Demographic and histopathologic features of dogs with abnormally high concentrations of hepatic copper
Abstract
Background
Copper associated hepatopathy (CAH) has become an important and prevalent disease since the 1990's, coincidental with changes in copper (Cu) content in commercial dog foods. Knowing the demographic and histopathologic features related to hepatic Cu concentrations might aid in diagnosing CAH in dogs.
Hypothesis/Objectives
The primary aim was to identify demographic and histopathologic features associated with abnormally high hepatic Cu concentrations.
Animals
Dogs that underwent liver histopathology and Cu quantification at a veterinary diagnostic laboratory between July 2010 and February 2020.
Methods
Data was retrospectively collected from an electronic database. A Gaussian multiple regression model on the log scale was used to evaluate associations between hepatic Cu and a set of demographic and histologic features selected with machine learning methods.
Results
Of 4559 cases meeting criteria, 50% had hepatic Cu > 400 and 19% had Cu > 1000 ppm (parts per million) dry weight (reference range 120-400). Median hepatic Cu was 391 ppm, range 4.5 to 31500. Age was negatively associated (P < .02), but specific breeds (Doberman pinscher, Labrador retriever, and West Highland white terrier) were positively associated with abnormally high hepatic Cu (P < .001). Severity of inflammation (mild, moderate, and severe) and necrosis/apoptosis were associated with abnormally high hepatic Cu (P < .01).
Conclusion and Clinical Importance
Abnormally high hepatic Cu is prevalent in hepatic biopsies from dogs. Machine learning modeling showed that necroinflammation, not cholestasis or cirrhosis, on hepatic histopathology, is predictive of higher hepatic Cu and might be a reliable histologic predictor of CAH.
Abbreviations
-
- CAH
-
- copper associated hepatopathy
-
- Cu
-
- copper
-
- ppm
-
- part per million
1 INTRODUCTION
Copper associated hepatopathy (CAH), is a common and important hepatic disorder in dogs and the prevalence of CAH has increased over time.1-7 Median copper (Cu) has increased 3fold from the 1980's to 2000's.2, 4 One study reported that one-third of dogs with chronic hepatitis have copper associated disease.3
A genetic etiology of CAH is strongly suspected given breed predispositions in the Labrador retriever,4, 5, 8 Bedlington terrier,9-11 Doberman pinscher,12 West Highland white terrier,13, 14 and Dalmatian.15 Mutations in Cu transport genes, such as Cu metabolism domain containing 1 (COMMD1) and ABCA12 genes of Bedlington Terriers16-19 or the ATP7B gene of Labrador retrievers and Doberman pinschers, have been associated with CAH.20, 21 Environmental factors such as dietary Cu have also been highly implicated.22 Dramatic increases in hepatic Cu were observed after the National Research Council and Association of American Feed Control Officials (AAFCO) regulations mandated more bioavailable Cu compounds in commercial dog food.2, 4, 22, 23 While Cu accumulation has been observed secondary to cholestatic or inflammatory disease in cats and humans,24-31 the impact of these factors has not been thoroughly evaluated and might be minimal in dogs.32
Dogs with CAH are typically young to middle-age and present with asymptomatic elevations in liver enzyme activity that later progress to liver failure.3, 6, 7 Excessive hepatic Cu causes oxidative stress and hepatocyte injury causing cell death (necrosis or apoptosis) and chronic hepatitis.33-35 Treatment with copper chelation,36-40 dietary Cu restriction,41-43 or a combination is essential to prevent progression. If untreated, some dogs can develop renal Cu accumulation causing kidney injury and Fanconi's syndrome.44, 45
Diagnosis of CAH requires quantification of Cu concentrations and should optimally be accompanied by Cu staining to document excess hepatic Cu stores. Staining typically shows Cu accumulation in a centrilobular location initially, but the distribution can progress periportal to widespread throughout the liver with time.1 However, variation in Cu distribution within the liver,46, 47 necroinflammation,4 parenchymal remodeling,47 sampling technique and specimen size,48-50 tissue fixation,49 and an individual dog's threshold for Cu accumulation4 can affect accurate estimates of Cu and obfuscate the diagnosis of CAH. Thus, knowing the demographic and histologic features most predictive of abnormally high hepatic Cu helps proactively detect, accurately diagnose, and treat CAH to prevent end-stage disease. It is also important to know whether factors such as cirrhosis and cholestasis impact hepatic Cu concentration and the diagnosis of CAH.
