Serum amyloid A as a marker to detect sepsis and predict outcome in hospitalized neonatal foals
Emma Hoeberg and Alexandra Sånge shared first authorship.
Abstract
Background
Serum amyloid A (SAA) has been reported to hold promise as diagnostic and prognostic marker in foals. This has not been investigated thoroughly.
Objectives
Evaluate admission SAA concentrations as predictor of sepsis and outcome.
Animals
Five hundred and ninety hospitalized foals <14 days old.
Methods
Retrospective multicenter study. Foals were scored with sepsis and survival scores, grouped according to health category (septic, sick but nonseptic, uncertain sepsis status) and outcome; septic foals were further categorized according to severity (normal sepsis, severe sepsis, and septic shock). SAA was compared between groups using Mann-Whitney test and Kruskal-Wallis test. Receiver operating characteristic curves identified optimal SAA cut off values for detecting sepsis and predicting outcome.
Results
Admission SAA concentrations differed significantly between sick nonseptic foals (312.1 ± 685.4 mg/L) and septic foals (1079.7 ± 1254.5 mg/L) and increased with increasing sepsis score. SAA did not differ between sepsis severity groups. The optimal cut off for sepsis detection was 1050 mg/L (sensitivity 30.2%, specificity 90.7%). Admission SAA concentrations were lower in surviving (435.0 ± 723.6 mg/L) compared to nonsurviving foals (1062.7 ± 1440.1 mg/L) and decreased with increasing survival score. The optimal cut off for nonsurvival prediction was 1250 mg/L (sensitivity 22.1%, specificity 90.8%).
Conclusions and Clinical Importance
SAA concentration was higher in septic foals and nonsurviving foals. Even though optimal cut offs for SAA to detect sepsis and predict outcome had low sensitivity, they had good specificity. SAA can therefore be used as a marker to rule out sepsis and nonsurvival.
Abbreviations
-
- ANOVA
-
- analysis of variance
-
- CARS
-
- compensatory anti-inflammatory response syndrome
-
- IgG
-
- immunoglobulin G
-
- MODS
-
- multi organ dysfunction syndrome
-
- ROC
-
- receiver operating characteristic
-
- SAA
-
- serum amyloid A
-
- SIRS
-
- systemic inflammatory response syndrome
-
- WBC
-
- white blood cell
1 INTRODUCTION
Sepsis in neonatal foals has a high case fatality rate and a large economic impact.1, 2 The diagnosis of sepsis can be challenging as it has nonpathognomonic clinical, hematological and biochemical signs.3, 4 The diagnostic methods currently used, sepsis scores and blood cultures, are limited by variable sensitivity and specificity.5-7 The sepsis scores have a sensitivity ranging from 52% to 94% and specificity of 73.4% to 86%.7-10 With blood cultures, a minimum of 48 hours are needed to get the final result and false negative results occur frequently.5 Sepsis is a condition that requires immediate action and early treatment with antibiotics to improve outcome, and a method with good screening test characteristics (fast, convenient, high sensitivity) is therefore desirable.1, 11, 12 Predicting outcome early in the disease process is valuable, as treatment can be expensive.6
Serum amyloid A (SAA), a major acute phase protein in horses, is used to identify inflammatory and infectious conditions. SAA increases within few hours after the onset of inflammatory conditions, it has low concentrations in the healthy individual, a wide dynamic range with several thousand-fold concentration increases, and its concentration decreases rapidly once inflammation subsides.13, 14
Foals are capable of producing SAA.15 Healthy neonatal foals have SAA concentrations slightly above reference range for at least 72 hours, most likely because of an inflammatory response originating from the parturition.16 However, SAA remains generally below 100 mg/L in healthy equine neonates.17 In human neonates, SAA has a high sensitivity and specificity as a diagnostic tool for sepsis and SAA concentrations are inversely related to sepsis-related death.18-22 Few studies have investigated SAA in sick foals. Based on small numbers of animals, foals with sepsis or a focal infection have higher SAA concentration compared to healthy foals and that foals suffering from a bacterial infection have higher SAA levels compared to foals suffering from a nonbacterial infection.17, 23, 24 SAA could be useful in the diagnosis of sepsis in foals.25 Cut off values of 100 mg/L SAA are suggested for diagnosing infection17 including sepsis in foals.25 The prognostic ability of SAA has only been studied in 1 study that evaluated SAA in relation to outcome of sepsis in 34 neonatal foals, and found concentrations of SAA not to be different between surviving and nonsurviving foals.24
As there is currently limited information on the use of SAA in foals, the objectives of this study were to evaluate whether admission concentrations of SAA are useful to detect sepsis and predict outcome in hospitalized neonatal foals.
