Multicenter retrospective evaluation of transmural migration of subcutaneous ureteral bypass devices within the digestive tract in cats
Abstract
Background
Placement of a subcutaneous ureteral bypass (SUB) device is an effective method to relieve all causes of ureteral obstruction in cats. Complications involving migration within the gastrointestinal tract have been seldomly described.
Objectives
To characterize transmural migration of SUB devices within the digestive tract in cats.
Animals
Eleven migrated SUB catheters identified in 8 cats between 2017 and 2021.
Methods
Retrospective review of medical records of cats with a SUB device in which migration into the gastrointestinal tract was identified.
Results
The median time from SUB device placement to implant migration was 928 days (201-2298 days). Seven cats had obstruction of the SUB device and a positive urine culture at diagnosis. The migration was identified by ultrasound in 6/11, pre-operative contrast radiography in 2/2, and only at time of surgery in 3 SUB devices. All cats underwent surgical correction. Four nephrostomy and 7 cystotomy catheters migrated. Migration occurred into the duodenum (3/11), jejunum (7/11), and colon (1/11). SUB devices were removed in 7 cats and replaced in 2 cats, with 1 cat diagnosed with 2 migration events. Gastrointestinal resection and anastomosis were performed in 7/8 cats and an enterotomy in 2 cats. Six cats survived to discharge. The median follow-up time after migration diagnosis was 365 days (range, 0-1114 days) and 2 cats are still alive.
Conclusions and Clinical Importance
Although a rare complication, migration of SUB device should be considered in cats with SUB device obstruction and a positive urine culture.
Abbreviation
-
- SUB
-
- subcutaneous ureteral bypass
1 INTRODUCTION
Ureteral obstruction is an increasingly recognized and potentially life-threatening condition in cats.1, 2 Ureterolithiasis is most frequently identified as the cause of obstruction. Other causes include ureteral stricture, infection, dried solidified blood stones, iatrogenic ureteral ligature, neoplasia and as a complication after renal transplant.1
Medical management of ureterolithiasis is successful in a minority of cats, reaching 13% in 1 study.2 Surgical options include ureterotomy, ureteral resection and anastomosis, neoureterocystotomy, and ureteronephrectomy and ureteral stent placement.2-5
The subcutaneous ureteral bypass (SUB) device has been successfully used in cats to relieve ureteral obstruction, regardless of the underlying cause.4, 6-12 SUB device placement is considered a safe and effective option in cats with ureteral obstruction.4, 5, 13, 14 Long-term complications include occlusion of the device (blood clot, mineralization), kinking of the catheters, chronic urinary tract infection, and intermittent dysuria.4, 5, 13-15
Transmural migration of foreign bodies is well documented in humans and most commonly occurs as a result of a forgotten surgical sponge or as a complication after surgical implants such as a hernia mesh.16, 17 The transmural migration of abdominally retained foreign bodies into the gastrointestinal tract is seldomly reported in dogs and cats.18-20 Migration of the dacron cuff of the nephrostomy catheter in 2 cats with a SUB device has been reported.20
Interest arose for this study after postings to the Veterinary Interventional Radiology and Interventional Endoscopy Society (VIRIES) list serve of cats presenting with displacement, migration, and gastrointestinal complications after SUB placement.20
The aim of this retrospective study was to describe the transmural migration of SUB devices within the digestive tract in cats and its outcome.
2 MATERIALS AND METHODS
To identify cats with transmural migration of SUB devices within the digestive tract, supervising clinicians from reference institutions having reported this complication on the VIRIES list serve were contacted directly and agreed to participate in a multi-institutional retrospective case series. Cases were eligible for inclusion if transmural migration of SUB devices within the digestive tract was confirmed surgically.
Information retrieved from the medical file included: information at the time of identification of migration including: signalment, body weight, clinical signs, time elapsed between the time of SUB device placement and identification of migration, clinical laboratory findings, diagnostic modalities used, and surgical reports; information at the time of SUB device placement including: cause of the ureteral obstruction, side of obstruction, ultrasound report, type of SUB device placed, complications pre, peri and postoperatively; follow-up information including: frequency of rechecks and SUB device flushing, obstruction of the device (kink, luminal), use of a Tetra-EDTA protocol (for flushing/obstruction) based on the Norfolk Vet Products instructions for use manual for T-Flo-Loc (T-FloLoc 2% Tetra-EDTA Flush and Lock Solution, Norfolk Vet Products, Skokie, Illinois) and clinical laboratory findings.
Descriptive data are presented as means, medians, and ranges unless otherwise specified. The incidence of SUB device migration in the population of cats with a SUB device was calculated as the number of cats included in the study divided by the number of all cats with a SUB device placed in participating institutions up to February 2021. The incidence of SUB device migration was calculated as the number of devices migrated divided by the number of all devices placed in participating institutions up to February 2021.
3 RESULTS
3.1 SUB device migration; signalment and reason for presentation
A total of 8 cats were identified from 6 Universities and 1 private referral center between April 2017 and February 2021 in which the SUB device migrated transmurally into the gastrointestinal tract. Cases included 7 spayed females and 1 neutered male. There were 5 domestic shorthairs and 1 of each: Siamese, Ragdoll, and Sphinx. The median age was 8.5 years (mean: 10 years, range, 5.5-19.5). The median weight was 3.68 kg (mean: 3.67, range, 2.87-5.18). The median time from SUB placement to implant migration was 928 days (mean: 1092 days, range, 201-2298).