Studies reporting the demographic and histologic features of CAH evaluated small cohorts of select predisposed breeds7-16 and most studies reported descriptive features. Furthermore, the prevalence of CAH has grown in both predisposed and nonpredisposed breeds. Thus, reassessing previously noted breed associations and identifying new at-risk breeds is warranted. Further research is also needed to clarify contradictory findings published in previous studies.6 Hepatic Cu was significantly correlated with necroinflammatory activity in 1 study,4 but not in 2 others.51, 52 Hepatic Cu was associated with cholestasis in Skye terriers,53 but not in dogs with experimentally induced acute cholestasis or naturally occurring chronic extrahepatic cholestasis.32, 54 Thus, the present study aimed to analyze a large dataset using machine learning methods to identify demographic and histopathological features, other than histologic staining with rhodanine, that correlated with abnormally high hepatic Cu in dogs. We hypothesized that middle-aged dogs and breeds such as Labrador retrievers4, 5, 8 and Doberman pinchers would have higher hepatic Cu.12 We also expected necroinflammation to be positively correlated with hepatic Cu.
2 METHODS
2.1 Datasets
2.1.1 Retrospective dataset (4559 cases)
The Colorado State University Diagnostic Veterinary Laboratory database was searched to include dogs that had liver tissue submitted for histopathologic analysis and hepatic Cu quantification (performed by flame atomic absorption spectrometry)51 and reported in ppm (parts per million) dry weight between July 2010 and February 2020. Date of biopsy submission, age, sex, and the presence or absence of histopathologic features in the liver biopsy report were collected and required for inclusion of each case. Histopathologic features of interest included presence and severity of inflammation within the liver, necrosis or apoptosis, cirrhosis, cholangitis or cholangiohepatitis, intrahepatic bile cholestasis, interface hepatitis, and nodular regeneration. If a biopsy report did not contain information regarding severity of inflammation, the case was excluded. Breed information was not required for inclusion, but demographic features including age, sex, intact status, and breed were recorded for all cases where available. Exclusion criteria were cases with duplicate submissions in which case the duplicate was removed, severe tissue artifact or autolysis when mentioned in the histopathologic report, and insufficient sample quantity to perform histopathologic analysis or Cu quantification. A sample quantity of <10 mg was considered inadequate for Cu quantification. Information regarding biopsy technique and specimen quality was not consistently available in the submission or histopathologic report and therefore was not an exclusion criterion. Of 5587 cases, 740 were excluded due to unavailable age or sex information, 177 due to incomplete information regarding histopathologic features, 64 due to duplicate reports, 34 due to severe tissue autolysis or artifact, and 13 due to insufficient sample quantity, which left 4559 cases that met the screening criteria.
Histopathologic reports for each case were reviewed using a coding and scoring scheme developed from WSAVA criteria and definitions agreed upon by a board-certified veterinary histopathologist (Tawfik Aboellail) and 2 board-certified internists (Tarini Vedantham Ullal and David C. Twedt), all with an academic and clinical interest in hepatology. The histopathologist and both internists reviewed 20 randomly selected cases independently and then jointly to reach a consensus on the coding and scoring scheme to be applied. One of the board-certified internists (TVU) independently coded and scored all cases. All data was rechecked and reviewed several times by this board-certified internist to ensure data accuracy and adherence to the coding and scoring scheme prior to statistical analysis. The coding and scoring scheme was as follows. Each histopathologic feature was marked as present or absent based on the following criteria. Presence of inflammation was defined as any inflammatory cell infiltrate (lymphocytes, plasma cells, neutrophils, or histiocytes) within the liver. Periportal, centrilobular, midzonal, or pericholangial infiltrate was considered inflammation within the liver, but perihepatitis, subcapsular, or serosal inflammation was not. Severity of inflammation within the liver was scored as absent (0), mild (1), moderate (2), or severe (3) based on descriptions in the histopathology reports. If the level of inflammatory infiltrate was described in the report as minimal, scant, very mild, extremely mild, few, inconsistently, scattered, low or very low, severity was scored as 0.5. If the description of inflammation was small, mild, or modest, it was scored as 1. If the inflammation was described as moderate, it was scored as 2 and if the inflammation was described as severe, heavy, or marked, it was scored as 3. If there were contradictory descriptors of severity in the report, the grade in the final histopathologic diagnosis was used or the case was scored as 1.5 (mild to moderate) or 2.5 (moderate to severe inflammation). If the severity of inflammation was not described, scoring could not be performed. Necrosis or apoptosis and cirrhosis were marked present if there was any mention of these features in the report. Cholangitis or cholangiohepatitis was marked as present if there was mention in the report or if there were inflammatory cells in the bile ducts with or without inflammatory cells in the hepatic parenchyma. Intrahepatic bile cholestasis was considered present if the report mentioned canalicular or biliary stasis, inspissated bile plugs in dilated bile canaliculi, entrapped bile, accumulated bile pigment, or bile pigment in hepatocytes. Interface hepatitis was marked as present if the report explicitly mentioned presence of interface hepatitis or if there was a description of inflammatory infiltrate percolating beyond the limiting plate into the liver parenchyma as per WSAVA (World Small Animal Veterinary Association) criteria.35 Nodular regeneration was marked as present if the report mentioned nodular regeneration or benign hepatic nodules.
