Oral cytarabine ocfosfate pharmacokinetics and assessment of leukocyte biomarkers in normal dogs
Abstract
Background
Cytosine arabinoside (Ara-C) is a nucleoside analog prodrug utilized for immunomodulatory effects mediated by its active metabolite Ara-CTP. Optimal dosing protocols for immunomodulation in dogs have not been defined. Cytarabine ocfosfate (CO) is a lipophilic prodrug of Ara-C that can be administered PO and provides prolonged serum concentrations of Ara-C.
Objectives
Provide pharmacokinetic data for orally administered CO and determine accumulation and functional consequences of Ara-CTP within peripheral blood leukocytes.
Animals
Three healthy female hound dogs and 1 healthy male Beagle.
Methods
Prospective study. Dogs received 200 mg/m2 of CO PO q24h for 7 doses. Serum and cerebrospinal fluid (CSF) CO and Ara-C concentrations were measured by liquid chromatography-tandem mass spectroscopy (LC-MS/MS). Complete blood counts, flow cytometry, and leukocyte activation assays were done up to 21 days. Incorporation of Ara-CTP within leukocyte DNA was determined by LC-MS/MS.
Results
Maximum serum concentration (Cmax) for Ara-C was 456.1-724.0 ng/mL (1.88-2.98 μM) and terminal half-life was 23.3 to 29.4 hours. Cerebrospinal fluid: serum Ara-C ratios ranged from 0.54 to 1.2. Peripheral blood lymphocyte concentrations remained within the reference range, but proliferation rates poststimulation were decreased at 6 days. Incorporation of Ara-CTP was not saturated and remained >25% of peak concentration at 13 days.
Conclusions and Clinical Importance
Oral CO may produce prolonged serum Ara-C half-lives at concentrations sufficient to induce functional changes in peripheral leukocytes and is associated with prolonged retention of DNA-incorporated Ara-CTP. Application of functional and active metabolite assessment is feasible and may provide more relevant data to determine optimal dosing regimens for Ara-C-based treatments.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- Ara-C
-
- cytosine arabinoside (1-β-d-arabinofuranosylcytosine)
-
- Ara-CDP
-
- AraC-diphosphate
-
- Ara-CMP
-
- AraC-monophosphate
-
- Ara-CTP
-
- AraC-triphosphate
-
- Ara-U
-
- 1-β-d-arabinofuranosyluracil
-
- AUC
-
- area under the curve
-
- BrdU
-
- bromodeoxyuridine
-
- Cmax
-
- maximum serum concentration
-
- CNS
-
- central nervous system
-
- CO
-
- cytarabine ocfosfate
-
- CSF
-
- cerebrospinal fluid
-
- dCK
-
- deoxycytidine kinase
-
- dG
-
- deoxyguanosine
-
- DNA
-
- deoxyribonucleic acid
-
- ENT
-
- equilibrative nucleoside transporter
-
- ISF
-
- interstitial fluid
-
- LC-MS/MS
-
- liquid chromatography-tandem mass spectrometry
-
- LOD
-
- limit of detection
-
- LOQ
-
- limit of quantification
-
- PBMC
-
- peripheral blood mononuclear cell
-
- VCOG-CTCAE
-
- Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events
1 INTRODUCTION
Cytarabine (1-β-d-arabinofuranosylcytosine/cytosine arabinoside/Ara-C) is a hydrophilic pyrimidine nucleoside analog of deoxycytidine commonly used to treat immune-mediated inflammatory brain disease in dogs, and presumed to be acting by modulation of leukocyte function. The metabolism and mechanism of action of Ara-C are complex with interplay of many factors including natural nucleotides, membrane transportation, intracellular activation, intra- and extra-cellular deactivation, interaction with cellular targets, and pharmacogenomics.1 Pyrimidine analogs are essentially prodrugs, and their plasma pharmacokinetics provide only limited data relating to efficacy.2-4 The entry of Ara-C into cells is rapid,5 and mediated primarily by equilibrative nucleoside transporter proteins (ENT), specifically ENT1 (SLC29A1 gene).6 It must be phosphorylated intracellularly through a pyrimidine salvage pathway to the biologically active Ara-C-triphosphate (Ara-CTP) that exerts its effects after integration into DNA as Ara-CMP (Figure 1).4, 7-10 Cytosine arabinoside is an S-phase cell-cycle-specific drug11 thought to act by inhibition of DNA polymerase, altered DNA synthesis and generation of chromosomal abnormalities,3, 9, 12-14 although an AMP-activated protein kinase mechanism15 and alterations in phospholipid and glycoprotein metabolism also have been reported.16-18 In humans, Ara-C is rapidly converted to 1-β-d-arabinofuranosyluracil (Ara-U) by cytidine deaminase which is found at high concentrations in liver, and at lower concentrations in kidney, red blood cells, and granulocytes.19-22 Dephosphorylation by 5′-nucleotidase enzymes also contributes to drug deactivation.
