Clinical performance of the IMMY cryptococcal antigen lateral flow assay in dogs and cats
Abstract
Background
Cryptococcal lateral flow antigen assays (CLFAs) have been assessed in comparison to the latex cryptococcal antigen agglutination test but their clinical performance is unknown.
Objective
Determine clinical performance of IMMY CLFA (Immuno-Mycologics Inc, Oklahoma) using patients with and without cryptococcosis as the reference standard.
Animals
One-hundred ninety-seven serum samples from client-owned dogs and cats.
Methods
Review of medical records of a referral population of dogs and cats that had CLFA performed between 2012 and 2020. Animals were classified as cryptococcosis positive (Cr+) or negative (Cr−) based on clinical information. Clinical diagnosis was used to calculate positive and negative percent agreement of the CLFA.
Results
Twelve specimens (4 canine, 8 feline) were obtained from Cr+ animals and had positive CLFA results. One-hundred eighty-five specimens (139 canine, 46 feline) were collected from Cr− animals. Negative CLFA results were recorded in 129 canine and 44 feline Cr− samples. Positive CLFA results were noted for 10 canine and 2 feline Cr− samples. Positive percent agreement of CLFA was 100% (confidence interval [CI], 39.8%-100% dogs; 63.1%-100% cats). Negative percent agreements were 92.8% (CI, 87.2%-96.5%) for dogs and 95.7% (CI, 85.2%-99.5%) for cats.
Conclusions and Clinical Importance
A negative IMMY CLFA result enables reliable exclusion of cryptococcal infection in dogs and cats. By contrast, a positive result must be interpreted cautiously and further testing should be performed to verify a diagnosis of cryptococcosis.
Abbreviations
-
- C
-
- Cryptococcus
-
- CALAS
-
- Cryptococcal Antigen Latex Agglutination System
-
- CLFA
-
- cryptococcal lateral flow antigen assay
-
- CNS
-
- central nervous system
-
- Cr−
-
- cryptococcosis negative
-
- Cr+
-
- cryptococcosis positive
-
- CT
-
- computed tomography
-
- LCAT
-
- latex cryptococcal antigen agglutination test
-
- MUO
-
- meningoencephalitis of unknown origin
-
- NPA
-
- negative percent agreement
-
- PPA
-
- positive percent agreement
1 INTRODUCTION
Cryptococcosis is a potentially life-threatening fungal disease that affects many species including humans, cats and dogs.1-3 Inhalation of basidiospores or direct inoculation of fungal organisms can result in disease of the respiratory tract, central nervous system (CNS) and skin or can result in disseminated disease.3-5 In severe cases, cryptococcosis can be fatal and an early and accurate diagnosis is warranted to decrease morbidity and mortality. Definitive diagnosis of cryptococcal infection requires fungal cultures of clinical specimens, although visualization of fungal organisms in cytological or histopathological samples is sufficient for diagnosing cryptococcosis in many patients. Serological tests such as latex cryptococcal antigen agglutination test (LCAT) and cryptococcal antigen lateral flow assay (CLFA) detect circulating cryptococcal polysaccharide capsular antigen in serum avoiding the need for invasive testing and allowing monitoring of antifungal treatment.6 The LCAT is considered a very accurate test for diagnosing cryptococcal infection in various species, including dogs and cats.7-11 In human medicine, CLFA is increasingly used because of its capacity as a patient-side test.12, 13 Several studies reported higher sensitivity for CLFA compared to LCAT for the diagnosis of cryptococcal infections in humans.12, 14, 15 In addition, CLFA allows early detection of infection before development of clinical signs of cryptococcosis by several months.16
Studies evaluating accuracy of CLFA for diagnosing cryptococcosis in animals are sparse.17, 18 A recent study assessed CLFA results in comparison to LCAT as the reference method in dogs, cats and koalas.17 Sensitivities of 100% for dogs and 92% for cats were determined. Although concordance of negative test results was excellent, several LCAT-negative patients had positive CLFA results. Consequently, specificities of CLFA were 81% in dogs and 84% in cats and thus substantially lower than reported in humans. A second study compared the performance of 2 different CLFAs in dogs and cats using LCAT results as the reference standard.18 In this study, concordance of positive results of CLFA and LCAT were high, with specificities of 93.2% and 94.9% for the 2 CLFAs. Sensitivities for the 2 tests were 92% and 80%, respectively. Although both studies found good performance of CLFA, LCAT results, but not clinical findings, were used as the reference method to determine the presence or absence of cryptococcosis. Absence of infection based on the clinical course was not reliably established, which may have hampered accurate assessment of CLFA performance.