2 METHOD AND MATERIALS
The study was designed as a multicentric, retrospective study performed with clinical data from 5 referral hospitals in Scandinavia (Table S1). The foals were hospitalized between 2007 and 2017. Regardless of the reason for hospitalization, foals with the following criteria were included: (1) blood analysis, including SAA, performed on admission or within the first 24 hours following admission, and (2) maximum 14 days of age. Recorded variables from the case files included signalment, history, clinical variables on admission, admission blood results, response to therapeutics, blood culture results, outcome and necropsy findings.
2.1 Laboratory analysis
The participating hospitals used different methods and machines for analyzing SAA (Table S1). Two of the hospitals did not fully dilute SAA concentrations but reported values of SAA as continuous until a certain value or being above that value (dichotomous data). Foals (n = 181) with not fully diluted (dichotomous) results were excluded from statistical analysis but were included in the descriptive part of this study. Foals (n = 409) with fully diluted SAA values (continuous data) were used for the statistical and descriptive analysis.
2.2 Scores
2.2.1 Sepsis score
The sepsis score used for this study was the original sepsis score created by Koterba et al. (Table S2).8 The variables “long transport of the mare” and “basophilic cytoplasm” were excluded and “Zink sulphate turbidity test” was replaced by the SNAP foal test. All foals received a total maximum sepsis score and a total minimum sepsis score; this was done by giving them for each individual sepsis score variable with missing data (1) a maximum score (variable with missing data scored as high as possible based on the additional clinical data) and (2) a minimum score (variable with missing data scored at 0). Per foal, the sum of all complete data variable scores together with the missing variable maximum scores resulted in the total maximum sepsis score, and the sum of all complete data variable scores together with the missing variable minimum scores resulted in the total minimum sepsis score.
2.2.2 Survival score
A survival score using presence of prematurity, cold extremities, 2 or more infectious or inflammatory sites, white blood cell counts and blood glucose concentration was used to predict survival in all foals (Table S3).6 The score has been validated under Scandinavian circumstances and with slightly older foals (until 14 days of age) than the original American sample and found to perform reasonably well.26 A high survival score indicates a high chance for survival.
2.3 Groups
2.3.1 SIRS classification
All foals were classified as having or not having systemic inflammatory response syndrome (SIRS) using SIRS criteria (Table S4).27
2.3.2 Sepsis classification
The foals were divided into groups of: septic, sick nonseptic, healthy, and uncertain sepsis status.
- positive blood culture;
- necropsy findings indicating sepsis;
- systemic inflammatory response (SIRS) in combination with a site of infection; and
- maximum and minimum total sepsis score both above 11.
The septic foals were further subdivided according to sepsis severity into normal sepsis, severe sepsis and septic shock (definitions are provided in Table S5).
Foals classified as sick nonseptic were diseased but did not fulfill any of the sepsis criteria mentioned above. Their minimal and maximal sepsis score were both 11 or below.
Foals classified as healthy were foals that had no disease and were not classified as having SIRS; they were typically hospitalized because of disease in the mare.
In absence of other variables indicating sepsis, foals with a maximum score above 11, yet a minimum score of 11 or lower were classified as uncertain sepsis status.
2.3.3 Outcome
Foals were divided according to outcome into survivors or nonsurvivors. Foals surviving were foals discharged from the hospital. Nonsurviving foals either died or were euthanized during hospitalization. Foals euthanized because of financial restrictions were identified and excluded from the outcome part of the study.
2.4 Data analysis
2.4.1 Descriptive analysis
For numerical variables median and range were calculated and for quantitative variables response rate (n) and prevalence (N) were calculated. Admission SAA concentrations of all foals (including not fully diluted dichotomous results) were grouped in the ranges low (<300 mg/L), intermediate (300-900 mg/L) and high (>900 mg/L) and were described according to sepsis status, sepsis severity and outcome. The levels of SAA for the low, intermediate and high category were set based on the authors' own experience with SAA.
