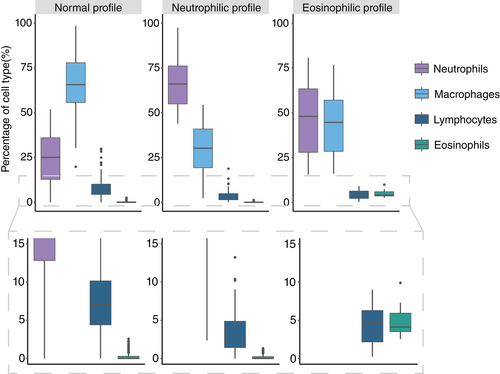
| Normal | Neutrophilic | Eosinophilic | |
|---|---|---|---|
| Neutrophils | 25.1 ± 23.2 [0.0-51.9]a | 66.0 ± 21.2 [43.9-97.4]b | 48.0 ± 35.5 [15.4-80.6]c |
| Macrophages | 65.7 ± 22.2 [19.8-98.5]a | 30.2 ± 21.6 [2.4-54.4]b | 44.6 ± 28.6 [16.0-76.6]c |
| Lymphocytes | 7.1 ± 5.8 [0.0-29.9]a | 2.8 ± 3.5 [0.0-18.8]b | 4.7 ± 4.1 [0.3-9.0]b |
| Eosinophils* | 0.0 ± 0.3 [0.0-2.5] | 0.0 ± 0.2 [0.0-1.3] | 4.1 ± 2.4 [2.5-9.9] |
- Note: Data are presented as medians ± interquartile range [min-max]. Statistical difference (P < .05) is marked by different letters in superscript.
- * Multilevel linear regression was not performed.
3.2.1 Associations of cytological profiles with categorical parameters
The cytological profile was not associated with breed, weight category, age category, head tilt, nasal and ocular discharge, spontaneous cough, tracheal cough reflex, Wisconsin score, Davis score, lung auscultation, consolidation ≥1 cm on TUS, consolidation ≥3 cm on TUS, multiple comet tails on TUS, the final diagnosis based on the combination of clinical and ultrasonographic examination, the percentage of volume retrieved with nBAL or macroscopically visible blood contamination. The proportion of positive samples for bacteriological and cytological criteria in the different profiles and their associations are presented in Table 2. Samples from the neutrophilic profile (43.2%) were more frequently positive for P. multocida than samples of the normal profile (26.5%, P = .001). The occurrence of M. haemolytica, H. somni, or M. bovis did not differ significantly among the profiles. Toxic granulation of neutrophils occurred more in the neutrophilic profile (49.6%) than in the normal profile (30.0%, P = < .001), whereas this did not differ between the eosinophilic profile and other profiles. Phagocytosis of bacteria was more likely in samples from the neutrophilic profiles (35.6%) compared to the normal profile (10.8%, P = .011). The presence of multinucleated macrophages was less likely in the neutrophilic profile (64.1%) compared to the normal profile (77.4%, P = .016). For the different profiles, no statistical difference was present among the presence of cellular phagocytosis by macrophages, the presence of squamous epithelial cells, and the presence of extracellular bacteria. Mast cells were significantly more encountered in samples of the eosinophilic profile (40% vs 9.4%-14.8%, P = .042), whereas there was no statistically significant difference between the 2 other profiles.
| Normal | Neutrophilic | Eosinophilic | ||||
|---|---|---|---|---|---|---|
| %pos | N | %pos | N | %pos | N | |
| P. multocida | 26.5a | 189 | 43.2b | 139 | 20.0ab | 10 |
| M. haemolytica | 16.4 | 189 | 16.6 | 139 | 20.0 | 10 |
| H. somni | 5.3 | 189 | 1.4 | 139 | 0.0* | 10 |
| M. bovis | 2.7 | 189 | 4.3 | 139 | 0.0* | 10 |
| Toxic neutrophils | 20.2a | 183 | 49.6b | 129 | 30.0ab | 10 |
| Phagocytosis bacteria by neutrophils | 10.8a | 185 | 35.6b | 135 | 30.0ab | 10 |
| Multinucleated macrophages | 77.4a | 183 | 64.1b | 128 | 70.0ab | 10 |
| Cellular phagocytosis by macrophage | 35.0 | 183 | 35.2 | 128 | 30.0 | 10 |
| Squamous epithelial cells | 72.1 | 183 | 64.1 | 128 | 60.0 | 10 |
| Extracellular bacteria | 23.5 | 183 | 21.9 | 128 | 40.0 | 10 |
| Mast cells | 14.8a | 183 | 9.4a | 128 | 40.0b | 10 |
- Note: Statistical difference (P < .05) is marked by different letters in superscript.
