Efficacy of an elemental diet in achieving clinical remission in dogs with chronic enteropathy
Abstract
Background
Diet may induce clinical remission in dogs with chronic enteropathy (CE). Elemental diets (EDs), providing protein as amino acids, modulate intestinal immunity and microbiome in rodents and humans.
Hypothesis
Evaluate the impact of an amino acid-based kibble (EL) on CE clinical activity and gastrointestinal (GI)-relevant variables.
Animals
Client-owned dogs (n = 23) with inadequately controlled CE.
Methods
Prospective, uncontrolled clinical trial. Diagnostic evaluation including upper and lower GI endoscopy was performed before study entry. Canine chronic enteropathy clinical activity index (CCECAI), serum biomarkers, and fecal microbiome were evaluated before and after 2 weeks of EL. Dogs with stable or improved CE remained in the study for another 6 weeks. Pre- and post-EL clinical and microbiological variables were compared statistically using a mixed model.
Results
After 2 weeks of EL, 15 of 22 dogs (68%; 95% confidence interval [CI], 47%-84%) consuming the diet were classified as responders with a median (range) decrease in CCECAI from 6 (3-12) to 2 (0-9; P < .001). Fourteen of 15 responders and 2/7 nonresponders at 2 weeks completed the trial; all 16 were experiencing adequate control at week 8 with a median CCECAI of 2 (0-3). In total, 16/23 dogs (70%; 95% CI, 49%-84%) were responders. Feeding EL caused shifts in fecal bacterial communities, which differed between responders and nonresponders. Serum biomarker concentrations were unchanged throughout the study apart from serum alkaline phosphatase activity.
Conclusions
Exclusive feeding of EL improved clinical signs in 16 of 23 dogs with uncontrolled CE. Fecal microbiome shifts were associated with response to diet and may represent a mechanism for clinical improvement.
Abbreviations
-
- ANCOM
-
- analysis of composition of microbiomes
-
- ASV
-
- amplicon sequence variant
-
- BA
-
- bile acid
-
- BCS
-
- body condition score
-
- CCECAI
-
- canine chronic enteropathy clinical activity index
-
- CD
-
- Crohn's disease
-
- CE
-
- chronic enteropathy
-
- CI
-
- confidence interval
-
- CRP
-
- C-reactive protein
-
- CSU-VTH
-
- Colorado State University Veterinary Teaching Hospital
-
- EDs
-
- elemental diets
-
- EEN
-
- exclusive enteral nutrition
-
- EL
-
- Purina ProPlan Veterinary Diets EL
-
- GM-CSF
-
- granulocyte monocyte colony stimulating factor
-
- IFN-γ
-
- interferon-gamma
-
- IL
-
- interleukin
-
- IP-10
-
- interferon-gamma induced protein 10
-
- KC
-
- keratinocyte chemotactic
-
- MCP-1
-
- macrophage chemotactic protein-1
-
- MFI
-
- mean fluorescence intensity
-
- NR
-
- nonresponder
-
- PARR
-
- polymerase antigen receptor rearrangement
-
- PCoA
-
- principal coordinate analysis
-
- PERMANOVA
-
- permutational multivariate analysis of variances
-
- R
-
- responder
-
- TNF
-
- tumor necrosis factor
1 INTRODUCTION
Chronic enteropathy (CE) is a common condition in dogs characterized by persistent clinical signs referrable to gastrointestinal (GI) dysfunction such as diarrhea, vomiting, and poor appetite. Dietary therapy is a mainstay of CE management in dogs, with resolution of clinical signs reported in up to 69% of dogs after dietary intervention.1-4 Treatment success has been documented with hydrolyzed protein, limited ingredient, and fat-restricted diets.3, 5 The mechanisms underlying clinical improvement after diet change are incompletely understood, but likely involve host, microbiome, and dietary factors. Compared with other strategies such as antibiotics and immunosuppressive drugs, dietary therapy has been associated with more robust, longer-lasting periods of clinical remission in dogs with CE.6
Food components are 1 of the many environmental factors that may incite and perpetuate CE in dogs7; effects may be direct, indirect, or both. Elemental diets (EDs) provide protein in the form of individual amino acids rather than as polypeptides (hydrolyzed diets) or intact proteins.8, 9 Carbohydrate, fat, vitamins, and minerals are added to make a complete and balanced diet. Nutrition provided in this manner is intended to be nonimmunogenic and easily assimilated. Clinically, EDs are utilized during exclusive enteral nutrition (EEN) in human patients, whereby liquid formulations are administered to meet nutritional needs.10 Pediatric Crohn's disease (CD) patients treated with EEN experience decreased disease activity, mucosal healing, and improved growth. The mechanisms underlying these improvements are incompletely understood.8-10 Chronic enteropathy in dogs and CD in human are not analogous, but appear to share disruptions in host/microbiome interactions11 and intestinal epithelial barrier structure and function.12, 13 Dietary therapy for CE mimics EEN in that a single diet is provided exclusively for a defined period of time. In affected dogs, clinical improvement may be associated with inclusion or exclusion of certain ingredients, altered nutrient profiles (eg, lower fat), improved digestibility, or other factors.14, 15
Given the potential for adverse reactions against diet and intestinal microbiota as well fat intolerance in CE dogs, we conducted a clinical trial with 23 dogs with inadequately-controlled CE to determine the impact of a novel diet for dogs deriving protein from individual aminos acids (Purina ProPlan Veterinary Diets EL, Nestlé Purina, St Louis, Missouri; EL) on clinical and biochemical disease manifestations, including the fecal microbiome. Our primary hypothesis was that clinical remission would be achieved in at least 60% of dogs with CE willing to eat EL for at least 2 weeks; clinical remission from CE also was assessed at 8 weeks in dogs remaining in the study.