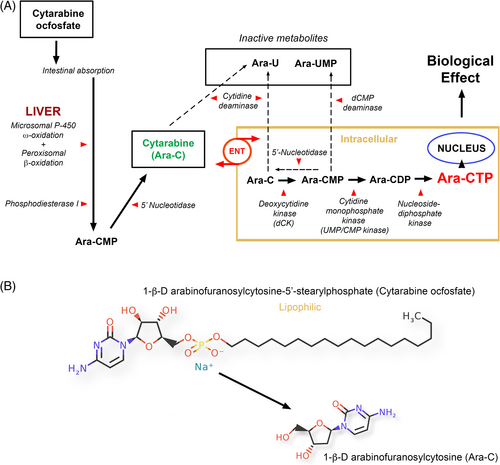
Key parameters have been described in humans defining rate-limiting steps in the processing of Ara-C to its active metabolites,5, 23-32 the nonlinear relationship between Ara-C and Ara-CTP,27, 33, 34 and the correlation of Ara-CTP rather than Ara-C with clinical efficacy.24, 34-39 Prolonged exposure to Ara-CTP, determined by elimination and steady-state kinetics, may be more important than peak concentrations or area under the curve,25, 34, 35, 39-42 and in vitro, cell death correlates better with Ara-CTP incorporation in DNA than basic cellular Ara-CTP pools.3 Similar data relating to key Ara-CTP-based parameters in dogs are lacking.
The alkyl phosphate derivative of Ara-C, 1-β-d-arabinofuranosylcytosine-5′-(n-stearyl-phosphate) (cytarabine ocfosfate (CO)/OcdP-AraC/C18PCA/YNK01) is characterized by the addition of an 18-carbon-long alkyl group and is a lipophilic, PO active prodrug of cytarabine 5′-monophosphate that is resistant to cytosine deaminase inactivation (Figure 1).43, 44 The combined effects of intestinal absorption, deaminase resistance, and ongoing conversion result in prolonged plasma half-life of Ara-C, albeit at lower concentrations. Use of CO has been reported in a single dog,45 but no pharmacokinetic data are available. We hypothesized that PO CO would result in prolonged serum Ara-C exposure times, accumulation of DNA-incorporated Ara-CMP, and alterations in lymphocyte subsets and functional parameters, even in the absence of lymphopenia.
Our objectives were to (1) define baseline pharmacokinetic data in dogs for an PO administered Ara-C pro-drug, (2) determine DNA incorporation of Ara-CMP in peripheral blood leukocytes, and (3) determine downstream effects of Ara-C-based treatment on hematological variables, lymphocyte subsets, and T-cell proliferative capacity.
2 MATERIALS AND METHODS
A prospective pharmacokinetic and pharmacodynamic study was conducted. The research protocol was approved by the University of California, Davis Institutional Animal Care and Use Committee [protocol #18431].
2.1 Study population
One healthy male Beagle, 20 months-old and weighing 10.8 kg, was used to provide pilot data. Three healthy female hound dogs (Marshall Farms USA Inc, New York) were studied. The hound dogs were 13 to 14 months old and weighed 17.5 to 20.8 kg. A 7-day acclimation period was provided before the start of the study. Jugular catheters were placed under general anesthesia in all dogs 24 hours before the first dose of CO and baseline samples also were collected at this time. The dogs were examined daily and any observed adverse effects were graded according to the Veterinary Cooperative Oncology Group Common Terminology Criteria for Adverse Events (VCOG-CTCAE) v2 (2021).46 All dogs were neutered and adopted to permanent homes after the study was completed.
2.2 Drug administration
The pilot study dog received a single dose of 200 mg/m2 CO PO (Starasid; inactive ingredients: hydroxypropyl cellulose, D mannitol, sodium carbonate hydrate, magnesium stearate, sodium dodecyl sulfate, erythrosine, and brilliant blue FCF. Nippon Kayaku Co., Ltd; Tokyo, Japan). Four weeks after the initial dose, the dog received 200 mg/m2 CO PO q24h for 3 consecutive days. Sampling times and data are found in Data S1.
Three hound dogs received 200 mg/m2 of CO PO q24h for 7 consecutive days or until the dog became leukopenic (VCOG-CTCAE v2 Grade 2). Dogs were fasted for 12 hours before CO administration. The time of the first dose was designated time 0 and day 0.
2.3 Pharmacokinetic analysis
2.3.1 Sample collection
Blood was collected via the jugular catheter from 20 minutes (min) to 20 days (d) after administration of the first dose. Serum was separated and stored at −80°C until analyzed.
Cerebellomedullary cistern cerebrospinal fluid (CSF) collection was done under general anesthesia 1 day before the first CO dose and at 24 hours, and 6 days. Dogs were premedicated with butorphanol 0.2 mg/kg IM (Torbugesic, Zoetis, Parsippany-Troy Hills, NJ) and atropine sulfate 0.02 mg/kg IM (Hikma Pharmaceuticals, Berkley Heights, NJ). General anesthesia was induced with propofol 4 to 5 mg/kg IV (Rapanofal, Ivaoes, Miami, FL) and maintained using isoflurane (Fluriso, VetOne, Boise, ID). Samples were stored at −80°C until analyzed.
2.3.2 Serum and CSF Ara-C analysis
Quantitative analysis of Ara-C in serum and CSF was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) with Ara-C-13C3 as the internal standard on an LTQ Orbitrap XL mass spectrometer (Thermo Scientific, San Jose, CA) coupled with an Acquity UPLC (Waters Milford, MA) essentially as previously described.47 Details are found in Data S1.