2.1.2 Breed dataset (1490 cases)
Of the 4559 cases, 1490 had breed information. Information regarding whether the dog was a mixed breed was not available in the electronic database.
2.2 Statistical methods
2.2.1 Simple linear regression
For datasets 1.1 and 1.2, demographic and histopathologic features were evaluated univariately for strength of association with the dependent variable hepatic Cu level (ppm) using the “glm” function in R. This did not consider confounding variables and was therefore considered exploratory. The parameters examined included date of biopsy submission, sex (male or female), intact status (spayed or neutered vs intact), breed, and hepatic histopathologic features including presence of inflammation in the liver, severity of inflammation, necrosis or apoptosis, cirrhosis, cholangitis or cholangiohepatitis, intrahepatic bile cholestasis, interface hepatitis, and nodular regeneration. For univariate analyses, severity of inflammation was scored in 0.5 increments, but for subsequent feature selection and multiple regression analyses, scoring was simplified to treat 0.5 scores as 0 (inflammation absent), because the inflammation was minimal and considered clinically insignificant, and 1.5 or 2.5 scores as 2 (moderate) or 3 (severe), respectively, to reflect the highest level of severity conveyed in the report. Descriptions of severity were not sufficiently specific in most cases to grade inflammation in 0.5 increments. Additionally, a sensitivity analysis was performed to ensure that the direction and magnitude of statistical effects did not change between the 2 different methods of encoding. P-values <.05 were considered significant. Strength of an association was further assessed using percent deviance, which is defined as percent of the null model deviance accounted for by inclusion of a feature in the regression model.
2.2.2 Feature importance and selection
From the demographic and histologic features, a subset of candidate predictors was selected by examining results from machine learning methods: forward/backward stepwise selection (“stepAIC” in the MASS R package v7.3.51.6),55 random forest feature importance criteria (randomForest R package v4.6.14),56 and feature weights calculated by penalized regression (glmnet R package v4.0.2).57 Penalized regression included ridge, lasso, and elastic net methods.58 The ridge-lasso tradeoff parameter for elastic net was optimized by minimizing the mean squared error using grid search at evenly spaced intervals from [0, 1] with a step size of 0.1. Penalized regression coefficients were optimized using 10-fold cross-validation as implemented in the “cv.glmnet” function (glmnet R package v4.0.2). Features were deprioritized from further analysis if they were selected against by stepwise selection, showed low feature importance scores by random forest, or had small or nonexistent weights by penalized regression.
2.2.3 Multiple regression generalized linear modeling and machine learning
Once a set of optimal features was selected, a Gaussian generalized linear model on the log-scale was fit to the retrospective (1.1) and breed datasets (1.2). Selected features and their interactions were included in the models. Candidate models were then compared using the Akaike Information Criterion (AIC)59 with the selected model having the lowest and optimal AIC. Final regression model residuals were visually checked for compliance with assumptions of homoscedasticity and potential outliers were assessed using quantile-quantile and residuals vs leverage plots.
3 RESULTS
3.1 Copper distribution
Complete data except for breed information were available for 4559 cases. Due to missing demographic data, only cases from 2012 to 2020 had complete information. Copper concentrations ranged from 4.5 to 31500 ppm with a median of 391 (Q1-Q3: 225-763), mean 704 ± SD (SD) 1058. Copper distribution showed a right-skewed distribution with a high degree of overdispersion and a heavy right-sided tail. However, on the log-scale, the distribution was roughly Normal with a small amount of right skew. Of 4559 cases, 50% (2233/4559) had hepatic Cu > 400 ppm, 19% (861/4559) had hepatic Cu > 1000 ppm, 33% (1505/4559) had normal Cu (120-400 ppm), and 13% (595/4559) had copper below the normal reference range.
3.2 Demographic and histologic features
The number of days between the first and last case submitted during the examined study period was 2715. Mean age in the study cohort was 8.1 ± SD 3.5 years. Sex composition consisted of 55% female (2499/4559), 45% were male (2060/4559) and 89% (4037/4559) were neutered or spayed. Breed information was available for 1490 cases of which there were 118 unique breeds. The 3 most abundant breeds were the Labrador retriever (206/1490, 14%), Chihuahua (68/1490, 4.6%), and Shih Tzu (52/1490, 3.5%). Of the dogs with Cu > 1000 ppm, most common breeds were Labrador retriever (65/861, 7.5%), Doberman pinscher (25/861, 2.9%), and West Highland white terrier (13/861, 1.5%). Specimen quality and size were not routinely reported, but all 4559 cases described portal areas, suggesting that there were adequate numbers of portal triads in all samples. Additionally, 3566/4599 reports evaluated sections from 2 or more lobes. Of 4559 cases, 63% (2854/4559) had hepatic inflammation scores characterized as mild in 45% (1292/2854), moderate in 36% (1039/2854), and severe in 18% (523/2854). Other common histopathologic features included necrosis or apoptosis observed in 30% (1378/4559), intrahepatic bile cholestasis in 20% (934/4559), and cholangitis or cholangiohepatitis in 16% (719/4559). Less commonly observed features were cirrhosis in 9% (402/4559), nodular regeneration in 2% (94/4559), and interface hepatitis in 1% (54/4559).