We investigated the use of a CLFA in a clinical setting by correlating test results to clinically determined presence or absence of cryptococcosis in dogs and cats. We hypothesized that CLFA would be able to exclude cryptococcal infection but not allow reliable confirmation of the disease.
2 METHODOLOGY
2.1 Animals
The patient database of a university hospital receiving referrals from Western Australia was searched for dogs and cats that had been billed for CLFA (IMMY cryptococcal antigen lateral flow assay, Immuno-Mycologics Inc, Oklahoma) between August 2012 to December 2020. Cases were included if a CLFA result was recorded and a complete medical record was available. If the CLFA was performed more than once in an animal, only the first result was included for analysis. All medical records were independently reviewed by 2 internists (WY, KFAL) and assigned to the categories cryptococcosis positive (Cr+) or negative (Cr−). Animals were classified as Cr+ if cryptococcal organisms were detected in cytological, histopathological or fungal culture specimens of the affected animal. Dogs and cats also were classified as Cr+ if the patient had a positive cryptococcal antigen titer measured by LCAT (Remel Cryptococcus Antigen Latex Test, Remel, Kansas) or Cryptococcal Antigen Latex Agglutination System (CALAS; Meridian Bioscience Inc, Ohio) and if signs resolved with antifungal treatment. Positive LCAT results were defined as a reciprocal titer ≥2 as per manufacturer recommendations. Animals were classified as Cr− if the affected patient had stable, improving or resolving signs for at least 3 months after the CLFA and if animals were not receiving antifungal drugs for >7 days during this period. If available, LCAT results were recorded but were not used to determine the absence of cryptococcal infection. Presenting signs, clinical course, follow-up times and outcomes were recorded for all patients. If the patient had returned to the referring veterinarian for further treatment, the practices were contacted to obtain the follow-up information. Patients with insufficient information to confirm or exclude cryptococcosis as defined above were excluded. No Institutional Review Board approval was required for data collection.
2.2 Cryptococcal antigen lateral flow assay
The CLFA was performed as part of the diagnostic evaluation or for monitoring of antifungal treatment. Blood samples were collected from patients by venipuncture and serum separated. As per manufacturer's instructions, 1 drop of specimen diluent was added into a test tube and mixed with 40 μL of patient serum. The white end of the test strip was submerged into the diluent/specimen mixture and evaluated after 10 minutes. Results were considered valid if a test line was visible. The presence of an additional line was consistent with a positive test result. Heat or pronase treatments were not applied to the samples.
2.3 Statistical analysis
The recorded CLFA results were compared with the clinical diagnosis established for each patient. Positive and negative percent agreement (PPA and NPA) were calculated for CLFA with a 95% confidence interval (CI) using the clinical diagnosis as the reference standard. Calculations were performed using statistical software (MedCalc Software Ltd, Belgium). Results from dogs and cats with undetermined diagnosis were excluded from the calculations. Post hoc comparison of discordant results over time was performed by the Fisher's exact test by a commercial software (GraphPad Prism 4, California). A P-value ≤.05 was considered significant.
3 RESULTS
3.1 Animals
The database search identified 337 entries for CLFA performed in dogs and cats between August 2012 and December 2020 (Figure 1). Eight entries were excluded because they were serial measurements. Four animals were excluded because of erroneous billing, 2 for lack of documentation of CLFA results and 1 for lack of clinical information. Of the 322 eligible animals, 12 were classified as Cr+ and 185 as Cr−. For 85 dogs and 40 cats, absence of cryptococcal infection could not be established because of insufficient follow-up time (n = 119), use of antifungal drugs for >7 days within 3 months after CLFA (n = 5) or worsening of clinical signs within 3 months after the CLFA (n = 1).