2 METHODS
2.1 Case selection criteria
The primary study investigator (ACM) directly oversaw enrollment into this Colorado State University Institutional Animal Care and Use Committee (protocol # 1440)-approved study. Dogs presented to Colorado State University's James L. Voss Veterinary Teaching Hospital (CSU-VTH) for increased defecation or decreased fecal consistency, vomiting, or decreased appetite with or without weight loss, regurgitation or flatulence were considered for entry into the study. Owners of eligible dogs reported inadequate control of CE signs, answering no to the question “is your dog currently experiencing adequate relief from its CE signs?”16 Clinical signs were present persistently for ≥3 weeks or intermittently for ≥6 months, despite interventions including dietary changes, antibiotics, immunomodulatory treatments, and other treatments. Each owner also completed a canine chronic enteropathy clinical activity index (CCECAI) survey,17 and to be included in the study the cumulative score needed to be ≥3.
The clinical trial schedule is presented in Figure 1. At the initial visit (T0), after obtaining informed written consent, dogs underwent a thorough diagnostic evaluation to exclude specific infectious, structural, and metabolic causes for CE clinical signs. Exclusion criteria included diagnosis of hypoadrenocorticism or exocrine pancreatic insufficiency, GI anatomic abnormalities that could cause CE signs, severe hypoalbuminemia (≤2 g/dL), azotemia (serum creatinine concentration >2 mg/dL), and clinically relevant anemia (Hct <30%). After the initial study visit, all dogs were treated with a 5-day course of PO fenbendazole (50 mg/kg). Previously prescribed medications that did not appear to be necessary were discontinued. Medications deemed essential based on owner and primary investigator discussions were continued at a constant dose throughout the study.
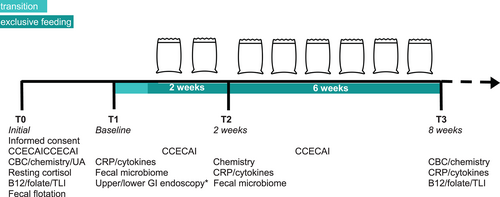
After the initial visit, dogs were enrolled in the study and returned within 3 weeks for the baseline (T1) visit and diagnostic evaluation (Figure 1). At each visit, owners again were asked about adequate response. They provided a fecal sample from their dogs (naturally passed within 12 hours of the appointment and kept at refrigerator temperature until arrival at the hospital) at this and all subsequent study visits. The primary investigator scored the fecal sample using Purina's fecal scoring chart (https://www.proplanveterinarydiets.ca/sites/g/files/2021-02/180107_PPPVD-Fecal-Scoring-Chart-UPDATE-EN-FINAL.pdf). Dogs were fasted for at least 12 hours before each visit. Upper and lower GI flexible endoscopy was completed in routine fashion at T1 by the primary investigator (ACM) in 22/23 dogs enrolled in the study (endoscopy was not performed in 1 dog because of the owner's risk aversion). During the procedure, ≥10 biopsy samples were collected from the duodenum and ileum and ≥6 samples were collected from the stomach and colon. Samples were preserved in formalin and submitted to the Colorado State University Veterinary Diagnostic Laboratory for routine histopathological analysis; dogs were excluded if findings included anything other than inflammation without an identifiable inciting cause (ie, infectious agent). Dogs with hypocobalaminemia (<300 ng/L) identified at the initial visit received cyanocobalamin SC once at the T1 visit (250-1000 μg depending on body weight). Owners were provided with an adequate supply of EL in unbranded packaging, along with specific feeding instructions, an 8-oz measuring cup, and a study diary in which they noted the amount of kibble consumed, fecal scores, and any other observations.
Owners were instructed to gradually transition their dogs to EL over 5 to 7 days. For dogs with normal or high body condition score (BCS), the recommended daily allocation of EL was extrapolated from the dog's current caloric intake with the goal of weight maintenance. This amount was matched with an appropriate volume of EL (357 kcal/cup). In dogs with BCS <4/9, an ideal body weight estimate18 was used to calculate a recommended daily caloric intake. After 2 weeks of exclusive EL feeding, the owner returned with their dog and another fecal sample (identical guidance as T1 sample) for physical examination and blood collection (T2; Figure 1). For dogs with stable or improved CE compared with T1, another 6-week supply of EL was provided with instructions to continue feeding the diet exclusively. Throughout the study, owners had the option to remove their dog if clinical response was insufficient. The primary investigator removed dogs from the study if they failed to ingest ≥60% of calculated caloric needs for 2 consecutive days or had unacceptably severe CE signs.