3.3 Univariate analysis
In the univariate analysis, numerous features were significantly associated with hepatic Cu but to varying strengths (Table 1). Strength of the association was assessed not only with significance of the P-value (<.05), but also percent deviance. Date of biopsy submission (P = .21), sex (P = .87) and intact status (P = .02) had minimally weak to no associations with hepatic Cu level, accounting for ≤0.1% of the deviance. Age in years was significantly negatively associated with hepatic Cu (P < .001, 1.6% of deviance). Breed accounted for 18% of the deviance and the Doberman pinscher was the breed most significantly correlated with abnormally high Cu (P < .001).
| Feature | Coefficient estimate | SE | P-value | % Deviance of data |
|---|---|---|---|---|
| Day | 6.1 | 0.01 | .21 | 0.03 |
| Age (years) | −0.04 | 0.004 | <.001* | 1.6 |
| Male/female | −0.004 | 0.03 | .87 | 0 |
| Spayed/neutered or intact | −0.10 | 0.04 | .02* | 0.1 |
| Breed | 18 | |||
| Doberman | 0.66 | 0.20 | <.001* | |
| Presence of inflammation | 0.54 | 0.03 | <.001* | 4.6 |
| Severity of inflammation | 13 | |||
| Score 0.5 | 0.13 | 0.04 | <.001* | |
| Score 1 | 0.39 | 0.04 | <.001* | |
| Score 1.5 | 0.72 | 0.08 | <.001* | |
| Score 2.0 | 0.89 | 0.04 | <.001* | |
| Score 2.5 | 0.89 | 0.13 | <.001* | |
| Score 3.0 | 0.94 | 0.05 | <.001* | |
| Necrosis or Apoptosis | 0.57 | 0.03 | <.001* | 7 |
| Intrahepatic Bile Cholestasis | 0.01 | 0.03 | .65 | 0.004 |
| Cholangitis or Cholangiohepatitis | 0.20 | 0.04 | <.001* | 0.6 |
| Cirrhosis | 0.57 | 0.05 | <.001* | 2.5 |
| Nodular regeneration | 0.31 | 0.08 | <.001* | 0.3 |
| Interface hepatitis | 0.30 | 0.13 | <.02* | 0.1 |
- Note: This table shows the results of the univariate analyses evaluating the association of each demographic or histologic feature with hepatic copper (Cu) concentrations. For each demographic or histologic feature, coefficient estimate, SE of estimate, and percent deviance are listed. Coefficient estimates and SEs are given on the natural log scale. Significant P values <.05 are marked with an asterisk.
Presence of inflammation (4.6% of deviance), each increasing level of severity of inflammation (13% of deviance), necrosis or apoptosis (7% of deviance), and cirrhosis (2.5% of deviance) were all significantly and strongly associated with abnormally high hepatic Cu level (P < .001; Figure 1A-C and Table 1). Other histopathologic features such as cholangitis or cholangiohepatitis (P < .001), nodular regeneration (P < .001), and interface hepatitis (P < .02), had significant, but weaker associations with Cu level (Table 1) accounting for only 0.1% to 0.6% of the deviance. Intrahepatic bile cholestasis showed no association with Cu level (P = .65; Table 1).
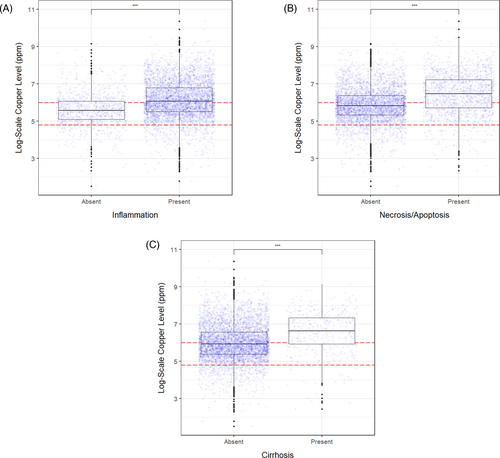
3.4 Feature selection
Feature selection revealed that severity of inflammation was the predominant predictor of hepatic Cu concentrations. Of the 1490 cases where breed information was available, a select subset of breeds (Doberman pinscher, corgi, Dalmatian, Labrador retriever, West Highland white terrier, Cavalier King Charles spaniel, and Great Dane) were the most predictive of abnormally high hepatic Cu. Following feature selection, the optimal predictors of hepatic Cu included age, the subset of breeds previously mentioned, severity of inflammation, and necrosis or apoptosis. The least important features by penalized regression and random forest were sex (male/female), intact status, cholangitis or cholangiohepatitis, cirrhosis, nodular regeneration, intrahepatic bile cholestasis, and interface hepatitis.