2.3.3 Serum and CSF cytarabine ocfosfate analysis
The analytical reference standard for CO was obtained from Nippon Kayaku Co Ltd (Marunochi Chiyoda-Ku, Japan), and working solutions, quality control samples, and calibration curves (0.1-1000 ng/mL) were prepared as described above. Quantitative analysis of CO in serum and CSF was performed by LC-MS/MS using the methodology as previously described.48
2.3.4 Data analysis
Noncompartmental analysis was performed on serum cytarabine and CO concentrations by commercially available software (Phoenix WinNonlin Version 8.1, Certara, Princeton, NJ). Details are found in Data S1.
2.4 Determination of DNA-incorporated Ara-CMP within peripheral blood leukocytes
Buffy coat samples for DNA-incorporated Ara-CMP in peripheral blood leukocytes were collected at 24, 48, 72, 80 hours, 5, 6, 7, 8, 9, 10, and 13 days. Deoxyribonucleic acid (DNA) hydrolysis was done essentially as described previously.49 Details are found in Data S1. Quantitative LC-MS/MS analysis was performed on the instrument as described above for CO with the same mass spectrometer settings. Chromatography employed an EZ:faast 25 cm × 2.0 mm, 4 μm column (Phenomenex, Torrance, CA), and a linear gradient of acetonitrile in water with 0.2% formic acid, at a flow rate of 0.2 mL/min. The response for the product ions for Ara-C (m/z 112.1), dG (m/z 151.9), and the internal standard (m/z 115.1) were plotted and peaks at the proper retention time integrated with Quanbrowser software (Thermo Scientific, San Jose, CA). The DNA-incorporated Ara-CMP was reported as pg/mL Ara-C per μg/mL dG.
2.5 Peripheral blood leukocyte evaluation
Blood was collected into EDTA tubes at time 0, 3, 6, 7, 8, 9, 10, 13, and 20 days after the first CO dose. Complete blood counts were performed at the diagnostic hematology laboratory at the UC Davis, Veterinary Medical Teaching Hospital, with an automated hematology analyzer (ADVIA 120, Siemens Healthineers, Erlangen, Germany). All differential leukocyte counts were confirmed by microscopic observation of a modified Wright stained (Model 7151 Wescor Aerospray Hematology Pro, ELITech Bio-Medical Systems, Logan, UT) blood smear. For the lymphocyte phenotype and ex vivo proliferation experiments, blood was collected (BD Vacutainer CPT cell preparation tube with sodium heparinN; Becton, Dickinson and Company, Franklin Lakes, NJ) at 0, 3, 6, 10, and 20 days after the first CO dose and peripheral blood mononuclear cells (PBMCs) processed as previously described.50 The PBMCs were either incubated with 8 panels of primary conjugated antibodies (Table 1) to define leukocyte subsets (CD45RA-CD4-CD8, CD3-CD4-CD8, CD3-CD21, CD25-CD21, CD4-CD8-CD25, Ki67-CD3-CD4, Ki67-CD21, and CD3-CD4-FoxP3), or stimulated for proliferation analysis. A minimum of 25 000 live cells were collected for each experiment and all panels included a viability dye for exclusion of dead cells (Fixable Viability Dye eFlour780; eBioscience, San Diego, CA). For stimulation experiments, PBMCs were collected and resuspended in activation medium (Dulbecco's modified Eagle medium + 10% heat-inactivated fetal bovine serum + 1% Penicillin-Streptomycin), and stored on ice until plating. The PBMCs were activated with 5 μg/mL concanavalin A (Sigma-Aldrich, St Louis, MO) and spiked with 10 μM bromodeoxyuridine (BrdU) 3 days poststimulation. Cells were collected 4 days poststimulation, processed according to the manufacturer's instructions (BrdU Flow Kit; BD Biosciences, San Jose, CA), stained with a viability dye and a primary conjugated anti-BrdU antibody, and analyzed on a flow cytometer (Cytomics FC500, Beckman Coulter Inc, Brea, CA). Flow cytometry data were analyzed by FlowJo flow cytometry software (Tree Star Inc, Ashland, OR).
| Target epitope | Cell type | Fluorophore | Clone | Vendor |
|---|---|---|---|---|
| CD3 | T cell | Alexa Fluor 488 | CA17.2A12 | Leukocyte Antigen Biology Lab, UCD |
| CD4 | T helper | Phycoerythrin (PE) | CA13.1E4 | Leukocyte Antigen Biology Lab, UCD |
| CD8 | T cytotoxic | Pacific Blue | MCA1039PB | AbD Serotec |
| CD21 | B cell | PE | CA2.ID6 | Leukocyte Antigen Biology Lab, UCD |
| CD25 | Activated B and T cell | Alexa Fluor 488 | P4A10 | eBiosciences |
| CD45RA | T cell (naive) | Fluorescein (FITC) | CA4.1D3 | Leukocyte Antigen Biology Lab, UCD |
| FoxP3 | T-cell regulatory | Alexa Fluor 647 | FJK-16s | eBiosciences |
| Ki67 | Cell proliferation | eFlour 660 | 20Raj1 | eBiosciences |
| BrdU | Cell proliferation | Alexa Fluor 647 | MoBU-1 | Invitrogen |
2.5.1 Statistical analysis
For peripheral blood leukocyte evaluations, data were analyzed statistically with GraphPad Prism software (GraphPad Dotmatics, San Diego, CA) Repeated measures data were analyzed by the Friedman test with Dunn's post hoc multiple comparison test. P < .05 was considered statistically significant.