At the end of 8 weeks of exclusive EL feeding (T3 visit), the owner returned with their dog and a final fecal sample. Repeat physical examination and study diagnostic tests were performed (Figure 1) and study participation concluded.
2.2 Serum biomarker assessment
Serum biochemistry results were evaluated at the CSU-VTH Clinical Pathology Laboratory. Serum C-reactive protein (CRP) was measured in serum samples using the Gentian particle-enhanced turbidometric immunoassay as previously described (Texas A&M Gastrointestinal Laboratory, College Station, Texas).15 The lower limit of quantification was 9.9 mg/L.
2.3 Sample handling
After analysis of the biochemistry panel, remnant sera were collected from the CSU-VTH Clinical Pathology Laboratory, and aliquoted and frozen at −80°C until analysis. Fecal samples provided by owners or collected during study visits were stored at 4°C before aliquoting and freezing at −80°C.
Serum cytokines were measured using the commercially available canine cytokine magnetic bead panel (MILLIPLEX, MilliporeSigma, Burlington, Massachusetts) according to the manufacturer's instructions. Measured cytokines included granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-gamma (IFN-γ), keratinocyte chemotactic (KC)-like, IP-10, interleukin (IL)-2, IL-6, IL-7, IL-8, IL-10, IL-15, IL-18, (monocyte chemotactic protein-1 (MCP-1), and tumor necrosis factor (TNF). Results were reported and compared in terms of mean fluorescence intensity (MFI).
2.4 Fecal DNA extraction
Frozen fecal samples were thawed on ice and DNA was extracted from a 100 mg aliquot using Qiagen's PowerSoil Pro kit. The DNA was eluted in 200 mL of nuclease-free water to optimize concentration for downstream analyses. Quantity and purity of DNA were assessed using a NanoDrop One spectrophotometer (Thermo Scientific, Waltham, Massachusetts) and samples were stored at −80°C.
2.5 Illumina 16S sequencing for bacterial ribosomal DNA
Frozen fecal bacterial DNA samples were sequenced by Novogene Inc. (Sacramento, California). Twenty-five base pair paired-end V3/V4 sequencing was done using the Illumina NovoSeq 6000. Microbial sequencing and analysis were performed as previously described.19 Reads with Qscore ≤5 or N >10 or length <60 bp were removed. Sequence analyses were performed using QIIME2 software.19 Microbial community similarity was displayed with principal coordinate analysis (PCoA) plots. Alpha diversity was determined using Shannon, Faith, and Pielou indices. Beta diversity on weighted and unweighted UniFrac, Bray Curtis, and Jaccard measures were calculated in QIIME2. Alpha diversity indices were compared using a paired T test, and β-diversity metrics were compared using permutational multivariate analysis of variances (PERMANOVA). Analysis of composition of microbiomes (ANCOM) was employed to determine the individual amplicon sequence variants (ASV) that differed significantly between time points within the responder (R) and nonresponder (NR) groups.20
2.6 Outcome assessments
The primary outcome was subjective response to EL after 2 and 8 weeks of exclusive feeding, which was determined by the CCECAI score and the owner's impression of adequate relief of their dog's CE clinical signs. If owners asserted that their dogs were experiencing adequate relief from their CE and their CCECAI scores decreased by >50% or to a value ≤3, the dogs were classified as Rs. Dogs not meeting both of those criteria were considered NRs. If outcomes at the 2 time points were discordant, the 8 week response was used to determine overall outcome. Secondary outcome measures included changes in biochemistry variables and CRP concentrations, as well as alterations in the fecal microbiome after 2 weeks of EL feeding.
2.7 Statistical analysis
Body weight, clinical activity scores, and serum biomarker concentrations were assessed for normality using visual inspection of histograms and QQ plots. Data was generally skewed, and thus numerical data was reported as median and range. Categorical data was reported in terms of frequency and proportion along with 95% confidence intervals (CI). Differences in variables before and during the exclusive feeding period were compared using either a 2-way repeated measures ANOVA (no missing values) or a mixed model (missing values) between Rs and NRs at T2. Fixed effects included subject, treatment, time, and treatment-by-time interaction. For comparison of serum cytokine concentrations, MFI values were compared using a mixed model. P values < .05, adjusted for multiple comparisons using Sidak's test, were considered significant. All statistical analysis was completed using Prism 9 for macOS.