- * Multilevel logistic regression was not performed.
3.2.2 Associations of cytological profiles with continuous parameters
The median, interquartile range, and the association with continuous factors are shown in Table 3. A higher breathing frequency was associated with samples of the neutrophilic profile, compared to the normal (P = .024) and the eosinophilic profile (P = .037). No association was found between the cytological profile and body temperature. In contrast, the TNCC was higher in samples from the neutrophilic profile than in the samples from the normal profile (P < .001). The amount of epithelial cells was higher in the normal profile compared to the neutrophilic profile (P < .001).
| Normal | Neutrophilic | Eosinophilic | |
|---|---|---|---|
| Breathing frequency (rate/min) | 32 ± 16 [14-80]a | 36 ± 16 [20-116]b | 28 ± 11 [16-44]a |
| Temperature (°C) | 39.0 ± 0.6 [37.5-41.2] | 39.0 ± 0.7 [36.6-40.4] | 38.6 ± 0.8 [37.9-39.4] |
| TNCC (×109 cells/L) | 3.4 ± 3.4 [0.2-24.9]a | 7.5 ± 6.8 [0.3-44.6]b | 4.0 ± 2.8 [2.5-10.2]ab |
| Epithelial cells (%) | 16.2 ± 17.2 [0.0-95.9]a | 7.07 ± 10.21 [0.0-35.8]b | 10.1 ± 16.7 [1.0-39.0]ab |
- Note: Data are presented as medians ± interquartile range [min-max]. Statistical difference (P < .05) is marked by different letters in superscript.
- * Multilevel linear regression was not possible on this parameter.
3.3 Reference ranges and decision tree
The reference ranges for the normal cytological profile are presented with their 90% confidence interval in Table 4. A classification and regression decision tree analysis resulted in a tree with an accuracy of 98.0% (Figure 3). The sensitivity, specificity, and accuracy of each cytological profile is shown in Table 5. Suggested values for detection of an abnormal cytological profile were ≥44.5% of neutrophils and either ≥2.4% of eosinophils without concurrent neutrophilia or ≥1.6% in case of concurrent neutrophilia.
| Neutrophils | Macrophages | Lymphocytes | Eosinophils | |
|---|---|---|---|---|
| Reference values (%) | 2.3-47.4 | 35.1-95.1 | 0.4-22.9 | 0.0-1.9 |
| Lower 90 %CI (%) | 0.0-5.1 | 19.8-46.0 | 0.0-1.1 | 0.0-0.0 |
| Upper 90 %CI (%) | 44.4-51.9 | 90.5-98.5 | 17.4-29.9 | 1.1-2.6 |
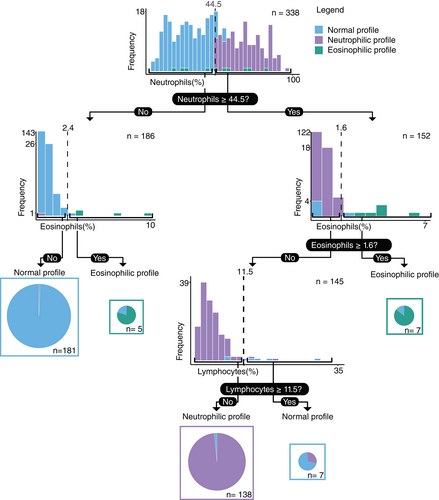
| Normal profile | Neutrophilic profile | Eosinophilic profile | |
|---|---|---|---|
| Sensitivity (%) | 97.9 | 97.8 | 1.0 |
| Specificity (%) | 98.0 | 99.0 | 99.4 |
| Accuracy (%) | 97.9 | 98.4 | 99.7 |
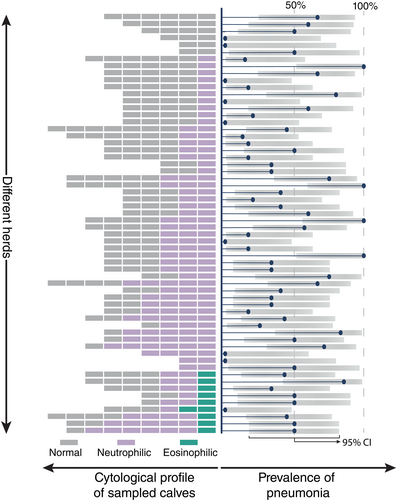
3.4 Within herd distribution of cytological profiles
The prevalence of the different cytological profiles is shown per herd together with the prevalence of pneumonia (Figure 4) and the proportion of pathogen-positive nBALs (Figure 5). Only in a minority of the herds, all sampled calves showed a normal profile (10.0%, 6/60). The occurrence of both, normal and neutrophilic profiles within one herd was found in 70.0% (42/60) of the herds. In 15.0% (9/60) of the herds, sampled calves displayed a normal, neutrophilic or eosinophilic profile. The remaining 5% (3/60) of the herds consisted of calves with a neutrophilic profile only.