3 RESULTS
3.1 Drug administration and adverse reactions
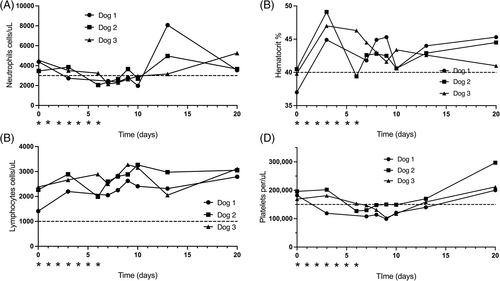
All dogs received a total of 7 doses of CO at 200 mg/m2 PO. The CBC data were not available for Dog 1 on day 6 because of technical issues. All dogs developed VCOG grade 1 neutropenia (1500-3000 cells/μL; Figure 4). Neutropenia resolved in all dogs by day 13. No dogs developed lymphopenia based on UC Davis Veterinary Medical Teaching Hospital reference intervals (<1000 cells/μL). One dog developed VCOG grade 1 decrease in hematocrit (hematocrit = 30-40%) after drug administration on day 6 (Figure 4). Hematocrit was within reference intervals by day 7 in all dogs. One dog developed VCOG grade 2 thrombocytopenia (99 000 cells/μL; VCOG Grade 2 = 50 000-100 000 cells/μl) and all dogs had VCOG grade 1 thrombocytopenia (100 000-150 000 cells/μL; Figure 4). Thrombocytopenia resolved between day 13 and day 20. No other adverse events were noted.
3.2 Assay validation
The response for Ara-C and CO was linear and gave correlation coefficients ≥0.99. The intra-day and inter-day precision and accuracy of the assay were determined by assaying quality control samples in replicates (n = 6). Accuracy was reported as percent nominal concentration and precision was reported as percent relative SD. For Ara-C, accuracy was 99%, 100%, 101%, and 90% for 0.6, 15, 80, and 750 ng/mL, respectively. Precision for Ara-C was 12%, 5%, 2%, and 5% for 0.6, 15, 80, and 750 ng/mL, respectively. For CO, accuracy was 97%, 94%, 92%, and 101% for 0.6, 15, 80, and 750 ng/mL, respectively. Precision was 16%, 4%, 2%, and 5% for 0.6, 15, 80, and 750 ng/mL, respectively. The technique was optimized to provide a limit of quantitation (LOQ) and limit of detection (LOD) of 0.1 and 0.08 ng/mL for Ara-C and 0.2 and 0.1 ng/mL, respectively, for CO. The LOQ was 5 times signal-to-noise with no more than ±20% deviation from the nominal concentration at the LOQ, and the LOD was 3 times signal-to-noise.
3.3 Pharmacokinetic analysis
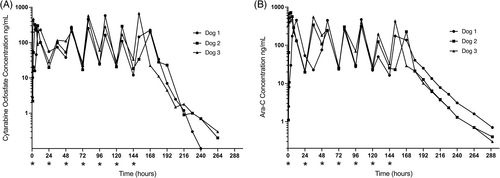
Cytosine arabinoside and CO were detectable in serum of all dogs after PO administration of the pre-prodrug CO (Figure 2, Table 2). Preliminary data from 1 beagle dog was used to define appropriate sampling time points for the main study and provided similar results to the 3 study dogs (Data S2).
| Dog | CO | Ara-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/mL) | Tmax (h) | T1/2 (h) | AUC (h ng/mL) | AI | Cmax (ng/mL) | Tmax (h) | T1/2 (h) | AUC (h ng/mL) | AI | |
| 1 | 229.3 (0.37 μM) | 12 | 6.7 | 3609 (5.86 μM) | 1.09 | 456.1 (1.88 μM) | 12 | 23.3 | 3252 (13.4 μM) | 1.96 |
| 2 | 324.6 (0.53 μM) | 4 | 15.1 | 2076 (3.37 μM) | 1.5 | 724.0 (2.98 μM) | 4 | 29.4 | 2194 (9.0 μM) | 2.32 |
| 3 | 473.2 (0.77 μM) | 2 | 20.5 | 5843 (9.50 μM) | 1.8 | 709.4 (2.92 μM) | 2 | 24 | 4282 (17.6 μM) | 1.98 |
| Mean | 342.4 (0.56 μM) | 6 | 14.1 | 3843 (6.24 μM) | 1.46 | 629.8 (2.59 μM) | 6 | 25.6 | 3243 (13.3 μM) | 2.10 |
- Note: 1 μg/mL Ara-C = 4.1 μM.
- Abbreviations: AI, accumulation index; AUC, area under the curve; Cmax, maximum serum concentration after first CO dose; Tmax, time of maximum serum concentration after first CO dose; T1/2, half-life.