3 RESULTS
3.1 Patient study population
Twenty-eight dogs with inadequately controlled CE were screened for enrollment. Reasons for exclusion included hypoalbuminemia (<2.0 g/dL), hookworm infection, exocrine pancreatic insufficiency, perianal fistulae, and chronic hypertrophic pyloric gastropathy in 1 dog each. The median age of the 23 dogs enrolled was 3.5 years (0.9-11) with a median weight of 29 kg (4.4-65) and BCS 4/9 (2-7). Neutered males made up 78% (17/23) of the group, 5 were spayed females and 1 was an intact male. The most common breed was mixed (n = 7) followed by German Shepherd (n = 4), Great Dane (n = 2) and 1 each of miniature Schnauzer, English Setter, Staffordshire terrier, standard poodle, Weimaraner, Australian shepherd, Boykin spaniel, Labrador Retriever, Boston terrier, and Belgian Malinois. Duration of clinical signs before study enrollment ranged from 5 to 72 months (median, 14). The most common clinical sign was abnormal defecation frequency or fecal consistency (22/23; 96%) followed by vomiting in 13/23 (57%) and poor appetite in 11/23 (48%). Median fecal score was 4 (2-7). Seventeen dogs had small bowel diarrhea and 4 had mixed bowel diarrhea. No dogs had isolated signs of large bowel diarrhea. Owners of 7/23 (30%) dogs reported weight loss or difficulty maintaining weight despite good appetite. All dogs' CE had failed to improve with a diet change (including commercial well pet, veterinary therapeutic, home-prepared diets or some combination of these); 18 of 23 (78%) dogs had failed a trial with a veterinary therapeutic GI diet (limited ingredient, highly digestible, hydrolyzed, or high fiber; Table S1). These included 9/18 (50%) dogs that had previously failed a ≥14 day trial with ≥1 hydrolyzed protein diet.
Comorbidities in the study population included anxiety (n = 8), atopic dermatitis (n = 6), excess body weight (n = 3), and 1 dog each with atrial septal defect, dilated cardiomyopathy, chin furunculosis, osteoarthritis, cutaneous lupus erythematosus, and dermal endocrine cysts.
All dogs had serum albumin concentrations >2 g/dL, but 3/23 were below the laboratory's reference range for dogs (3 g/dL). Two dogs had baseline serum cholesterol concentrations below the reference interval (Figure 3). Six of 23 dogs had serum cobalamin concentrations <300 ng/L and 1/23 had hypofolatemia. Fecal flotation identified parasitism at T0 in 2/19 dogs (1 Giardia and 1 Monienzia spp.). Giardia was absent after fenbendazole treatment and the Monienzia was ignored. All dogs had abdominal ultrasound examinations performed within 6 weeks of enrollment and none were found to have GI tract structural abnormalities or abdominal masses.
Gastroduodenoscopy and ileocolonoscopy with biopsies were performed in 22/23 dogs (1 owner refused given risk aversion). The duodenum was biopsied in all dogs and the ileum in 18/22. Duodenal biopsy sample findings with hematoxylin and eosin staining included lymphoplasmacytic enteritis with or without an eosinophilic component in 21/22 study dogs. The other dog had primarily plasmacytic enteritis. Evidence of duodenal lacteal dilatation was noted in 10/22 (45%) dogs. Details of histopathological findings are reported in Table S3. Because of excessive numbers of intraepithelial lymphocytes in duodenal biopsy samples from 2 dogs, the polymerase antigen receptor rearrangement (PARR) assay was submitted to CSU-VTH Clinical Hematopathology Laboratory. A polyclonal rearrangement was documented in the immunoglobulin and T cell receptor genes in 1 dog. The other dog had a “possibly clonal” T cell receptor gene PARR result. This dog was enrolled nonetheless given the absence of weight loss, anemia, intestinal muscularis thickening and biopsy architecture distortion suggestive of small cell lymphoma.
Eight of 23 dogs were continued on medications or supplements throughout the study period (see Table S2). One dog was restarted on metronidazole (8.5 mg/kg PO q12h) after endoscopy but before introduction of EL because of intractable diarrhea. The 6 dogs with a serum cobalamin concentration <300 ng/L received a single dose of cyanocobalamin (250 to 1000 μg depending on weight) SC at the T1 visit. No dogs received additional cobalamin supplementation before study exit. This deviation from commonly recommended protocols21 was intended to normalize dogs at the time of diet initiation.
3.2 Clinical response
All but 1 of the 23 study dogs ate an amount more than or equal to the recommended amount of EL during the initial 2 weeks. One dog (#4) refused to eat EL; this dog was considered a treatment failure and excluded from further analysis.
Our primary endpoint was the impact of EL on CE clinical signs. After 2 weeks of EL feeding, no significant differences in BCS or body weight were identified. Owners of 16/22 (73%; 95% CI, 52-87) dogs felt their pet was experiencing adequate relief from CE (Figure 2). Individual dog (P = .01), time (P < .0001), and the interaction of time with response to treatment (P = .01) impacted CCECAI score at T2, with a significant decrease in both R and NR dogs (Figure 2). A CCECAI ≤3 or >50% reduction occurred in 17/22 dogs (77%; 95% CI, 57-90). Considering these measures together, at T2, 15/22 (68%; 95% CI, 47-84) dogs were Rs and 7/22 (32%; 95% CI, 16-53) were NRs. Median T2 Purina fecal score assessed by the supervising clinician was 2 (1-7). The proportion of dogs with normal fecal consistency based on owner response to the CCECAI survey question increased from 1/22 (5%; 95% CI, 0-22) to 16/22 (73%; 95% CI, 52-87) after 2 weeks of EL; owners of 8 dogs noted firm, dry feces.