2.4.2 Statistical analysis
- Groups:
- Receiver operating characteristic (ROC) curves:
Receiver operating characteristic (ROC) curves were used to identify optimal SAA cut off values for predicting sepsis and nonsurvival. For the ROC curve regarding health status, septic vs nonseptic and healthy foals were used. For the ROC curve regarding outcome, survivors vs nonsurvivors were used. Statistical analysis was performed using a statistical software Rstudio 3.4.0 (PBC 250 Boston, Massachusetts) and STATA/SE Acad. 14.2 (Stata Corp., College Station, Texas).
3 RESULTS
3.1 Study sample data
A total of 590 foals were included in the study. An overview of their signalment is provided in Table 1. Foals presented for a variety of reasons, most commonly because they were suspected to suffer from sepsis (20.5%, n = 121), meconium impaction (16.9%, n = 100) and weakness (12.0%, n = 71).
| Response rate | Prevalence | ||||
|---|---|---|---|---|---|
| n | % | N | % | ||
| Sex | Filly | 570 | 96.6 | 233 | 40.9 |
| Colt | 570 | 96.6 | 337 | 59.1 | |
| Breed | Pony | 577 | 97.8 | 37 | 6.4 |
| Trotter | 577 | 97.8 | 151 | 26.2 | |
| Thoroughbred | 577 | 97.8 | 17 | 2.9 | |
| Warmblood | 577 | 97.8 | 278 | 48.2 | |
| Other | 577 | 97.8 | 94 | 16.3 | |
| Median (range) | |||||
| Age (days) | 2 (1-14) | ||||
- Note: n = response rate; N = prevalence. Response rate indicates the number of foals where this information was available, and prevalence indicates the number of foals fulfilling these specific characteristics.
A fully diluted SAA concentration (continuous data) was available for 452 foals. For the dichotomous data, SAA concentrations were available for 138 foals. Of the 138 foals with dichotomous data, 4 foals had SAA concentrations of >800 mg/L; therefore they could not be grouped in the intermediate (300-900 mg/L) or the high (>900 mg/L) SAA category and were omitted from all analyses.
3.2 Scores
3.2.1 Sepsis score
There was an acceptable level of missing data on the sepsis score: an average of 11.1 out of the 14 variables of the sepsis score was obtained, and 508 foals had data available on ≥10 sepsis score variables. Overall, the foals had an average (± SD) sepsis score of 7.2 ± 4.8, ranging from 0 to 24. Low SAA concentrations (<300 mg/L) were mainly seen in foals with lower sepsis scores, and the median SAA concentration per sepsis score increased with increasing scores (Table 2). There were however foals with high SAA concentrations that scored low in the sepsis score, and the other way around.
| Sepsis score | Serum amyloid A concentration (mg/L) | |||
|---|---|---|---|---|
| Score | No. of foals | % of foals | Median (range) | No. of foals with SAA >300 |
| 0 | 13 | 3.4 | 55.0 (0.3-1675) | 4 (30.8%) |
| 1 | 24 | 6.3 | 86.5 (5.0-907) | 5 (20.8%) |
| 2 | 22 | 5.8 | 77.5 (0.0-1539) | 5 (22.7%) |
| 3 | 36 | 9.5 | 60.0 (4.0-2856) | 6 (16.7%) |
| 4 | 26 | 6.9 | 119.9 (0.1-2027) | 6 (23.1%) |
| 5 | 21 | 5.5 | 41.0 (0.5-5997) | 4 (19.0%) |
| 6 | 31 | 8.2 | 72.0 (0.1-3077) | 3 (9.7%) |
| 7 | 28 | 7.4 | 155.8 (0.1-2256) | 11 (39.3%) |
| 8 | 28 | 7.4 | 371.5 (5.0-4220) | 15 (53.6%) |
| 9 | 33 | 8.7 | 231.0 (0.2-2597) | 16 (48.5%) |
| 10 | 20 | 5.3 | 197.5 (9.0-2194) | 7 (35.0%) |
| 11 | 21 | 5.5 | 181.6 (5.0-3232) | 8 (38.1%) |
| 12 | 19 | 5.0 | 635.0 (86.0-5604) | 14 (73.7%) |
| 13 | 15 | 4.0 | 567.4 (0.0-2820) | 8 (53.3%) |
| 14 | 5 | 1.3 | 677.0 (514.9-2632) | 5 (100%) |
| 15 | 7 | 1.8 | 606.2 (0.0-1595) | 4 (57.1%) |
| 16 | 12 | 3.2 | 1105.0 (17.0-4871) | 9 (75.0%) |
| 17 | 11 | 2.9 | 521.0 (13.0-2460) | 9 (81.8%) |
| 18 | 1 | 0.3 | 528.0 (−) | 1 (100%) |
| 19 | 3 | 0.8 | 784.9 (259.0-995) | 2 (66.7%) |
| 20 | 1 | 0.3 | 2369.0 (−) | 1 (100%) |
| 22 | 1 | 0.3 | 904.0 (−) | 1 (100%) |
| 24 | 1 | 0.3 | 21.0 (−) | 0 (0.0%) |
- Note: n = 379. The minimum sepsis score was used, where 0 was attributed for an unknown variable. Foals with <10/14 known variables for the sepsis score were excluded from this table. Only foals with fully diluted (continuous) serum amyloid A concentrations were included.