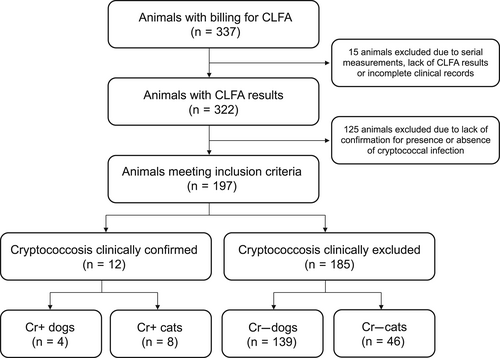
3.2 Cr+ animals
Four Cr+ dogs (Table S3) and 8 Cr+ cats (Table S4) were identified from the database (Figure 1). Clinical signs and details of diagnosis are summarized in Table 1.
| Dogs | Cats | |
|---|---|---|
| Number of animals | 4 | 8 |
| Presenting signs | ||
| Upper respiratory tract signs | 2 | 5 |
| Neurological signs | 2 | 3 |
| Cutaneous or mass lesions | 2 | 3 |
| Diagnosis of cryptococcosis | ||
| Cytology | 4 | 5 |
| Histopathology | 3 | 3 |
| Fungal culture | 4 (3 C. gattii, 1 C. neoformans) | 3 (C. neoformans) |
| Positive LCAT and response to antifungal treatment | 0 | 3 |
| Median follow-up time in months (range) | 5 (0-36) | 23 (9-58) |
- Abbreviations: C, Cryptococcus; LCAT, latex agglutination cryptococcal antigen test.
Of the 4 Cr+ dogs, 3 were diagnosed with CNS cryptococcosis; 2 of these dogs also had extension into the nasal cavity. One dog was diagnosed with naso-orbital cryptococcosis. Three dogs underwent antifungal treatment and 1 dog was euthanized after diagnosis. All treated dogs achieved clinical remission. The median follow-up time was 5 months (range, 5-36).
The 8 Cr+ cats were diagnosed with nasopharyngeal cryptococcosis (n = 3), concurrent nasopharyngeal and CNS cryptococcosis (n = 2), CNS cryptococcosis, facial cutaneous cryptococcosis, and disseminated disease (each n = 1). Detection of cryptococcal organisms confirmed the diagnosis in 5 cats (Table 1). In the remaining 3 cats, a positive LCAT and resolution of signs with antifungal treatment were used to establish a diagnosis of cryptococcal infection. The first cat was presented for stertor and neurological signs. A sphenoidal mass with intracranial extra-axial extension and concurrent bilateral retropharyngeal lymphadenopathy were found on computed tomography (CT) imaging. Clinical signs resolved with fluconazole administration and did not recur throughout the follow-up time of 58 months. The LCAT titer at the time of diagnosis was 1:1280 and became negative after a year of treatment. The second cat was presented for multiple neurological abnormalities. Advanced imaging was not performed. Fluconazole treatment resolved clinical signs and the cat remained neurologically normal until euthanasia 30 months later for hepatic carcinoma. The LCAT titer was 1:1024 at diagnosis and became negative 9 months into treatment. The third cat presented for generalized seizures and sneezing. Cerebrospinal fluid analysis and CT imaging of the head did not identify structural abnormalities. Clinical signs resolved with fluconazole administration, recurred once treatment was ceased and resolved again after administration was continued. The cat was euthanized because of renal failure 2 years after first presentation. The LCAT titer before commencing treatment was 1:2 (Remel) and 1:4 (CALAS). The LCAT titer (CALAS) at the time of relapse was 1:2. At the time of diagnosis, expert opinion (Dr Richard Malik) was sought and confirmed the suspicion for cryptococcosis in this patient. All 8 Cr+ cats were treated with antifungal drugs and achieved full clinical recovery. The median follow-up time was 23 months (range, 9-58).