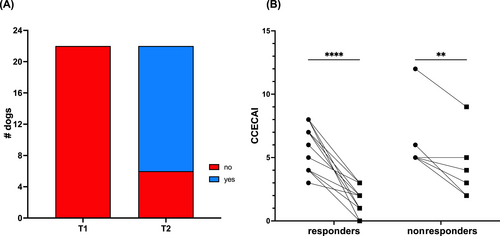
Owners of 15/15 T2 Rs and 6/7 T2 NRs consented to continue study participation. One owner elected exit at T2 because of the dog's insatiable appetite, persistent flatulence, and coprophagia (#22; T2 NR). Within 11 days of the T2 visit, 4 additional dogs (all T2 NRs) exited the study. All exited because of unacceptable CE signs including unformed feces in 3 dogs (#s 2, 3, 17) and worsened pruritus and very firm, dry feces in 1 dog (#7). A fifth dog (#16; T2 R) developed a fibrocartilaginous embolus resulting in acute paraparesis after 5 weeks of EL feeding. The dog was started on PO prednisolone (0.67 mg/kg/day) and was removed from the study at that point. Results from these 6 dogs were truncated at the T2 visit.
The remaining 16 dogs returned after ~8 weeks of EL feeding (T3). Neither body weight nor BCS were significantly different compared with T1. Although 6/6 dogs assessed to have suboptimal BCS at T1 gained weight (median, 6%; range, 4-19), none achieved their goal weight calculated from initial BCS.18 Median fecal score at T3 was 2 (1-4). Feces were deemed normal consistency by the owners in 11/16 dogs (69%; 95% CI, 44-86). Of the 16 dogs completing the 8-week trial, all 16 were experiencing adequate relief from their CE and median CCECAI was 2 (0-3). Using the combined criteria, the overall clinical response rate was 70% (16/23; 95% CI, 49-84); 2 dogs classified as T2 NRs were Rs at T3.
3.3 Serum biomarkers
No significant differences were noted in serum albumin concentration after 2 weeks of EL feeding (Figure 3A). Median serum cholesterol concentration was higher at T2 (204.5 mg/dL; range, 105-306) compared with before EL (197.5 mg/dL; range, 110-461) with a significant impact of time; this difference was only significant for Rs (Figure 3B; P = .01). Regardless of R or NR status, median serum alkaline phosphatase (ALK-PHOS) activity was higher at T2 (68.5 U/L; range, 26-281; Figure 3C) compared with before EL (41.5 U/L; range, 10-142; P .04).
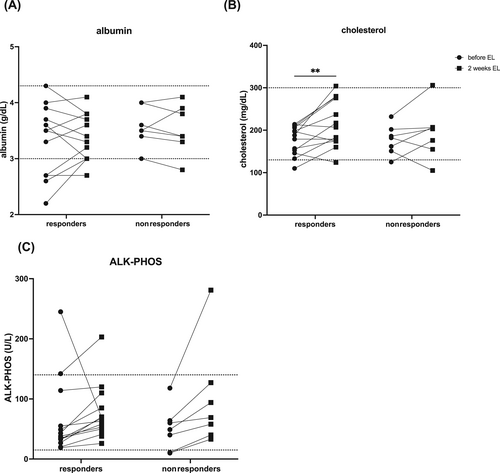
Serum CRP concentration was below the lower limit of detection in 39/42 samples and was not significantly different in available sera from 21 dogs at T2 compared with before EL. None of the serum cytokine MFIs were significantly different comparing these 2 time points, regardless of clinical response status (Figure S1).
After 8 weeks of EL feeding, all 16 R dogs had serum cobalamin concentrations >300 ng/L (median, 578; range, 338-1000) despite no supplementation during that period (5/16 dogs received single SC dose at T1). Serum folate concentrations in the Rs were significantly higher at T3 (median, 18.2 mg/L; range, 13.8-27.1) compared with before the diet (median, 13.1; range 5.1-18.5). Neither serum CRP concentrations nor cytokine MFIs were significantly different in samples from 15 Rs after 8 weeks of EL feeding compared with pre-EL (Figure S1).
3.4 Fecal microbiome
We concurrently investigated the impact of EL feeding on the fecal microbiome using 16S rRNA gene sequencing on available fecal samples from 18/22 study dogs before and after 2 weeks of EL feeding. Samples from 4 dogs were not included in the analysis because of lack of sufficient fecal material in either the T1 or T2 sample in 3 dogs (2 Rs: #13, 23; 1 NR: #22) and initiation of metronidazole between T1 and T2 in 1 dog (NR; #17). The predominant bacterial phyla present in feces before EL were, in order of decreasing relative abundance, Firmicutes, Fusobacteria, Proteobacteria, Bacteroides, and Actinobacteria. This same relative ranking of bacterial prevalence was present after 2 weeks of EL. No significant difference was found in alpha diversity (Faith, Pielou's evenness or Shannon) in fecal microbiota before EL versus T2 regardless of clinical response (Table S4).