Pharmacokinetic data were similar for all 3 hound dogs (Figure 2). After the first dose, the mean Cmax for CO was 342.4 ng/mL (0.56 μM; range, 229.3-473.2 ng/mL), and mean Cmax for Ara-C was 629.8 ng/mL (2.59 μM; range, 456.1-724.0 ng/mL). Mean elimination half-life for CO was 14.1 hours (range, 6.7-20.5 hours), and mean elimination half-life for Ara-C was 25.6 hours (range, 23.3-29.4 hours). With a dosing interval of 24 hours, the mean accumulation indices for CO and Ara-C were 1.4 and 2.1, respectively. Cerebrospinal fluid was collected successfully from all 3 dogs at days 0 and 1, and from 2/3 dogs at day 6 (Table 3). Cytarabine ocfosfate was not detected in CSF of any dog at day 1 or day 6. Cytosine arabinoside was detected at 24 hours in all dogs and CSF Ara-C concentrations ranged from 9 to 64.9 ng/mL (0.04-0.27 μM) with CSF:serum ratios ranging from 0.54 to 1.2.
| Time (days) | Dog 1 | Dog 2 | Dog 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Serum (ng/mL) | CSF (ng/mL) | CSF:Serum | Serum (ng/mL) | CSF (ng/mL) | CSF:Serum | Serum (ng/mL) | CSF (ng/mL) | CSF:Serum | |
| 0 | 0 | 0 | — | 0 | 0 | — | 0 | 0 | — |
| 1 | 53.7 (0.2 μM) | 64.9 (0.27 μM) | 1.2 | 19.7 (0.08 μM) | 15 (0.06 μM) | 0.76 | 21.2 (0.09 μM) | 16.5 (0.07 μM) | 0.77 |
| 6 | 16.4 (0.07 μM) | 9 (0.04 μM) | 0.55 | 25.4 (0.1 μM) | 13.7 (0.06 μM) | 0.54 | ND | ND | ND |
- Abbreviations: CSF:Serum, CSF, serum Ara-C concentration ratio; ND, not done.
3.4 Intranuclear Ara-CTP within normal peripheral blood leukocytes
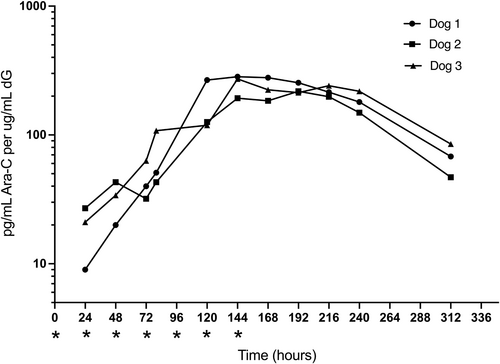
The DNA-incorporated Ara-CMP (reported as pg/mL Ara-C per μg/mL dG) was first assayed within peripheral leukocytes 24 hours after the first administration of CO (Figure 3). Final CO administration was at 144 hours (+6 days) and incorporated Ara-CMP increased progressively with maximum concentrations of 218.3 to 283.3 pg/mL Ara-C per μg/mL of deoxyguanosine (dG) at 144 to 192 hours. The DNA incorporation of Ara-CMP did not appear to be saturated after 7 doses based on continuing increases in Ara-CMP concentrations up to or beyond the last dose at 144 hours (+6 days). Elimination half-life for DNA-incorporated Ara-CMP was estimated to be 45.4 to 60.9 hours for the 3 dogs and mean DNA-incorporated Ara-CMP concentrations at +13 days remained >25% mean maximum concentrations.
3.5 Peripheral blood lymphocyte evaluation
Peripheral blood lymphopenia (<1000 cells/μL) was not documented in any dog, but a decreasing trend was seen in 2 dogs at day 6 (Figure 4).
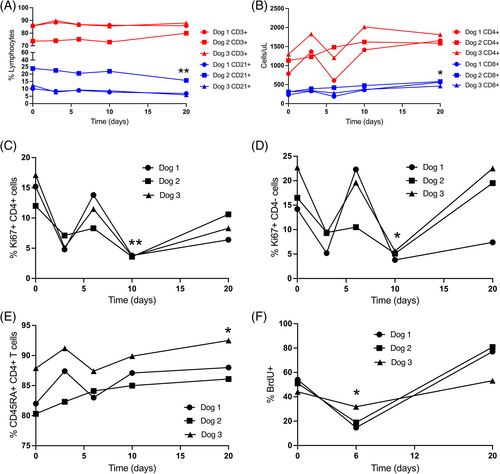
A small but significant (P = .01) decrease in CD21+ B-cell proportion was present on day 20 compared with baseline (Figure 5), but no significant difference in absolute number of B cells was noted. Moreover, parallel changes in T-cell numbers were not recorded. Within the T-cell compartment, there was a moderate but significant (P = .04) increase in absolute CD8+ T-cell count at day 20 compared with baseline, and a significant increase (P = .02) in CD45RA+ (naive) CD4 T-cell proportion on day 20 compared with baseline. A significant decrease in both proliferating Ki67+ CD4+ T cells (P = .01) and proliferating Ki67+ CD4- T cells (P = .04) was noted on day 10, compared with baseline, and ex vivo lymphocyte proliferation rates after exogenous mitogenic stimulation were decreased in all 3 dogs on day 6 compared to day 0, with a significant difference between day 6 and day 20 (P = .04; Figure 5).