3.3 Cr− animals
A total of 139 dogs (Tables S1 and S5) and 46 cats (Tables S2 and S6) were identified from the database (Figure 1).
A definitive or presumptive diagnosis was established in 103/139 dogs (74%). Of the 139 dogs, 12 underwent testing with LCAT, and all test results were negative. Except for 2 dogs, none of the Cr− dogs was treated with antifungal drugs. One dog with meningoencephalitis of unknown origin (MUO) received fluconazole PO for 6 days after CLFA. One dog with nasal aspergillosis underwent a single nasal irrigation with clotrimazole but did not receive treatment with PO antifungal drugs. Sixty-one dogs (44%) experienced full recovery from their presenting complaints whereas in 44 dogs (32%) clinical signs improved and in 34 dogs (24%) clinical signs did not progress. The median follow-up time was 19 months (range, 3-101) for all Cr− dogs. Presenting signs, clinical course and follow-up times for all Cr− dogs are summarized in Table 2.
| Main presenting signs | Diagnosis established | Clinical course | Median follow-up time in months (range) |
|---|---|---|---|
| Neurological signs (n = 125) | Yes (n = 89)a | Stable (n = 22) | 15 (4-75) |
| Improved (n = 31) | 20 (4-99) | ||
| Recovered (n = 36) | 17 (3-72) | ||
| No (n = 36) | Stable (n = 7) | 11 (3-23) | |
| Improved (n = 7) | 14 (4-96) | ||
| Recovered (n = 22) | 24.5 (7-101) | ||
| Upper respiratory signs (n = 9) | Yes (n = 9)b | Stable (n = 7) | 7 (4-20) |
| Improved (n = 3) | 25 (3-85) | ||
| Recovered (n = 1) | 83 | ||
| Retrobulbar or facial bone associated masses (n = 5) | Yes (n = 5)c | Stable (n = 1) | 5.5 |
| Improved (n = 1) | 6 | ||
| Recovered (n = 3) | 36 (22-85) |
- a Diagnoses included idiopathic epilepsy (n = 25), meningoencephalitis of unknown origin (n = 22) steroid-responsive meningitis-arteritis (n = 9), liver failure (n = 4), brain neoplasia, otitis media, behavioral disorders, Chiari-like malformation with syringohydromyelia (each n = 3), vascular accident, degenerative disc disease, idiopathic facial neuritis (each n = 2), concurrent congenital hydrocephalus and vestibular disease, idiopathic trigeminal neuritis, sudden acquired retinal degeneration syndrome, uveitis because of multicentric lymphoma, polyradiculoneuritis, neosporosis, geriatric vestibular disease, hypoadrenocorticism, metastatic hemangiosarcoma, hypercalcemia of malignancy and orthopedic disease (each n = 1).
- b Diagnoses included nasal neoplasia (n = 4), idiopathic lymphoplasmacytic rhinitis (n = 3), nasal aspergillosis and sialocele (each n = 1).
- c Diagnoses included facial bone neoplasia (n = 3) and retrobulbar abscess (n = 2).
In 26/46 (57%) Cr− cats, a definitive or presumptive diagnosis was established. In 4 cats, LCAT was performed and was negative. None of the Cr− cats received antifungal drugs for the first 3 months of follow-up. Full recovery from presenting clinical signs was recorded in 23 cats (50%), improvement in 9 cats (20%) and stable disease in 14 cats (30%). The median follow-up times were 16 months (range, 3-87) for all cats. Presenting signs, clinical course and follow-up times for all Cr− cats are summarized in Table 3.