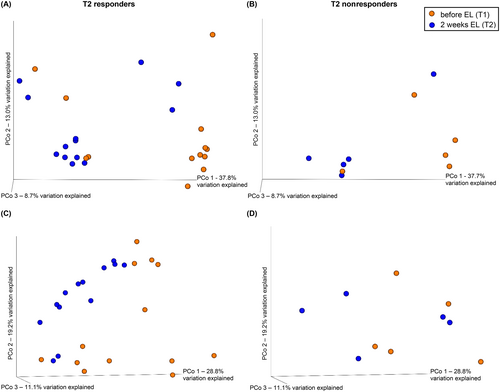
Principal coordinate analysis allowed visualization of the similarities between fecal microbial community compositions before treatment and at T2 (Figure 4). All of the similarity metrics (unweighted and weighted UniFrac, Bray Curtis, Jaccard) indicated heterogeneity, with overlap between the before and T2 samples. The PERMANOVA testing identified significant differences in β-diversity comparing before diet to T2 samples in the 13 Rs that were not present in the 5 NRs (Table 1; Figure 5).
| Similarity | Responders | Nonresponders | ||
|---|---|---|---|---|
| P value | Pseudo-F statistic | P value | Pseudo-F statistic | |
| Unweighted UniFrac | .01 | 4.364 | .18 | 1.749 |
| Weighted UniFrac | .01 | 2.98 | .66 | 0.732 |
| Bray Curtis | .01 | 2.108 | .58 | 0.844 |
| Jaccard | .01 | 2.618 | .12 | 1.443 |
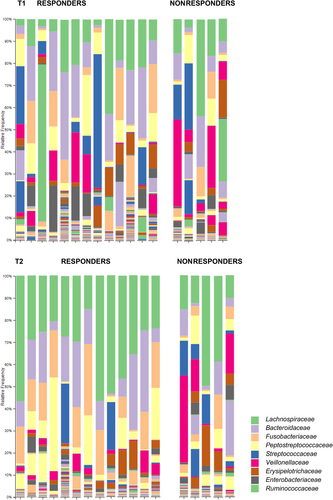
To identify the ASVs contributing to changes in bacterial communities, ANCOM was applied to compare pre-diet to T2 samples within the R and NR groups. Results identified a differential impact of EL feeding on the fecal microbiome of Rs compared with NRs (Figure 5). The 3 significant differences in NRs were a decrease in the phylum Tenericutes, the class Mollicutes, and the order Anaeroplasmatales (Table 2). Within the Rs, significant changes in relative abundances were seen at the phylum, class, order, family, and genus levels; these included reduced relative abundances of Bifidobacteriaceae, Lactobacillaceae, Streptococcaceae, and Desulfovibrionales (Table 2).
| T2 responders | Level | ASV | T2 vs. T1 | W value | F statistic |
|---|---|---|---|---|---|
| Phylum | Nitrospirae | Increase | 4 | 1.53 | |
| Tenericutes | Decrease | 3 | −1.77 | ||
| Firmicutes | Decrease | 2 | −0.8 | ||
| Verrucomicrobia | Increase | 2 | 1.08 | ||
| Class | Deltaproteobacteria | Decrease | 10 | 2.58 | |
| [Spartobacteria] | Increase | 7 | −1.45 | ||
| Bacilli | Decrease | 4 | 1.98 | ||
| Nitrospira | Increase | 4 | −1.9 | ||
| Order | Bifidobacteriales | Decrease | 17 | 3.36 | |
| [Chthoniobacterales] | Increase | 11 | −1.53 | ||
| Desulfovibrionales | Decrease | 11 | 2.5 | ||
| Lactobacillales | Decrease | 8 | 2.51 | ||
| Family | Bifidobacteriaceae | Decrease | 15 | −3.23 | |
| Lactobacillaceae | Decrease | 14 | −3 | ||
| S24-7 | Decrease | 9 | −2.71 | ||
| Desulfovibrionaceae | Decrease | 9 | −2.37 | ||
| Streptococcaceae | Decrease | 8 | −2.81 | ||
| Genus | Bifidobacterium | Decrease | 14 | −3.16 | |
| S24-7 unnamed genus | Decrease | 12 | −2.63 | ||
| Prevotellaceae unnamed genus | Decrease | 11 | −2.29 | ||
| DA101 | Increase | 11 | 1.74 | ||
| Clostridium | Decrease | 10 | −2.36 | ||
| Desulfovibrio | Decrease | 10 | −2.29 |
| T2 nonresponders | Level | ASV | T2 vs. T1 | W value | F statistic |
|---|---|---|---|---|---|
| Phylum | Tenericutes | Decrease | 7 | 2.98 | |
| Class | Mollicutes | Decrease | 8 | 2.83 | |
| Order | Anaeroplasmatales | Decrease | 9 | −2.84 |
- Note: Clinical responders were dogs whose owners answered “yes” to the adequate relief question and whose CCECAI scores reduced by >50% or to a value <3.