3.5 Multiple linear regression model or Gaussian Generalized Linear Modeling (GLM)
Results of Gaussian Generalized Linear Modeling (GLM) showing associations with the optimal features identified by feature selection and hepatic Cu are in Table 2. Severity of inflammation was the most strongly predictive of Cu level with each level of severity (1 = mild, 2 = moderate, 3 = severe) increasingly associated with abnormally high Cu level (mild P < .001, moderate P < .001, and severe, P < .001). Necrosis or apoptosis was significantly associated with hepatic Cu (P = .003), particularly when inflammation was moderate (.004) or severe (P = .05). Years of age was significantly associated with decreased hepatic Cu level (P = .02), particularly when necrosis and apoptosis was present (P < .001) and when inflammation was moderate (P = .002) or severe (P < .001).
| Predictor | Coefficient estimate | SE | P value | |
|---|---|---|---|---|
| Main effects | Intercept | 5.77 | 0.05 | <.001* |
| Years of age | −0.01 | 0.006 | .02* | |
| Necrosis/apoptosis | 0.34 | 0.11 | .003* | |
| Inflammation Severity 1 (mild) | 0.40 | 0.09 | <.001* | |
| Inflammation Severity 2 (moderate) | 0.93 | 0.10 | <.001* | |
| Inflammation Severity 3 (severe) | 1.00 | 0.15 | <.001* | |
| Interaction effects | Years of age Necrosis/apoptosis |
−0.03 | 0.01 | <.001* |
Years of age Inflammation Severity 1 (mild) |
−0.01 | 0.009 | .19 | |
Years of age Inflammation Severity 2 (moderate) |
−0.04 | 0.01 | .002* | |
Years of age Inflammation Severity 3 (severe) |
−0.05 | 0.01 | <.001* | |
Necrosis/apoptosis Inflammation Severity 1 (mild) |
0.13 | 0.10 | .20 | |
Necrosis/apoptosis Inflammation Severity 2 (moderate) |
0.28 | 0.10 | .004* | |
Necrosis/apoptosis Inflammation Severity 3 (severe) |
0.24 | 0.12 | .05 |
- Note: Coefficient estimates, SEs, and P-values of the Generalized Linear Modeling on large dataset 1.1 for the best fitted model by AIC (Akaike Information Criterion) are shown. Coefficient estimates and standard errors are given on the natural log scale. Significant P-values <.05 are marked with an asterisk.
Amongst the breed retrospective dataset (1490 cases), severity of inflammation (mild: P = .002, moderate: P < .001, or severe: P < .001) and necrosis or apoptosis (P = .03) were also associated with abnormally high hepatic Cu (Table 3). Additionally, the following breeds had significant correlations with abnormally high hepatic Cu level: Doberman Pinscher (P < .001), Labrador retriever (P < .001), West Highland White Terrier (P < .001), Dalmatian (P = .001), Corgi (P = .03), and Cavalier King Charles spaniel (P = .01; Table 3). Bloodhounds had a strong correlation with decreased hepatic Cu level (P < .001).
| Predictor | Coefficient estimate | SE | P-value |
|---|---|---|---|
| Intercept | 5.57 | 0.08 | <.001* |
| Years of age | 0.004 | 0.009 | .66 |
| Necrosis/apoptosis | 0.36 | 0.16 | .03* |
| Inflammation Severity 1 | 0.46 | 0.15 | .002* |
| Inflammation Severity 2 | 1.03 | 0.18 | <.001* |
| Inflammation Severity 3 | 1.29 | 0.24 | <.001* |
| Breed-Corgi | 0.52 | 0.23 | .03* |
| Breed-Doberman | 0.69 | 0.13 | <.001* |
| Breed-Dalmatian | 0.70 | 0.21 | .001* |
| Breed-Labrador retriever | 0.28 | 0.07 | <.001* |
| Breed-West Highland White terrier | 0.79 | 0.16 | <.001* |
| Breed-Cavalier King Charles spaniel | 0.75 | 0.29 | .01* |
| Breed-Bloodhound | −3.63 | 0.86 | <.001* |
- Note: Coefficient estimates, standard errors, and P-values of the Generalized Linear Modeling on the breed dataset 1.2 for the best fitted model by AIC (Akaike Information Criterion) are shown. Coefficient estimates and standard errors are given on the natural log scale. Significant P-values <.05 are marked with an asterisk.