3.2.2 Survival score
There were few foals with missing data on the survival score: an average of 5.8 out of the 6 variables of the survival score was obtained, and 576 foals had data available on ≥5 survival score variables. Overall, the foals had an average (± SD) survival score of 5.8 ± 1.4 ranging from 1 to 7. Low SAA concentrations (<300 mg/L) were mainly seen in foals with a high survival score, and the median SAA concentration decreased with increasing scores (Table 3). There were however foals with high SAA concentrations that scored high in the survival score, and the other way around.
| Survival score | Serum amyloid A concentration (mg/L) | |||
|---|---|---|---|---|
| Score | No. of foals | % of foals | Median (range) | No. of foals with SAA >300 |
| 1 | 5 | 1.1 | 390.5 (21.0-2363) | 4 (80.0%) |
| 2 | 11 | 2.5 | 635.0 (0.0-4871) | 9 (81.8) |
| 3 | 17 | 3.8 | 166.0 (2.0-2134) | 5 (29.4%) |
| 4 | 30 | 6.8 | 236.5 (0.2-2597) | 14 (46.7%) |
| 5 | 74 | 16.7 | 183.3 (0.0-5604) | 27 (36.5%) |
| 6 | 134 | 30.2 | 236.5 (0.0-3795) | 59 (44.0%) |
| 7 | 172 | 38.8 | 129.9 (0.0-5997) | 50 (29.1%) |
- Note: n = 443. Foals with <5/6 known variables of the survival score (n = 10) were excluded from this table. Only foals with fully diluted (continuous) serum amyloid A concentrations were included. A high survival score indicates a high chance for survival.
3.3 Groups
3.3.1 Sepsis status
Dividing foals according to sepsis status resulted in 40.3% (n = 236) of the foals being classified as sick nonseptic, 32.4% (n = 190) as having an uncertain sepsis status, 26.6% (n = 156) as being septic and 0.7% (n = 4) as healthy. Out of the 156 foals classified as septic, 90 foals had a sepsis score >11, 30 foals had a positive blood culture, 13 foals had SIRS in combination with a site of infection, 13 foals had a sepsis score >11 in combination with positive blood culture, 6 foals a necropsy finding indicating sepsis and 4 foals with sepsis score >11 in combination with necropsy finding indicating sepsis. The septic foals were further divided into the sepsis severity subgroups of normal sepsis (60.4%, n = 89), severe sepsis (13.9%, n = 33) and septic shock (25.7%, n = 34). The 4 healthy foals had low SAA concentrations (58.2 ± 91.9 mg/L; range, 0-193.5 mg/L). They were excluded from further analysis because of the low number.