- Abbreviations: ASV, amplicon sequence variant; NR, nonresponder; R, responder.
4 DISCUSSION
We conducted the first observational, uncontrolled clinical trial involving feeding of an amino acid-based diet to manage CE in dogs. Crucial study findings were that the diet was palatable and that 16/23 dogs achieved clinical remission. Feeding of EL altered the fecal microbiome of dogs within the R group, suggesting a potential explanation for decreased clinical disease activity. These findings indicate that feeding EL could be effective for the management of CE in dogs and should be evaluated in future controlled studies.
In our study, 22/23 dogs readily ate EL and remission from CE was achieved in 73% (16/22) of dogs consuming EL. Clinical improvement was rapid, in agreement with previous studies observing positive responses within the first 10 to 14 days of dietary therapy for CE dogs.1, 3, 5, 22 In studies involving hydrolyzed protein diets, 66% to 89% of CE dogs achieved clinical remission.2, 3, 5 In our study, 8/9 dogs previously failing a feeding trial with a hydrolyzed protein diet achieved clinical remission with EL. Other factors (eg, lack of exclusive feeding, inadequate duration, concurrent comorbidities, unaddressed hypocobalaminemia) may have negatively impacted the success of the historical diet trial; these factors could not be fully evaluated given potential unreliability of owner recollections and incomplete medical records. Our results reinforce the importance of providing clear guidance for proper implementation of a diet trial. Moreover, failure to improve on 1 diet does not rule out the possibility of diet-responsive CE in dogs.2, 18, 23
Various mechanisms could explain the clinical improvement observed in the dogs in our study, including decreased antigenicity, altered nutrient profile, inclusion or exclusion of specific ingredients, and modification of the intestinal microbiome. Diet is a modifiable environmental risk factor for the onset and perpetuation of intestinal inflammation.24 A direct beneficial effect of feeding free amino acids is possible. One study found decreased pro-inflammatory cytokine release when inflamed human intestinal biopsy samples were incubated with amino acids from an ED ex vivo.25 Exclusion of dietary antigen also could decrease inflammatory stimuli. Although food-derived antigens trigger immunological reactions in humans with Celiac disease (gliadin26) and IgE-mediated food allergy,27 there is minimal evidence that food proteins drive CE in dogs.28 The ability of dietary protein to aggravate intestinal inflammation is also controversial in humans with inflammatory bowel diseases. Of note, a meta-analysis of CD patients treated with EEN found no difference in remission rates comparing utilization of diets sourcing protein from individual amino acids to those formulated with oligopeptides or intact proteins.29 A study directly comparing feeding of an intact protein to an amino acid-based diet to CE dogs with immunological monitoring would be required to determine the importance of protein source in this disease.
Fat is another nutrient of interest in CE in dogs. Maldigestion of fat may provoke malabsorption and ensuing osmotic or secretory diarrhea.30, 31 Moreover, dietary fat has been linked to increased intestinal permeability and aggravated immune responses locally and systemically in mice.32 Nonetheless, fat represents the most efficient means of delivering calories on a kcal per kg diet basis. The fat in EL was provided by vegetable, coconut, and fish oils, resulting in an energy density of 3322 kcal/kg and a fat digestibility of 95.5%. Feeding of EL (23.7% fat on metabolizable energy basis) represented a decrease in dietary fat for 10/15 Rs and 5/5 NRs (original diet macronutrient data available for 20/22 dogs ingesting EL), and thus it does not appear that clinical response can be linked to fat restriction alone. Dogs with CE represent a heterogeneous group, and provision of a moderate amount of readily assimilable fat may have contributed to the clinical improvement seen in some of these dogs. Given the well-recognized benefit of diet modification in CE in dogs,1, 5, 6, 12, 33 other factors correlating food components to GI-related clinical signs should be considered. Much data supports the ability of diet to modulate the GI immune system, with an indisputable role of the gut microbiome.32, 34, 35 Recent studies indicate that the antigenic target in dogs with CE may be the intestinal microbiota rather than food components,11 raising the possibility that microbiome shifts may underlie clinical improvement. Feeding EL had significant effects on the fecal microbiome, which varied based on T2 clinical response (Figures 4 and 5). This finding is consistent with studies in dogs3 and humans36 linking clinical response to diet with microbiome shifts. Contrary to results of studies employing polymeric diets for EEN in human IBD patients,36-38 changes did not include a decrease in bacterial richness or evenness with EL feeding.
How exactly changes in microbiome composition impacted T2 clinical outcome is unclear. It is possible that Rs benefited from the expansion of Verrucomicrobia. This phylum includes mucus-degrading Akkermansia spp. the abundance of which is inversely correlated to disease severity in various chronic GI conditions.39 Decreases in sulfate-reducing Desulfovibrionales bacteria may have promoted clinical improvement by limiting luminal hydrogen sulfide, a compound known to promote intestinal inflammation.40 Our approaches did not allow for assessment of total bacterial load, which has been found to decrease with ED feeding.41 Analysis of the intestinal mucosal microbiome may have yielded more immunologically relevant information. However, serial biopsy samples are impractical, and data from humans has identified comparable alpha and beta diversity comparing intestinal mucosal and fecal samples.42 Additional studies employing multiomics approaches are necessary to explore the functional consequences of these microbiome changes.