4 DISCUSSION
Optimal immunomodulatory dosing regimens for Ara-C-based treatments in dogs with inflammatory central nervous system (CNS) disease have not been defined. Optimization of veterinary regimens based on the pharmacokinetics of Ara-C prodrug in plasma or serum has been suggested, often aimed at increasing exposure time to specific plasma concentrations of prodrug (Table S1).51-57 However, serum Ara-C concentration, although important, has been shown to be a suboptimal biomarker on its own for prediction of active drug metabolites and biological efficacy in human oncology patients.24, 34-39, 58 Defining key parameters in the metabolism of Ara-C to its active metabolite in dog tissues both in vitro and in vivo, ideally combined with biological markers for cellular efficacy, will be important in determining appropriate and relevant targets for therapeutic serum Ara-C concentrations. Peak serum Ara-C concentrations in our study were <750 ng/mL (3 μM) and minimum Ara-C concentrations were <50 ng/mL (0.2 μM). At low plasma Ara-C concentrations (<1 μM) accumulation of cellular Ara-CTP active metabolite is limited by the number of membrane nucleotide transporters.23 Clinically, in human leukemic patients, cellular elimination rate of Ara-CTP remains relatively constant under different Ara-C infusion conditions, and although plasma Ara-C concentrations vary with different infusion doses, as long as steady-state plasma concentration is >7 to 10 μM, Ara-CTP accumulation is saturated and constant.24-26, 32 The rate-limiting step associated with saturation, both in peripheral and CNS tissue, appears to be the initial phosphorylation of intracellular Ara-C to Ara-CMP5, 23, 24, 27-31, 59 mediated by deoxycytidine kinase (dCK; Figure 1) which is under complex control via a variety of nucleotides.60 Deoxycytidine kinase is present at high concentrations in human peripheral lymphocytes and is critical for development of normal B and T lymphocytes.61, 62 As such, therapeutic targeting via the dCK salvage pathway utilizing nucleoside analogs such as Ara-C or disrupting normal dCK activity is a potent mechanism to inhibit lymphocyte function. Increased dCK activity is reported in several experimental autoimmune models, and inhibiting dCK activity has been shown to ameliorate clinical signs in experimental allergic encephalomyelitis.63 Saturation-related parameters for the salvage pathway for Ara-C, including plasma concentrations and duration of drug exposure, in normal dog leukocytes have not been defined, although maximal Ara-C concentrations in a majority of pharmacokinetic studies in dogs are in excess of human-defined saturation concentrations (Table S1).51-57, 64
Clinical response to different Ara-C regimens in dogs with inflammatory brain disease has been reported, and is the ultimate gold standard for efficacy, but reports have been variable with design limitations and contradictory results. Lack of histopathological diagnoses in a highly variable group of diseases, multiple immunomodulatory drugs, and different geographical and population features likely confound published data. In dogs, an initial Ara-C constant rate infusion (100 mg/m2 over 24 hours) was reported as superior for short-term survival compared to 50 mg/m2 SC q12h for 2 days.65 A subsequent study suggested additional SC Ara-C doses beyond the initial infusion had no clinical benefit.66 Other studies, however, have not identified a clinical benefit from protocols involving initial Ara-C infusions.67, 68
In our study, altered lymphocyte function was identified at Ara-C concentrations below commonly suggested target concentrations (mean Cmax = 0.6 μg/mL [2.6 μM]). A nominal target plasma concentration of 1 μg/mL (4.1 μM) Ara-C in dogs with inflammatory brain disease has been suggested based primarily on reported in vitro cytotoxic effects in leukemic cells. An Ara-C concentration of 1.0 μg/mL (4.1 μM) caused 90% loss of leukemia cells in vitro,20, 69, 70 and decreased viability at 0.625 μM with 50% decrease at 2.5 μM or 20 μM was reported in another study.15 However, only 0.1 μg/mL (0.41 μM) Ara-C decreased DNA synthesis in human leukemic cells by 50% to 97%, sufficient to inhibit cell division,20, 29, 69, 70 and increasing concentration > 0.25 μg/mL (1 μM) resulted in no additional suppression of DNA synthesis.29 Dog leukemia cells have variable reported half-maximal inhibitory concentrations with exposure to Ara-C, ranging from 0.2 to 4.1 μM.71, 72
Normal lymphocytes and bone marrow cells generally exhibit less variability in pharmacokinetics and response to Ara-C as compared to leukemia cells, and are generally more resistant to its cytolytic and proapoptotic effects.33, 73-76 Significant decrease in proliferation of activated normal T lymphocytes is seen at Ara-C concentrations of 0.35 μM with approximately 50% decrease at 1 μM (approximately 0.25 μg/mL), and parallel but less marked effects are seen on cell viability and release of cytokines, chemokines, and interleukins at 0.35 μM.75 Overall, biological effects on normal leukocytes (potentially more relevant to inflammatory versus neoplastic disease) are consistently reported at Ara-C concentrations <1.0 μg/mL (4.1 μM) as reflected in the functional decrease in lymphocyte proliferation seen at Ara-C concentrations in this range (Cmax, 1.88-2.98 μM) in our study.