| Main presenting signs | Diagnosis established | Clinical course | Median follow-up time in months (range) |
|---|---|---|---|
| Neurological signs (n = 27) | Yes (n = 10)a | Stable (n = 3) | 36 (30-87) |
| Improved (n = 1) | 48 | ||
| Recovered (n = 6) | 12 (3-55) | ||
| No (n = 17) | Stable (n = 1) | 11 | |
| Improved (n = 5) | 6 (3-21) | ||
| Recovered (n = 11) | 18 (3-53) | ||
| Upper respiratory signs (n = 17) | Yes (n = 14)b | Stable (n = 7) | 16 (8-44) |
| Improved (n = 3) | 6 (4-30) | ||
| Recovered (n = 4) | 18.5 (9-46) | ||
| No (n = 3) | Stable (n = 1) | 11 (3-23) | |
| Recovered (n = 2) | 54 (54) | ||
| Neurological and upper respiratory signs (n = 1) | Nasal neoplasia with brain invasion | Stable | 13 |
| Lymphadenomegaly (n = 1) | Multicentric lymphoma | Stable | 22 |
- a Diagnoses included idiopathic epilepsy (n = 3), bacterial meningitis (each n = 2), meningioma, thiamine deficiency, otitis media, behavioral disorder and adverse effect to chlorambucil administration (each n = 1).
- b Diagnoses included feline upper respiratory infection (n = 8), nasal neoplasia (n = 2), nasopharyngeal stenosis, nasopharyngeal polyp, bacterial pneumonia and feline asthma (each n = 1).
3.4 Comparison of CLFA results with clinical diagnosis
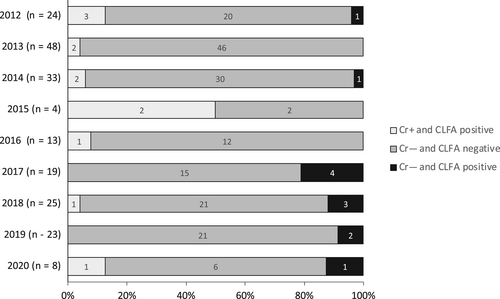
The CLFA results as recorded in the hospital database were compared with the assigned diagnosis for each animal. The CLFA was positive for all 12 Cr+ dogs and cats resulting in a PPA of 100% (CI, 39.8%-100% dogs; 63.1%-100% cats). The CLFA was negative in 173/185 Cr− animals. A discordant result was noted in 10 Cr− dogs and 2 Cr− cats (Tables S1 and S2). Four of these results were described as “weak positive” characterized by a faint band. The quality of the band was not described for the remaining animals. The CLFA was performed twice on the same serum sample in a dog with MUO whereas no information was available whether the test was repeated in the remaining animals. The frequency of discordant CLFA results varied over the years of the study (Figure 2). Four discordant results were noted in 2017 (number of total tests, 19), 3 in 2018 (25), 2 in 2019 (23) and 1 each in 2012 (24), 2014 (33) and 2020 (8). No discordant results were recorded in 2013 (48), 2015 (4) and 2016 (13). The number of discordant results was significantly higher in 2017 when compared to 2013 (P = .005) and 2014 (P = .05) and significantly higher in 2018 when compared to 2013 (P = .04). All patients with discordant CLFA results were presented for neurological abnormalities. An alternative diagnosis was established in 9/12 patients and included MUO (n = 3), idiopathic facial neuritis (each, n = 2), idiopathic epilepsy, meningioma, concurrent retrobulbar and periocular abscess and concurrent congenital hydrocephalus and vestibular disease (each, n = 1). In 2 dogs and 1 cat, a diagnosis was not available. The dogs both were presented for seizures and were presumptively diagnosed with idiopathic epilepsy given their young age, normal neurological examination and exclusion of extracranial disease. Both dogs responded favorably to administration of antiepileptic drugs as the sole treatment during the follow-up period of 4.5 and 42 months, respectively. Advanced imaging however was not performed in these 2 patients. The cat was presented for a single episode of transient weakness and disorientation and had complete resolution of neurological signs for 38 months of follow-up. An LCAT was performed in all dogs and cats with discordant results and was negative. One dog with MUO was treated with fluconazole PO for 6 days while the LCAT was pending before commencing immunosuppressive treatment with prednisolone, cytosine arabinoside and cyclosporine for 8 months. None of the other animals received antifungal drugs during the follow-up period. Four animals experienced full recovery, 2 improvement of signs and 6 stable disease. The median follow-up time for all 12 animals was 21 months (range, 4.5-42) with 18.5 months (range, 4.5-42) for dogs and 28 and 38 months for the 2 cats, respectively. Given lack of clinical evidence for cryptococcal infection in the 12 patients, the CLFA results were classified as false positives. Thus, NPA of the CLFA were 92.8% (CI, 87.2%-96.5%) for dogs and 95.7% (CI, 85.2%-99.5%) for cats.