Unlike the fecal microbiome analysis, we found no utility of monitoring serum inflammatory biomarkers (eg, cytokines, CRP) in these dogs. We attribute this finding to minimal systemic inflammation in our study cohort. This conclusion is not novel, with many previous investigations finding CRP concentrations to be within the reference range in most or all normoalbuminemic CE dogs.1, 12, 43 C-reactive protein also fails to correlate with CE disease activity.44 Our findings encourage analysis of GI-relevant samples, such as feces and biopsy tissue. Serum cobalamin concentrations were adequate (>300 ng/L) in all dogs remaining in the study at T3, including 5 dogs that were hypocobalaminemic at study enrollment and 2 with historical hypocobalaminemia. Of note, urine methylmalonic acid was not evaluated, and thus cobalamin deficiency at the cellular level cannot be ruled out.45 The circulating half-life of cobalamin in healthy dogs is approximately 2 months,46, 47 and thus T3 concentrations likely reflect dietary uptake rather than exogenous supplementation. It is possible that EL feeding improved intestinal absorption in Rs.
Feeding of EL was not associated with owner-reported adequate relief from CE in 6/22 dogs. We elected to utilize the owner's response to the “adequate relief” question to determine clinical response given the inherent limitations of the CCECAI score and well recognized utility of this variable in humans with irritable bowel syndrome. At T2, 4/22 dogs met the R criteria based on decrease in CCECAI but their owners felt CE was uncontrolled. Two of 4 dogs exited the study between T2 and T3 and ultimately were classifed as NRs. Thus, this question appears to be a useful adjunctive variable beyond the CCECAI. Age, baseline CCECAI, serum albumin concentration or cobalamin concentration, or presence of lacteal dilatation were not useful in predicting response to EL. The Rs had a median CCECAI of 6.5 (3-8) and age of 2.75 (0.9-11) years, which are consistent with publications reporting a wide range of ages and disease severity in diet-responsive CE dogs.12, 48, 49 Our results conflict with previous reports finding diet-responsive CE dogs to be younger, with lower clinical activity indices, as compared to dogs not responding to diet.1, 6 Given that diet-responsive CE dogs tend to have better outcomes compared with those treated using other strategies,6 multiple dedicated feeding trials are prudent before resorting to immunosuppressive drugs or antibiotics.
Serum ALK-PHOS activity doubled in 11/22 dogs fed EL in our trial, with activity increasing above the reference range in 5 dogs. One study in rats documented marked increases in hepatic lipid and cholesterol and significant decreases in fecal bile acid (BA) excretion during feeding of an ED.50 In those animals, fatty liver was hypothesized to be secondary to decreased cholesterol catabolism for BA synthesis. A separate study in rats showed a significant increase in jejunal and ileal mucosal ALK-PHOS activity with EL feeding.51 We did not measure ALK-PHOS isoenzymes or fecal BA concentrations, nor were hepatic biopsy samples obtained from any of the study dogs. Further study is required to determine the implications of these initial observations.
Our study had some limitations. It was uncontrolled, and thus we cannot draw any conclusions regarding the relative efficacy of EL compared with other diets and treatment modalities. Randomized clinical trials are required to make these comparisons. Medications administered throughout the feeding period could have impacted clinical response, but these were not changed during the study period, and thus it is unlikely that clinical improvement was falsely attributed to EL. Finally, fecal microbiome was only analyzed at T2; the impact of longer-term feeding is unknown.
Chronic enteropathy in dogs is a frustrating condition that may substantially decrease dog and owner quality of life. Diet-responsive dogs make up the largest subset of CE patients.1, 22, 49 Because of a lack of biomarkers that can predict diet responsiveness, dedicated feeding trials are of utmost importance. Additional dietary options may result in more dogs achieving long-lasting remission from their CE. Our results support a trial with EL in nonanorexic CE dogs before ruling out diet-responsive disease and further underscore the importance of the microbiome in chronic intestinal diseases.
ACKNOWLEDGMENT
Funding provided by Nestle Purina PetCare. The authors acknowledge the contributions of Patricia Lopes Sicupira Franco and Rae Isdale toward patient care and sample collection. They are also greatly appreciative of the technical support provided by Renata Impastato and Jennifer Hawley.
CONFLICT OF INTEREST DECLARATION
Dr Gagne is an employee of Nestle Purina PetCare. He was not involved in data collection, entry, or analysis. He provided guidance as a veterinary nutritionist and provided minor edits to the final document. None of the authors were paid for the work required to complete this study.
OFF-LABEL ANTIMICROBIAL DECLARATION
One dog was receiving metronidazole during the study period to improve stool quality, which is not an approved use of this drug.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by Colorado State University IACUC & Veterinary Teaching Hospital Clinical Review Board approved 1/21/2021, protocol 1440, and Nestle Purina PetCare.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