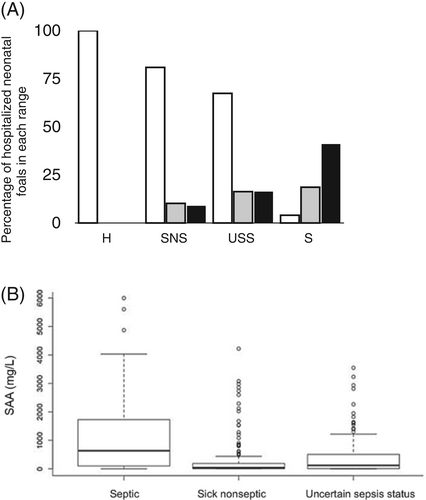
Through nonstatistical observation of the data, the proportion of foals with a low SAA (<300 mg/L) was found to be larger (80.9%) in the sick nonseptic group compared to the uncertain sepsis status group (67.4%), and larger in the uncertain sepsis status group compared to the septic group (40%; Figure 1A). Concentrations of SAA were statistically different between groups (P < .001) with median concentrations being 52 mg/L (0-4220 mg/L) in sick nonseptic foals, 123 mg/L (0-3232 mg/L) in foals with an uncertain sepsis status and 718 mg/L (0-5604 mg/L) in septic foals (Figure 1B).
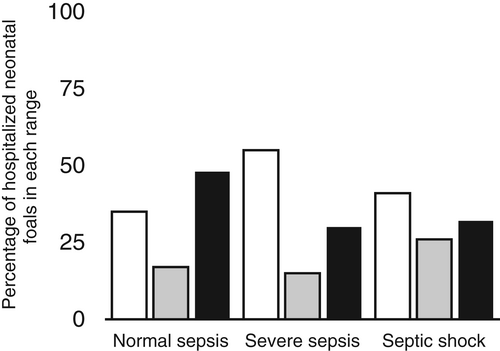
Through non-statistical observation of the data, a larger proportion of foals with severe sepsis and septic shock was found to have low SAA concentrations (< 300 mg/L) (54.5% and 41.2% respectively) than foals with normal sepsis (34.8%; Figure 2). Concentrations of SAA in the 3 sepsis severity groups (normal sepsis, severe sepsis, septic shock) were not significantly different (P = .5) with median concentrations being 887 mg/L (0-5604) in sepsis foals, 577 mg/L (0-2869 mg/L) in severe sepsis and 566 (0-2632) in septic shock.
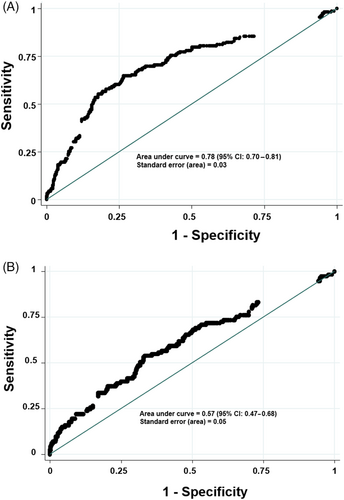
The optimal cut off value for SAA to predict sepsis was 1050 mg/L (sensitivity 30.2%, specificity 90.7%; Figure 3A). Following this cut off value, 273 out of 374 foals were correctly classified according to their sepsis status, but 110 out of 157 foals that were classified as septic had SAA concentrations lower than 1050 mg/L.
3.3.2 Outcome
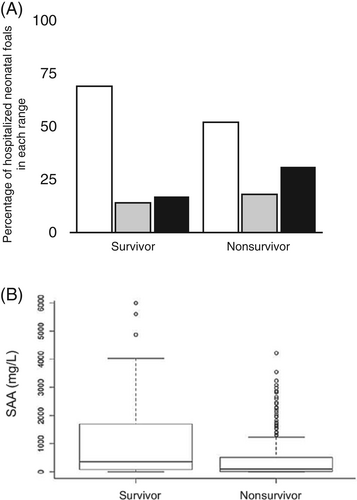
The outcome was recorded for 586 foals: 477 were survivors and 109 nonsurvivors. Out of the 109 nonsurvivors, 82 foals were euthanized, 17 foals died, and 10 foals were euthanized because of financial restrictions. Through nonstatistical observation of the data, the survival group had more foals with low SAA (<300 mg/L) than the nonsurvivor group (69% and 52%, respectively), and the nonsurvivor group had more foals in the high SAA range than the survivor group (31% and 17%, respectively; Figure 4A). Statistical analysis showed a significant difference between survivors and nonsurvivors (P < .001) with median SAA concentrations being 191 mg/L (0-5604 mg/L) in nonsurvivors and 93 mg/L (0-4220 mg/L) in survivors (Figure 4B).