4 DISCUSSION
The availability of an extensive dataset on cytological and bacteriological data from group-housed calves was a great opportunity to perform a cluster analysis to determine different cytological profiles, propose reference values, and determine the association between profiles with clinical and diagnostic data in calves. Subsequently, we created a decision tree that could support the interpretation of cytological results from BALf, and finally, we investigated the herd prevalence of specific profiles.
In our study, 3 cytological profiles in the individual BALf taken by nBAL from calves were observed, namely, the normal, neutrophilic, and eosinophilic profiles. The normal profile was the most common at the calf level, and showed a predominance of macrophages followed by neutrophils and lymphocytes. Eosinophils were present in very low numbers. The calculated reference values were 35.1% to 95.1% macrophages, 2.3% to 47.4% neutrophils, 0.4% to 22.9% lymphocytes, and 0.0% to 1.9% eosinophils. Although comparison of these with other species and studies is difficult because of different sampling protocols, the high neutrophil numbers are remarkable in comparison to previously published values in calves (1.25%, 1.7%),18, 21 horses (<5%),47, 48 and healthy children (0%-17%).49 For the other cell types, more similar reference values were previously observed. For example, Ollivett et al. mentioned a mean of 97% macrophages and 1% eosinophils for BALf obtained by bBAL of 4 clinical and ultrasonographical healthy calves.18 Likewise, values of 87.5% macrophages and 5% eosinophils were obtained from nBAL obtained BALf from calves weighing 350 kg.21 This is in line with proposed reference values for horses (50%-70% macrophages, <5% eosinophils)47, 48 and healthy children (34.6%-94% macrophages, 0.0%-3.6% eosinophils).49 The reference range for lymphocytes is comparable to the values previously published for nBAL in calves (10.3% lymphocytes)21 and to the numbers found in respiratory healthy children (1%-22%).49
The remarkably high neutrophil count in our study can be the result of different factors, such as the used sampling protocol or the etiology of the airway inflammation. The use of a nonbronchoscopic method could have caused an elevation of neutrophils because of a more proximal sampling of the airway's, as was observed when comparing nBAL to bBAL methods in humans.50 The use of a lower instillation volume and the use of one aliquot instead of multiple were associated with higher neutrophil count in horses51 and Holstein Friesian calves,19 and consequently could have had an influence on our results. Next to this, a higher neutrophil count could be caused by the presence of infectious or noninfectious airway inflammation. For example, intranasal vaccination causes a predominately neutrophilic inflammation reaction.52 Since the vaccination status of the calves was unknown, vaccination could have contributed to the higher neutrophil counts in our study. In addition, elevated neutrophil counts have been associated with exposure to particulate matter 10 in calves17 and low-grade airway inflammation caused by environmental factors such as airway pollution in other animal species.15 In our study, exposure of calves to a suboptimal barn climate, with high amounts of particulate matter or other air pollutants is probable, since animals were housed in commercial farms with a variety of barn features.