4 DISCUSSION
This retrospective study examined hepatic Cu concentrations in a large dataset of dogs over the span of nearly 10 years and used machine learning approaches with regression analyses to evaluate associations with several demographic and histopathologic features. Abnormally high hepatic Cu concentrations were commonly found in this group of dogs. Fifty percent of dogs had hepatic Cu above 400 ppm and 20% above 1000 ppm. Age was negatively associated with hepatic Cu, but select breeds (Doberman pinscher, Labrador retriever, West Highland white terrier, Dalmatian, Corgi, Cavalier King Charles spaniel), presence and severity of inflammation in the liver, and necrosis or apoptosis were associated with abnormally high hepatic Cu. Results highlighted the abundance of abnormally high hepatic Cu in this cohort, identified 2 new breed associations, and supported that necroinflammation, not cholestasis or cirrhosis, is a strong histologic marker of abnormally high hepatic Cu and potentially CAH.
A relatively high proportion of dogs had hepatic Cu above the reference range (> 400 ppm) and >1000 ppm in this study cohort, consistent with reported findings.2, 4 Frequency of hepatic Cu concentrations above 1000 ppm was reported in this study because necroinflammation is typically observed at levels >1000,1, 4, 9, 60 although histologic changes can be observed between 600 and 1000 ppm and histologic changes might not be present at levels above 1000.46 Copper concentrations and the prevalence of CAH increased after 193061-66 and dramatically rose in the 1990's after regulations23 increased the bioavailability and Cu content of commercial dog foods.2, 4, 22 However, Cu concentrations have reportedly plateaued in the 2000's.2 Our study also did not find a significant increase in hepatic Cu over the 2012 to 2020 study period. However, Cu concentrations might have been inaccurately underestimated due to variable biopsy technique,48, 50 specimen quality,49 and hepatic parenchymal remodeling.46, 47 Examining multiple sections of the liver using digital image analysis of rhodanine-stained liver biopsy sections might have improved the accuracy of Cu quantification.46, 47 However, underestimates of hepatic Cu do not detract from the significant positive associations with abnormally high hepatic Cu identified in this study though they could have resulted in underestimations of the proportions of dogs with abnormally high hepatic Cu (> 400 or >1000 ppm). These proportions likely do not reflect a true incidence or prevalence of CAH because dogs were not definitively diagnosed with CAH and the study cohort consisted of dogs already suspected of hepatic disease. Dogs with abnormally high hepatic Cu can be subclinical and biochemically normal and such dogs are less likely to undergo liver biopsy procedures. Cases in this study cohort were also submitted to a single Diagnostic Veterinary Laboratory and therefore findings might not translate to other geographic regions or the larger global population. However, this study and several other studies2-4, 22 demonstrate that abnormally high hepatic Cu is a common, pervasive problem in dogs. Therefore, high Cu in commercial dog foods67 and the connection to abnormally high hepatic Cu68 warrants reconsideration of the current regulations for dietary Cu.22
In addition to dietary Cu, based on previously reported breed associations (Labrador retriever,4, 5, 8 Doberman pinscher,12, 38 Bedlington terrier,9-11 West Highland white terrier,13, 14 Dalmatian15) and genetic mutations, genetic factors appear involved.16-21, 69 Abnormally high hepatic Cu is much more common in predisposed breeds compared with nonpredisposed breeds2 and CAH can be heritable.5, 70 The present study corroborated previously reported breed associations including the Labrador retriever, Doberman pinscher, West Highland white terrier, and Dalmatian. New associations were also found in the Corgi and Cavalier King Charles spaniel in this study. Two case reports of CAH have been published in a Pembroke Welsh Corgi36 and Cardigan Welsh Corgi45 but none in Cavaliers. Bedlington terriers were not represented in this dataset likely because of the rarity of this breed and genetic selection that bred out the COMMD1 mutation. A significant negative association with hepatic Cu was found in the Bloodhound breed. It should be considered that this breed might have genetic mutations and protective mechanisms that decrease hepatic Cu load or increase the possibility of Cu deficiency and have been implicated in Labrador retrievers and human patients with Wilson's and Menkes disease.20, 69, 71-73
Another key demographic finding in this study was that age was negatively associated with Cu, indicating that younger dogs had higher hepatic Cu. Since the mean age of the study cohort was 8 years, this finding was consistent with typical diagnoses of CAH, which occur between 4 and 8 years.2, 5, 8, 9, 12 A previous study found differing results in that Cu concentrations were significantly higher in older dogs, but in comparison, the dataset of this previous study was much smaller and the analysis was performed by grouping and comparing dogs ≥9 and < 9 years of age.46 Although hepatic Cu can progressively accumulate in dogs as they age,9, 42, 74 in a subset of dogs and possibly in certain breeds such as the West Highland white terrier and Bedlington Terrier, Cu levels can plateau or reduce over time9, 60, 70 perhaps because they eventually adapt to high levels of Cu,70 which has been observed in rodent models.75, 76 Adaptive mechanisms to reduce hepatic Cu by increasing renal excretion and decreasing intestinal absorption of Cu have also been identified in human patients with Wilson's disease.72, 73 Alternatively, older dogs might develop fibrosis, cirrhosis or nodular areas in which Cu does not accumulate.47, 49 The negative relationship with age in this study was even more significant in the presence of necrosis, apoptosis, and moderate to severe hepatic inflammation, which further supported that hepatic necroinflammation was associated with abnormally high Cu.