In human patients, metabolism of Ara-C by cytidine deaminase occurs within minutes and a majority of drug is excreted in urine, primarily as Ara-U, within 5 to 24 hours.20 Cytosine arabinoside (unlike CO) is not administered PO because of high first-pass elimination in the liver and metabolism in the gastrointestinal tract where high cytosine deaminase activity results in drug inactivation. Cytidine deaminase concentrations are significantly lower in dog tissues compared to human tissues21, 64, 77, 78; but metabolism of another cytidine deaminase-targeted nucleoside analog (gemcitabine) was similar in dogs and humans.79 Similarly, in our study and other Ara-C studies, half lives in humans are similar to those in dogs with a generally biphasic elimination and variably defined half lives in the range of approximately 60 to 250 minutes in both species (Tables S1 and S2).20, 51-58, 64, 80-86 Other compounding factors such as interspecies differences in kinetic properties of rate-limiting dCK activity also may determine overall similar Ara-C pharmacokinetics.87 In humans, these relatively short elimination half-lives have driven the development of modified Ara-C formulations such as PO CO and palmitic acid-conjugated Ara-C with altered pharmacokinetic profiles.43, 88
Cytarabine ocfosfate is absorbed from the gastrointestinal tract and distributed to depot sites including the CNS89 and Ara-C is released after peroxisomal β-oxidation, primarily occurring in the liver.44, 89, 90 Studies of CO in rodent models and human clinical patients have consistently reported prolonged plasma half-lives of Ara-C in the 24-hour range (Table S3)44, 91-94 comparable to the results of our study. Because of its ease of administration and prolonged Ara-C half-life, CO has been targeted in humans for low-dose regimens and in patients not eligible for intensive Ara-C protocols,93-98 an obvious parallel with low-dose therapeutic approaches in dogs with inflammatory brain disease. Cytarabine ocfosfate has been reported in the treatment of 9 cats99 and 1 dog45 with myelodysplastic syndromes, but no pharmacokinetic data were described.
Compared to the 1 to 2 hour Ara-C half-lives reported previously in dogs and humans using Ara-C protocols (Tables S1 and S2),20, 51-58, 64, 80-86 CO could offer potential advantages for immunomodulatory treatment if drug exposure time is an important factor and Ara-C concentrations are appropriate to drive Ara-CTP incorporation and downstream biological effects. Clinical benefits have been reported in several clinical and experimental leukemia CO studies in humans with resultant serum Ara-C concentrations in the 0.04 to 2.98 μM range.44, 94, 100-102 These findings and our study's CO/Ara-C half-life and maximum concentration data suggest that optimization of clinically relevant CO dosing regimens to achieve clinically relevant immunomodulation in dogs should be feasible.
Concentrations of Ara-C in CSF in our study ranged from 0.04 to 0.27 μM with CSF:serum ratios between 0.54 and 1.22 that are consistent with the variable range (approximately 0.1-0.6) reported previously in different species.20, 51, 84-86, 103, 104 Cytarabine ocfosfate was not detected in CSF, consistent with previous data and potentially because of its high plasma protein binding.93 Assessment of DNA incorporation of Ara-CTP and functional activity of CSF resident leukocytes was not done in our study, but Ara-C concentrations in matched serum and CSF samples were in similar, and overlapping ranges for the 3 dogs in which functional effects were documented (Table 3) and accumulation of Ara-C and metabolites in brain interstitial fluid (ISF) and neuropil at similar concentrations is likely based on published data.59, 105, 106 Accumulation of Ara-C and Ara-CTP within the CNS is of unknown importance in the context of immunomodulation of inflammatory CNS disease in dogs. Whether target cells for immunomodulation migrate into the CNS, reside within the CNS or both is not defined. In addition to mononuclear leukocytes, resident microglia are also likely to play an important role in the pathophysiology of inflammation and Ara-C selectively decreases microglial activation and proinflammatory cytokines in rodent CNS models.107, 108 Drug entry into CSF and brain ISF, and partitioning among plasma, CSF, and brain ISF depends on many factors including protein binding, lipophilicity, molecular weight and efflux mechanisms. Although specific data relating to Ara-C are not available, Ara-C has low plasma protein binding, and CSF is likely to be a potentially good indicator (if not a reliable measure) of brain ISF concentrations of drugs based on data from several studies.109-111 Cytosine arabinoside induces expression of efflux pump protein ABCB1 (PGp/MDR1),112 but multidrug resistance protein activity appears to have minimal effect on Ara-C activity.113, 114 Relating serum Ara-C concentrations to ISF concentrations is challenging. Cytosine arabinoside is taken up relatively slowly into the CNS, but it appears to be concentrated over time, potentially because of cellular uptake and deaminase activity that is lower in brain (and CSF) than in extra-CNS tissues20, 115, 116 Cerebrospinal fluid concentrations therefore may underestimate ISF concentrations because of this cellular uptake and also because of more rapid turnover of CSF compared to ISF.117 Relating CSF to serum concentrations is also challenging because CSF:serum ratios >1 are possible because of less rapid clearance from CSF compared to plasma depending on sampling times,83, 85, 86 consistent with the CSF:serum ratio > 1 at 1 sample time-point in our study. Cytosine arabinoside is delivered relatively homogenously to brain after IV administration,105 and disruption of the blood-brain barrier has been shown to increase Ara-C concentrations in brain parenchyma experimentally,118 which potentially is relevant to clinical inflammatory brain disease. However, although Ara-C CSF concentrations may be an indicator of ISF concentrations derived from CNS vasculature, diffusion of Ara-C specifically from CSF to the neuropil is limited to a few mm.105, 106
Toxicity from Ara-C treatment regimens (50-600 mg/m2) for immune-mediated inflammatory brain disease in dogs appears to be low, and overt myelosuppression and associated hematological abnormalities, as with our study, are rarely reported.51, 53, 56, 57, 65, 119-123 However, from a pharmacotoxicology perspective, dosing regimens resulting in plasma concentrations above those that cause saturation of intracellular triphosphate formation are questionable although both peak and temporal exposure to Ara-C must be considered. Lymphopenia was not identified in any of the dogs in our study but mild anemia (1/3), neutropenia (3/3), and thrombocytopenia (3/3) were seen by 144 hours (6 days) likely resulting from the shorter circulating half-lives and more prolific progenitor pools compared to lymphocytes (Figure 4).124, 125 Lymphocyte sub-type data should be interpreted cautiously because of small sample number and variability among dogs. Altered CD4:CD8 T cell ratios have been reported in ex vivo Ara-C studies, albeit at higher (44 μM) Ara-C concentrations.75 The clinical relevance of the increased proportion of naive CD4 and absolute numbers of CD8 T cells, and decreased proportion of naive CD21 B cells is unclear and should be confirmed in larger studies.