The second most observed profile, the neutrophilic profile, is characterized by an increase of neutrophils, a decrease of macrophages, and no increase of eosinophils compared to the normal profile. Neutrophilia in BALf is commonly linked to infectious pneumonia in different animal species.9, 10, 12, 18, 46 In our study, no association between the affiliation to the neutrophilic profile and the occurrence of ultrasound-confirmed pneumonia could be established. In contrast, Ollivett et al. described an association of neutrophilia with ultrasound-confirmed pneumonia when using bBAL. This difference could be because of the blind sampling technique used in our study, which equally targets cranial and caudal lobes in calves.28 Since lesions of bronchopneumonia are more frequently found in the cranial lobes, nBAL sampling will not always be representative of the affected lung part.28 However, a link between the neutrophilic profile and the presence of P. multocida was observed. This is in line with observations in BALf from humans with bacterial pneumonia.10 Moreover, an association was found between samples belonging to the neutrophilic profile with the presence of neutrophils with toxic granulation and phagocytosed bacteria. These are both signs of bacterial infections, where phagocytosed bacteria have been used in the diagnosis of ventilator-associated pneumonia in humans.53 These 3 associations suggest that animals with samples fitting to the neutrophilic profile are more probable to have a suppurative infection. Conversely, no association of this profile was found with M. haemolytica, H. somni, or M. bovis. The prevalence of the latter two was very low in this study, possibly resulting in limited power to detect any association. Concerning M. haemolytica, some serotypes (A1 and A6) are clearly associated with respiratory disease, whereas for other serotypes (A2), contribution to disease is more controversial.54, 55 It is probable that commensal serotypes will not induce neutrophilia. In addition, leukotoxins of M. haemolytica have been shown to induce neutrophilic extracellular trap formation.56 Neutrophils involved in these traps are likely masked for differential counts and subsequently result in a lower neutrophilic count. In addition, upper respiratory tract contamination of BALf could have influenced the results of BALf culture. However, this seems less likely following that only dominant cultures were considered positive in this study and that the presence of polymicrobial deep nasal swabs did not influence the presence of pure or negative culture results of BALf in a comparative study.8 Besides infectious causes, neutrophilia is also seen in several hypersensitivity diseases like recurrent airway obstruction in horses, COPD, and certain forms of asthma in humans.12, 25 Whether similar etiologies contribute to the observed neutrophilia in calves is currently unclear.
The third profile, only observed in the minority of the calves, is the eosinophilic profile. This profile was characterized by an elevation of eosinophils with or without concurrent elevation of neutrophils. The elevation of eosinophils was mostly mild and none of the cases had a percentage of >25%, which is the cut-off value for true eosinophilic pneumonia in humans.46 Nevertheless, mild elevation of eosinophils, with or without elevation of neutrophils, is seen in different phenotypes of human25 and equine15, 57 asthma. Both are multifactorial hypersensitivity disorders where environmental factors, such as organic dust exposure, have a great contribution.15, 58, 59 In our study, mast cells were seen more frequently in samples belonging to the eosinophilic profile, strengthening the resemblance with equine asthma.57 Alternatively, eosinophilia could be caused by parasitic disorders. However, infection with lungworm is impossible in this calf population because of indoor housing and the age of the studied population.60 Because of the mentioned similarities with some phenotypes of asthma in other species, the cases with an eosinophilic profile in the present study are more likely to be cases of bovine asthma, a disease thus far suspected but undocumented in cattle in contrast to humans,61 horses,57 and cats.62
To aid the classification of future BALf samples to the aforementioned profiles, a classification and regression tree was made based on a few cut-off values. For neutrophils, the cut-off value (44.5%) was almost similar to the value for suppurative infection and acute lung injury in human BALf (50%).46 For eosinophils, cut-off values (1.6% and 2.4%) slightly differed on the neutrophilic percentage of the sample, which are highly comparable to the recommended cut-off for eosinophilia in human46 and equine57 asthma (≤1%). The final cut-off value for lymphocyte percentage (≥11.5%) categorizes samples into the normal profile, though with a higher neutrophil and lymphocyte count. Possibly, these samples represent a 4th cytological profile that could not be separated with the cluster analysis because of the rarity of these characteristics in the studied population. A mixed inflammation of neutrophils and lymphocytes has previously been reported found in dogs, and was associated with lower airway infection, chronic bronchitis, and aspiration injury.63 Whether the observations of our study represent a similar mixed inflammation in this calf population needs to be determined.