It is well documented in humans34, 77, 78 and animal models76, 79-81 that intracellular hepatic Cu in its cupric form causes oxidative stress, mitochondrial damage, cell death, and subsequent inflammation. Similarly, dogs with excess hepatic Cu accumulation show signs of greater oxidative stress32, 82, 83 and varying degrees of necrosis or apoptosis and hepatitis.4, 11, 74 Additionally, dogs with hepatitis have significantly higher hepatic Cu.2, 4 Accordingly, our study found that histologic evidence of hepatocyte cell death (necrosis or apoptosis) and hepatic inflammation of increasing severity were significantly associated with abnormally high hepatic Cu. However, positive associations do not imply causation or explain pathogenesis. Excess Cu might incite a necroinflammatory response (primary Cu accumulation) or conversely, necroinflammation disrupts Cu homeostasis and causes secondary Cu accumulation, defined as copper accumulation secondary to cholestatic and necroinflammatory disorders.24-27 The distribution of Cu and inflammation in the liver can help differentiate primary from secondary Cu accumulation because primary Cu tends to develop centrilobular initially while secondary Cu accumulates periportal around areas of inflammation.1 A limitation of this study was that rhodanine staining was not performed on all histological samples but only samples requested by the submitting clinician. This compromised the ability to determine distribution of necroinflammation and correlation of copper location and staining intensity. The rhodanine staining data were considered inadequate to include in the machine learning model. Additionally, the clinical context for cases was lacking to diagnose CAH. Rhodanine stain results, inflammatory distribution, and clinical case details would have been useful to include in a model to predict CAH.
In humans with Wilson's disease, primary hepatic Cu accumulation is attributed to mutations in the ATP7b copper transporter gene and epigenetic factors, but hepatic Cu can also be mildly increased in cases of viral hepatitis,84, 85 auto-immune hepatitis,86 or alcoholic steatohepatitis87 and further elevated in auto-immune biliary diseases such as primary sclerosing and biliary cholangitis.28, 29, 86, 88 Cu excretion is disrupted in these biliary disorders particularly when there is bile duct injury, hepatic fibrosis, and lobular disarray.27, 30, 31, 84 However, in dogs, it is unclear whether analogous disease processes lead to higher Cu burden. Dogs diagnosed with idiopathic chronic hepatitis4, 89 or biliary diseases often have normal hepatic Cu.32, 46 Although 1 study found decreased biliary excretion of Cu in Dobermans with copper associated hepatitis,90 a separate study found that chronic extrahepatic bile duct obstruction did not increase hepatic Cu until dogs were administered supplemental Cu intravenously.54 Parenchymal remodeling and cirrhosis can reportedly lower Cu measurements because Cu does not typically accumulate within regenerative nodules.47, 49 However, in a study of 161 dogs with histologically confirmed cirrhosis, 80% had hepatic Cu > 400 ppm and 40% had Cu measurements >1000 ppm, suggesting that cirrhosis does not dramatically lower Cu levels.22 Results in the present study showed that histologic features of interface hepatitis (a feature of chronic hepatitis),35 cholangitis with or without hepatitis, intrahepatic bile cholestasis, nodular regeneration, and cirrhosis were not reliable predictors of hepatic Cu, which supports that these inflammatory, cholestatic, and fibrotic processes do not significantly increase Cu concentrations and that CAH can still be diagnosed in the presence of cirrhosis and nodular regeneration.