The optimal cut off value for SAA to predict nonsurvival was 1250 mg/L (sensitivity 22.2%, specificity 98%; classifying 455 out of 586 foals correctly; Figure 3A).
4 DISCUSSION
This study evaluates SAA concentrations and its diagnostic and prognostic use in a large Scandinavian group of hospitalized neonatal foals. Concentrations of SAA on admission were significantly higher in septic foals than in nonseptic foals, and SAA concentrations increased with the sepsis score. Concentrations of SAA were inversely related to a survival prediction score and differed between survivors and nonsurvivors. Diagnostic and prognostic sensitivities of SAA were low, while specificity was high.
Clinical signs of sepsis might be subtle and nonspecific, making it difficult to differentiate foals with sepsis from foals suffering from noninfectious diseases such as neonatal maladjustment syndrome. On 1 hand, this might lead to clinicians overtreating and starting empirical antimicrobial treatment in all foals with disease—an undesirable practice, that exposes the foal to unnecessary adverse drug effects and nosocomial complications, and that might lead to emergence of resistant bacterial strains. On the other hand, there are fewer negative consequences of unnecessarily treating an uninfected foal than not instigating treatment early in an infected one. Therefore for early diagnosis of sepsis, high sensitivity of a biomarker is more important than high specificity, which was unfortunately not what was found in the current study. Several technical (eg, detection limit of the assay used) or study-related factors (a-posteriori selected cutoff points, patient characteristics, inclusion criteria, sepsis definitions, etc.) might influence the sensitivity of a marker, but also its response characteristics are of great importance. Concentrations of SAA take 6-12 hours to change significantly in response to inflammatory stimuli and might not peak until 48 hours,28 which can result in low sensitivity in early phases of sepsis. The current study only included SAA concentrations measured on admission, and even though the time span between onset of disease and admission was unknown, many foals could have been in an early phase of disease. The low sensitivity found in our study for sepsis diagnosis (30.2%) was lower than the sensitivity found in a recent study about foals with sepsis (52.9%).25 Furthermore that study suggested a much lower cut off value as indicator for sepsis (100 mg/L).25 These differences in sensitivity and cut off values could be related to a different sample (the other study included foals from a breeding farm and a referral hospital; the current study only included foals from referral hospitals) with a different percentage of septic foals (40% in the current study vs 9% in the other study), different age groups (up to 14 days old in the current study vs less than 36 hours old in the other study), and possible different SAA assays and dilution techniques.25 These low sensitivities in foals stand in contrast to SAA's high sensitivity as marker of human neonatal sepsis.19-21 There was poor sensitivity in early stages of infection in human studies that used C-reactive protein (CRP, another important acute phase protein) for diagnosing sepsis in infants.29 Similar to CRP in sepsis in infants,29 SAA in foals had a high specificity. A high specificity makes a biomarker useful to rule out disease; in this case, SAA can be used to rule out sepsis. Furthermore, combining SAA measurements with other biomarkers that have a fast response could result in a biomarker panel with both high sensitivity and high specificity. Interesting fast reaction markers include leukocytes, cytokines, and procalcitonin, with the latter being widely used in diagnosis of sepsis in humans. Procalcitonin in septic foals has a high sensitivity and specificity,30 but more knowledge and relevant assay systems are needed before it can be used routinely in equine medicine. Furthermore, repeated assessment of SAA concentrations could result in improved sensitivity. In sepsis in infants, repeated assessment of CRP within the first 24 to 48 hours of hospitalization improved sensitivity markedly.31 Another way to increase SAA's sensitivity is to apply lower concentration cutoff points. Foals will then most likely be identified earlier during the inflammatory response, but this will be at the expense of its specificity. While a low clinical cutoff is appropriate in adult horses, the higher basal SAA in neonatal foals must be taken into consideration. SAA concentrations in foals and newborn donkeys undergo a physiological rise for at least 72 hours after birth.15, 32 These physiologic dynamics, as well as certain maternal and perinatal factors, might affect interpretation of what constitutes a “normal” SAA value in healthy neonates. Adopting a too low cutoff point could lead to misclassification of healthy foals as septic (or as nonsurvivor). Further assessments of SAA reference levels in neonatal and young foals are required to determine a clinically relevant cutoff.