4 DISCUSSION
We investigated the accuracy of the IMMY CLFA using dogs and cats with and without cryptococcosis as the reference. The CLFA correctly identified cryptococcal antigen in all 12 samples of dogs and cats with cryptococcosis. The test was negative in 174 of 185 animals with clinically-confirmed absence of the disease. However, positive CLFA results were recorded in 12 animals (10 dogs and 2 cats) without evidence of current or developing cryptococcosis, providing conclusive evidence that false positive results are encountered with this test in dogs and cats.
Positive CLFA results in the absence of cryptococcal infection have been described previously in 1 dog with disseminated aspergillosis.17 The manufacturer of CFLA reports that cross-reactivity between capsular antigens of both fungal organisms can occur. Positive CLFA results also were obtained in 2 dogs with coccidiomycosis, likely representing cross-reactivity, as described previously in humans.14, 18, 19 In our study, fungal infection was only encountered in 1 dog, and the disease was localized to the nasal cavity. This dog had a negative CLFA result. A second dog with disseminated aspergillosis (Aspergillus caninus) had a positive CLFA with concurrent negative LCAT. However, this dog was excluded from analysis because of treatment with PO antifungal drugs. Inclusion of this dog would have marginally decreased the PPA of the CLFA from 92.8% to 92.1%.
An earlier study on the performance of CLFAs described positive CLFA results in 1 cat and 1 dog previously infected by Cryptococcus species (spp.).18 In both animals, antifungal treatment was stopped once the LCAT was negative and, at the time of CLFA testing, the animals were clinically in remission and had concurrent negative LCAT results. Both animals were followed for 8 and 18 months, respectively, without developing signs of relapsed cryptococcosis. It was speculated that higher sensitivity of the CLFA as described in humans may be responsible for the discordance.12 Evidence for a higher sensitivity of the CLFA compared to the LCAT also has been found in koalas with cryptococcosis undergoing antifungal treatment, in which the LCAT became positive several months later compared to the CLFA.20 Similarly, equine serum samples analyzed concurrently with CLFA and LCAT had consistently higher titers with CLFA compared to LCAT.21 In our study, none of the patients with positive CLFA and clinically-confirmed absence of cryptococcosis had a history of previous cryptococcal infection or a history of treatment for cryptococcosis, rendering this possibility for the discordant results unlikely.
Positive CLFA results recently were described in 14 dogs without current or previous evidence for fungal infection.17 Laboratory error was excluded by repeating the test using additional pronase and heat treatment of the serum sample. Consequently, these CLFA results were interpreted as false-positive results. However, clinical follow-up was not available for these animals and early infection with cryptococcal organisms cannot be excluded. In the patients of our study, a follow-up of 3 months was carried out, and resolved, improved or stable clinical signs were required to reliably establish the absence of cryptococcosis in the animal. Therefore, early or undetected cryptococcosis as cause for the positive CLFA in the described 12 animals is unlikely. In addition, 3 of these animals received long-term immunosuppressive treatment (prednisolone, cytosine arabinoside, cyclosporine or some combination of these) and remained free of clinical signs of cryptococcosis. As such, the 12 discordant CLFA results are considered false-positive results.