There were inherent limitations of this retrospective study such as lack of standardization in biopsy evaluations, unknown biopsy collection methodologies, and incomplete data. Although samples were submitted to a single laboratory, biopsies were read by 21 different histopathologists over the course of the study period. Each histopathologist had preferred descriptors for features, which introduced variability in the reports. Each report had to be reviewed manually to code each parameter, which was time-consuming, labor-intensive, and could have introduced data entry errors. All reports were reviewed by 3 authors (Tarini Vedantham Ullal, Nick Sbardellati and Brooke Gallagher) 2 to 3 times with a standardized coding and scoring system, but in the future, artificial intelligence tools might help analyze such large datasets more efficiently. Given the numerous histopathologists involved in biopsy interpretation, differences in histologic descriptions could have also introduced variability in the results. Although WSAVA guidelines for liver histology interpretation are published, it is unknown whether these criteria were referenced during biopsy evaluations. Additionally, guidelines were published in 2007 and were updated in 2021, after the conclusion of the study. Furthermore, the consensus on canine chronic hepatitis was published in 2019 and was not available for the majority of cases in this study. Due to the small number of cases diagnosed with cirrhosis or interface hepatitis in this study, we suspect some features were underreported. Ideally, a standardized approach and scoring system such as those used in humans91-93 would have been used to assess and grade histologic features.94 However, even when a scoring scheme is applied, evaluation of fibrosis, necrosis or apoptosis, and presence and severity of inflammation is subjective and variable between pathologists4, 95 and therefore, there is currently no universally accepted method to score necroinflammation in veterinary medicine. Features such as cirrhosis96 and interface hepatitis97 can be underestimated in humans as well, which can affect the diagnosis of chronic hepatitis. However, this study evaluated associations of demographic and histopathologic features with hepatic Cu and did not require histopathologists and clinicians to accurately diagnose idiopathic chronic hepatitis or CAH. Thus, re-cutting slides for all 4559 cases to obtain standardized, blinded histopathologic evaluations and evaluate interobserver agreement between 1 and 2 histopathologists was not performed. Some bias could have been introduced because histopathologists were not blinded to clinical history, Cu quantification, or rhodanine staining results during biopsy review although this information was variably available. Treatment history was also inconsistently available for cases and therefore, dogs already being treated for CAH or other causes of chronic hepatitis could have been included in the dataset. For example, CAH dogs being treated with anti-inflammatory or immunomodulatory medications would have reduced hepatic inflammation without impacting Cu. However, inclusion of such cases would have biased the data towards not finding an association between hepatic Cu and necroinflammation.
Method of biopsy collection was not standardized in this study. Liver biopsy procedures were performed by many different veterinarians likely using different approaches (laparotomy, laparoscopy, needle biopsy, or necropsy). This variability could have impacted sample quality and consequently, Cu and histologic analyses.49, 50 Optimally, wedge biopsies would have been collected to maximize diagnostic quality and specimen size. Furthermore, at least 2 lobes should have been sampled to account for interlobular variability and ideally, 3 lobes to diagnose chronic hepatitis.1, 48, 98 Although biopsy method, specimen size, and quality were not routinely mentioned, most reports were able to evaluate portal areas and described findings in at least 2 or more liver lobes, which assured some level of diagnostic quality. However, because the specific liver lobes biopsied in each case were not reported, the authors could not guarantee that copper quantification was performed on the same lobes evaluated and identified to have necroinflammation histopathologically.
Due to the retrospective nature of the study, 1028 of 5587 cases were excluded due to incomplete age, sex, or histologic data. Ultimately, only cases from the years 2012 to 2020 could be included, which reduced the dataset to 4559. Additionally, only cases from October 2017 onwards had available breed data because the electronic database was not capturing this information in earlier years. This reduced the breed dataset to 1490 cases. Furthermore, breed information was only available for pure-bred dogs. This prevented analyzing whether mixed breed dogs such as Labradoodles were at risk for abnormally high hepatic Cu. Even with these reductions in sample size, this is 1 of the largest retrospective studies investigating hepatic Cu levels in dogs to date, which enabled performing machine learning analyses and strengthened the statistical power of the results. P-values were reported on the log-scale, which made statistically significant results even more significant with respect to absolute values.
In human medicine, machine learning methods similar to those used in this study have been used to develop diagnostic algorithms for many hepatic diseases99, 100 including nonalcoholic fatty liver disease,101 hepatocellular carcinoma,102 and even Wilson's disease.103 Most studies have prioritized using noninvasive data (clinical, imaging, and/or multiomics data) instead of liver histology to develop these models.99, 100 However, future prospective studies in dogs could utilize these machine learning methods on both clinical and histopathologic datasets to develop and validate artificial neural network algorithms similar to those developed for humans.100, 101, 103 Such algorithms could dramatically improve our ability to screen, predict, diagnose, phenotype, and treat CAH and other hepatic disorders.
5 CONCLUSION
In conclusion, younger dogs and predisposed breeds such as the Labrador retriever, Doberman pinscher, and West Highland white terriers had abnormally high hepatic Cu. New breed associations were documented in the Corgi and Cavalier King Charles spaniel. Hepatic inflammation of increasing severity and necrosis or apoptosis were the strongest predictors of abnormally high hepatic Cu. Cholestasis, nodular regeneration, or cirrhosis were not significantly associated with hepatic Cu levels, suggesting these processes do not increase hepatic Cu and therefore might not affect the diagnosis of CAH.
ACKNOWLEDGMENT
No funding was received for this study. This study was presented at the 2022 American College of Veterinary Internal Medicine (ACVIM) Forum, Austin, Texas. The authors acknowledge Dr. Tawfik Aboellail for his assistance and expertise in developing the coding and scoring scheme utilized to evaluate the liver histopathology reports.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
ETHICS STATEMENT
Authors declare human ethics approval was not needed for this study.