Because group-housed calves are commonly exposed to a similar stable climate and infectious agents, the distribution of the 3 cytological profiles, the prevalence of pneumonia, and major pathogens was explored in the different herds. At herd level, calves with the neutrophilic profile were present in 85.0% of the herds, whereas in 15.0% of these herds also an eosinophilic profile was present. Within the herds, the presence of animals with a normal and neutrophilic profile was most common. This suggests that neutrophilic airway inflammation is highly prevalent in Belgian herds. This may be associated with viral or bacterial infections or with exposure to air pollutants. Neutrophilic airway inflammation on a herd level did not seem associated with ultrasonographic lesions or the presence of bacterial pathogen detection. However, careful interpretation is warranted, as the lack of association with ultrasonographic lesions could possibly be caused by the use of the blinded sampling technique. Lack of an association with Pasteurellaceae and M. bovis on herd level could be because of certain isolated bacteria representing commensal bacteria, the presence of noninfectious airway inflammation, or a viral agent (not explored). Finally, the eosinophilic profile was only seen in 1 or 2 of the sampled calves of a few herds and only in 1 or 2 animals of the sampled calves, suggesting a more individual pathology (eg, asthma).
This study had some limitations. The use of nBAL with a small instillation volume has an impact on cytological results and is less suited for localized lung pathologies. Nevertheless, in this study, nBAL with small volumes was preferred for several reasons. A much lower complication rate of the nBAL and an cost reduction of 87% have been demonstrated when comparing the nBAL to bBAL in macaques.64 Moreover, the nBAL technique performed in our study does not require extended training, allows easy sterilization, and is already routinely used in several Western European countries.8, 64 Therefore, nBAL is the most suitable for large-scale usage in a farm setting, where the cost-benefit of diagnostics is crucial. The established decision tree should be interpreted carefully since the dataset was not divided into a test and train dataset, resulting in a limited internal validation (cross-validation). Hence, this tree is less suited for external use.65 Another limitation concerns the k-means clustering algorithm, which is sensitive to the outlier present in 2 of the 4 used variables in this study. To minimize this effect, a modified, more robust k-means algorithm was used. Manual counting of cells is subjected to high intraobserver and interobserver variability. To minimize the sources of variability all samples were evaluated by the same veterinarian. Besides, the results of logistic regression analysis should be interpreted carefully for the eosinophilic profile because this profile had a low number of samples. Similar to this, calves with a head tilt, ocular discharge, H. somni, or M. bovis were poorly present in this study and lack of associations with these factors should be interpreted with caution. Likewise, the limited sample size for some farms warrants careful interpretation of the estimated within herd prevalence. This information was only added for descriptive purposes, as the primary focus of this study was the individual calf level. Lastly, because of financial reasons and limited resources to detect all viral agents at once, viral etiologies were not determined in the samples. Including viruses in future research could result in a more precise association of cytological profiles to pneumonia and specific pathogens.
In conclusion, the present study provides a reference framework for the cytological assessment of airway health and inflammation in calves and has great potential for a convenient interpretation of nBAL obtained BALf cytology. BALf fitting to a neutrophilic profile in combination with neutrophils with toxic granulation and phagocytosed bacteria by neutrophils on cytology are promising factors for the detection of a suppurative infection. Whereas in the absence of Pasteurellaceae and M. bovis, the neutrophilic profile could indicate noninfectious airway inflammation or a viral infection. The eosinophilic profile showed similarities with eosinophilic phenotypes of human and equine asthma, increasing the suspicion of bovine asthma.
ACKNOWLEDGMENT
This research was partially funded by a special research fund of Ghent University (01D25016 & BOF/STA/202009/006). Bacterial species identification was done by MALDI-TOF MS financed by the Research Foundation-Flanders (FWO-Vlaanderen) as Hercules project AUGE/15/05 (G0H2516 N). The dataset of this study is available with the corresponding author, upon reasonable request.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Study conducted in compliance with the Ghent University rules of animal experiments with the approval of the university's animal experiment ethics committee.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