In cattle and humans, SAA concentration has been suggested to reflect extent/severity of underlying inflammation.28, 33 Concentrations of SAA were not able to distinguish the sepsis severity groups, and many foals classified with severe sepsis (54.5%) or septic shock (41.2%) had low serum SAA concentration (<300 mg/L). This can be explained by the sometimes peracute nature of severe sepsis and septic shock, where disease progresses so fast and overwhelmingly that the foal deteriorates before significant hepatic SAA synthesis takes place. Additional explanations for low blood concentrations of SAA on admission with increasing severity of sepsis could be related to organ failure and decreased organ perfusion that are both typically seen in severe sepsis and septic shock and could potentially affect hepatic synthesis and release of SAA in the bloodstream.3
Using biomarkers to predict prognosis is highly desirable for several reasons, not least from an animal welfare point-of-view (sparing the animal the ongoing suffering when prognosis is hopeless) and for financial reasons (avoiding costly treatments for foals with grave prognosis). Our results suggest that SAA can be included in decision-making, as concentrations differed between survivors and nonsurvivors and were inversely associated with a survival score (lower SAA concentrations with higher prediction of survival). Its high specificity indicates that it can be used to rule out nonsurvival, and the cutoff point for SAA as a predictor for nonsurvival was 1250 mg/L, which lead to correct classification of 77.6% of foals. However, its low sensitivity, its moderate concentration difference between survivors (median 93 mg/L) and nonsurvivors (median 191 mg/L) and the overlap between outcome categories highlight the fact that assessment of SAA at the time of admission cannot be used as a stand-alone prognostic marker. These prognostic limitations of SAA in foals can partially be explained by the fact that noninfectious conditions (without stimulation of SAA production) can have major effects on owner decisions on outcome, for example musculoskeletal abnormalities leading to poor sport prognosis. In addition, as opposed to SIRS, some severely sick foals might be suffering from compensatory anti-inflammatory response syndrome (CARS) with lower SAA concentrations.
A major limitation of this study was the lack of a reliable gold standard for classifying foals as truly septic to compare SAA against. It is well documented that both sepsis scores7-9, 34 and blood culture results15 suffer from poor sensitivity. These low sensitivities highlight the difficulty of diagnosing sepsis in neonatal foals, and misclassification of foals might thus have affected the assessment of diagnostic accuracy of SAA in our study. Furthermore, to make the sepsis diagnosis as solid as possible for this study, multiple diagnostic criteria were included, among which the sepsis score. Since the sepsis score played a critical role for case definition in this study, it was not possible to evaluate the sepsis score combined with SAA at the same time as a diagnostic test. Nonetheless, it would be interesting to test new sepsis scores that include SAA, as has been suggested in earlier publications.17, 23 Another limitation was the retrospective nature of the study. Retrospective studies have inherent drawbacks, for example, missing values and changes in management over time. However, a large portion of variables and information needed for the study was available from the files, with relatively few missing data. Using the same assay and laboratory for measurements of SAA concentrations would have strengthened the study, as SAA concentrations can differ between different assays and analysis approaches.28 To obtain the largest possible study group, the study was conducted as a multicenter study, and the authors feel that the drawbacks (differences in patient management and SAA analyses) were outweighed by the advantages (larger patient number, conclusions more generalizable).
In conclusion, concentrations of SAA increased with increasing sepsis score and differed between septic and nonseptic foals, and survivors and nonsurvivors. With its low sensitivity and high specificity, SAA was most useful for ruling out sepsis at the determined cutoff of 1050 mg/L and ruling out nonsurvival at the determined cutoff of 1250 mg/L. When combined with other available diagnostic and prognostication tools for neonatal foals and keeping the kinetics of the SAA response in mind, SAA might be useful to assess this patient group.
In future studies, diagnostic accuracy might be improved by combining SAA with infection markers that increase early in the course of infection, or as an integral part of the sepsis score. Studies assessing use of repeated measurements to increase sensitivity are also warranted, as are studies directed at elucidating dynamic and age-dependent reference values.
ACKNOWLEDGMENT
No funding was received for this study.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
The use of antimicrobials on these foals was not investigated or recorded for the purpose of this study, and therefore we cannot make any statement regarding off-label usage.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