False-positive results using the IMMY CLFA sporadically have been reported in human patients.22-24 In all instances, the CLFA was used in a semiquantitative way, and results yielded a low titer of ≤1 : 20. Although our study used only qualitative measurement of cryptococcal antigen, several of the false-positive tests identified a faint band, which is likely consistent with a low semiquantitative CLFA titer. A recent United Kingdom study cited recall of a specific lot of IMMY CLFA because of widespread occurrence of false-positive results.23 Interestingly, 7/12 false-positive results in our study occurred in 2017 and 2018. Another 4 animals had suspected discordant results in these 2 years, but were excluded because of insufficient follow-up or treatment with PO antifungal drugs. Therefore, it is possible that problems with reagent manufacturing may be responsible for a proportion of the false-positive results in our study. However, the batch numbers of the IMMY CLFA kits used for individual patients were not recorded, and it remains unclear whether the same or different kits had been used during the times when an increased number of discordant results was noted. Moreover, the samples in question had not been stored and the test could not be repeated.
Two previous studies have reported a small number of false-negative results for the IMMY CLFA in cats, all of which had confirmation of cryptococcosis by cytology, histopathology or fungal culture.17, 18 In 1 cat, focal infection with the minimally-encapsulated species Papiliotrema laurentii (synonym C. laurentii) was present and the 2 other cats had a prozone phenomenon because either dilution of the sample or repeat sampling once treatment was commenced showed positive CLFA results. In our study, 2 samples with LCAT titers of 1:4096 and 1 sample with a titer of 1:16384 from cats with nasal cryptococcosis yielded positive CLFA results without need for dilution of the sample. False-negative results for the CLFA in dogs as opposed to cats with confirmed cryptococcosis were not encountered in either of the previous studies.
Limitations of our study included the low number of animals diagnosed with cryptococcosis, which may have influenced the calculated NPA of the CLFA. However, other studies with higher numbers of dogs and cats with cryptococcosis using the LCTA as their reference standard have shown excellent sensitivity of the CLFA, rendering a statistical error in our study unlikely.17, 18 Three of the 8 Cr+ cats had a diagnosis established without the gold standard of the detection of cryptococcal organisms. This situation may have given rise to an incorrect classification further impacting the reported accuracy of the CLFA in our study. All of these cats however experienced complete and sustained resolution of their presenting clinical signs with antifungal treatment and were followed for at least 24 months after diagnosis. In addition, all animals in question had concurrently positive LCAT, which has demonstrated 100% specificity for the detection of cryptococcosis in dogs and cats.8, 10 For the purpose of our study, clinical follow-up rather than LCAT was used for the exclusion of cryptococcosis because the latter has been shown to produce false-negative results on rare occasions.8, 19 The follow-up period of 3 months for Cr− animals was chosen arbitrarily to exclude development of appreciable cryptococcosis in patients with a negative CLFA. The disease may have become evident with longer follow-up thereby decreasing the PPA of the CLFA reported in our study. Incubation periods of 6 weeks to 13 months have been reported for infections with Cryptococcus spp. in humans.25-27 In dogs and cats, incubation periods are less well understood but appear similarly variable depending on the subspecies of the fungus and immune status of the host.28 Once clinical signs are present, disease progression without treatment can be expected within days to weeks.29-31 The median follow-up time for all Cr− animals was >12 months and 21 months for animals with discordant CLFA results. In addition, all patients had clinical signs at the time of testing, and animals with progressive disease were excluded. Therefore, it is unlikely that cryptococcosis was the cause of the observed clinical signs in the Cr− group. Finally, carriers of Cryptococcus spp. without clinical signs have been described in dogs and cats and may explain a positive CLFA in an animal without previous or current cryptococcosis.4, 32, 33 It remains unknown whether the animals in our study had benign carriage of Cryptococcus spp. in the respiratory system because no nasal swabs for cytology and fungal culture were collected.
5 CONCLUSION
Our results suggest that the IMMY CLFA has excellent NPA and enables exclusion of cryptococcosis in dogs and cats without need for further testing. A positive CLFA result, especially if weak positive, should prompt the clinician to seek verification by additional testing such as LCAT to avoid misdiagnosis and unnecessary antifungal treatment.
ACKNOWLEDGMENT
No funding was received for this study. The authors acknowledge Shiromi Piyasena for her assistance with data collection.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