The efficacy of Ara-C as an immunomodulatory agent is not easily attributable to total lymphocyte numbers or major alterations in lymphocyte subpopulations based on our study or previous studies in dogs, and the effect of Ara-C at these lower doses may primarily reflect alterations in lymphocyte function. Decreased percentages of Ki67 CD4+ and Ki67CD4-lymphocytes were seen at certain time points (10 days), but the most consistent finding was a decreased ability to respond to mitogenic stimulation ex vivo (Figure 5). This functional effect on lymphocytes occurred at serum concentrations much lower than the proposed 1 μg/mL (4.1 μM) target concentration reported previously in dogs,52-57 but consistent with Ara-C concentrations associated with altered biological function in normal leukocytes..29, 69, 70, 75 As expected, the functional effects on stimulated lymphocytes were associated with increased DNA-incorporated Ara-CTP. Cellular or incorporated Ara-CTP concentrations, and more importantly Ara-CTP retention, have predictive value for Ara-C clinical efficacy in oncologic patients.34, 37, 38, 126 Specific cellular concentrations of Ara-CTP in humans resulting in clinical efficacy and biological effects in leukemic cells have been reported.3, 34, 36, 39-41, 127, 128 but direct comparison with current data is not possible because of methodological differences. Our study used a more recently validated methodology49 to determine DNA incorporation of Ara-CTP relative to the native DNA nucleoside deoxyguanosine (dG) which was suggested to have better reliability compared to using number of cells or total protein as normalizing variables. Importantly, although Ara-C concentrations were <1% Cmax by 6 days, DNA-incorporated Ara-CTP remained at >25% Cmax at 2 weeks. Prolonged retention of the active Ara-CTP metabolite (rather than Ara-C) during this time frame is consistent with anecdotal 3- to 4-week Ara-C treatment regimens proposed for inflammatory brain disease based on amelioration of clinical signs. Also, impaired lymphocyte function after chemotherapy (including Ara-C) has been demonstrated for >= 4 months in leukemic children, albeit after more intensive therapeutic regimens.129 Notably, Ara-CTP accumulation was not saturated after 7 consecutive (200 mg/m2) doses, but accumulation indices suggested that dosing intervals could be extended.
Our study is limited by the small number of dogs and single dosing regimen, but similarity to previous findings relating to CO in other species is supportive of clinical relevance. Relevance of assessment of peripheral leukocyte populations in the context of CNS disease is a major consideration, as is the relevance of specific in vitro functional assays to in vivo peripheral lymphocyte function. The data do however provide a rationale for more objective, and potentially more relevant, methods of comparison of Ara-C-based dosing regimens as well as expanded in vitro definition of basic Ara-C metabolite kinetics and functional sequelae in canine tissues.
Cytosine arabinoside pharmacokinetics and pharmacodynamics are complex and incompletely understood. At the individual level, human patients and in vitro cells from human patients receiving the same Ara-C and CO dose may have markedly different toxicities and pharmacokinetics for Ara-C and Ara-CTP.1, 22, 26, 27, 29, 32, 33, 42, 94 Several potential factors including pharmacogenomic and population variables such as sex22 and metabolic pathway gene polymorphisms and activity1, 22, 130, 131 are likely relevant and rarely considered in the context of inbred, geographically variable veterinary populations. It seems logical from current published data that optimal dosing of Ara-C and Ara-C analogs based on plasma Ara-C concentrations in isolation has limitations. Our data, based on a single dosing regimen, suggest that functional perturbation of leukocyte activity in dogs occurs at Ara-C concentrations below currently proposed target concentrations, and that functional changes are associated with accumulation of DNA-incorporated Ara-CTP. The 7-day PO CO regimen was well tolerated other than moderate subclinical neutropenia and thrombocytopenia, and a prolonged elimination half-life of Ara-C was obtained. Expanded in vitro and in vivo studies likely will be required to establish optimal dosing recommendations for this and other Ara-C-based drugs based on more detailed pharmacokinetic analysis of Ara-C and downstream metabolites, preferably in combination with disease-relevant functional biomarkers.
ACKNOWLEDGMENT
Funding was provided by the University of California, Davis Center for Companion Animal Health and the Holliday Neurology Resident Research Grant.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by the University of California, Davis, IACUC (protocol #18431).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