The most common presenting clinical signs were decreased appetite (n = 6), followed by vomiting and weight loss (n = 3), then pollakiuria, lethargy, foul-smelling urine (n = 2). Other clinical signs included hematuria and adipsia (1 of each). Clinical signs were noted in 8 cats for a median of 13 days (mean: 38 days, range, 1-180 days) before presentation.
Complete blood count and serum biochemistries were evaluated in all cats. Normocytic normochromic non-regenerative anemia was present in 4/8 cats with a median hematocrit of 21.6% (mean: 21.4%, range, 17-25.2). Neutrophilic leukocytosis was identified in 6/8 cats with a median leukocyte count of 30.164 × 10E9/L (mean: 25.14, range, 20.76-52.9) and a median neutrophil count of 22.44 × 10E9/L (mean: 26.88, range, 15.99-49.73), with mature neutrophilia. The median serum creatinine concentration was 229 μmol/L (mean: 225; range, 88-440; median: 2.6 mg/dL; mean: 2.56 mg/dL; range, 1-5).
3.2 SUB device placement
Of the 8 cats, 3 had a unilateral SUB (1 left, 2 right) and 5 had bilateral individual SUB devices placed. The median age at the time of presentation for ureteral obstruction and SUB device placement was 5.5 years (mean: 7.4 years, range, 2.11-13.2).
The original cause of ureteral obstruction was ureterolithiasis (n = 3), circumcaval ureter (n = 2), a suspected stricture (n = 1), suspected kidney rupture/infection (n = 1), and remained unknown in 1 cat (n = 1).
A complete abdominal ultrasound was performed on all cats before SUB device placement. One cat was reported to have a mildly thickened small intestinal submucosa compatible with a mild enteropathy. Abdominal ultrasound did not identify any digestive abnormalities in any of the other cats. No cat had a history of chronic gastrointestinal signs and none underwent gastrointestinal endoscopy nor intestinal biopsy at the time of SUB device placement.
SUB device placement was performed as previously described.4 In all cases, nephrostomy catheters were glued (cyanoacrylate; Vetbond tissue adhesive, 3M London, Ontario, Canada) to the caudal pole of the kidney and cystotomy catheters were glued and sutured in place using 3 interrupted sutures in 4 cats and 4 uninterrupted sutures in 4 cats. The following suture material was used: monocryl 3-0 (n = 4), PDS 4-0 (n = 2), PDS 3-0 (n = 1), and prolene 3-0 (n = 1). The SUB devices (SUB device, Norfolk Vet Products, Skokie, Illinois, USA) placed in the 8 cats were: 1.0 small port (n = 4), 1.0 large port (n = 1), and SUB 2.0 Swirl Port (n = 3). The cats with bilateral SUB devices had the same version of SUB device placed on each side.
No intra-operative nor anesthetic complications were reported. Four cats had a revision of their SUB device a median of 56 days after placement (mean: 57.3, range, 5-112). The reason for SUB device revision included obstruction by a blood clot (n = 3) and rotation of the SUB port secondary because of breakdown of the tacking sutures (n = 1).
3.3 Clinical characteristics before SUB device migration
Before detection of SUB device migration, 7 cats had a positive urine culture, and 6/7 had clinical signs compatible with a urinary tract infection (UTI). The median number of positive urine cultures obtained between SUB device placement and migration was 3.6 per cat (mean: 3; range, 2-9). Escherichia coli was cultured in 6 cats and Enterococcus spp. in 5 cats. Corynebacterium spp., Actinomyces spp., and Pasteurella multocida were identified in 2 cats each. Candida albicans and Enterobacter cloacae were cultured in 1 cat each (Table 1).
| Bacteria/yeast | Number of cats |
|---|---|
| Escherichia coli | 2 |
| Escherichia coli and Enterococcus faecalis | 1 |
| Pasteurella multocida and Corynebacterium spp. | 1 |
| Escherichia coli, Enterococcus spp., and Candida albicans | 1 |
| Enterococcus spp., Corynebacterium spp., and Actinomyces spp. | 1 |
| Escherichia coli, Enterococcus faecalis, Enterococcus faecium, and Enterobacter cloacae | 1 |
| Escherichia coli, Enterococcus faecium, Enterococcus faecalis, Pasteurella multocida, and Actinomyces spp. | 1 |
Seven cats received tetrasodium ethylenediaminetetraacetic acid (tEDTA) flushes, either tEDTA 4% (n = 2) or tEDTA 2% (n = 5), when infection (n = 6) or obstruction (n = 1) was diagnosed. The median tEDTA quantity infused was 3.55 mL (mean: 3.62, range, 1-35 mL).
Obstruction of the device was reported in 2 cats after SUB device placement and before the diagnosis of migration, with 1 obstructing twice. The obstructions occurred a median of 205 days before the diagnosis of migration (mean: 326 days, range, 128-646 days). No anomalies were reported during the previous flushes of the SUB device for the other cats.
When migration was diagnosed, complete obstruction of the SUB device was suspected in 7/8 cats based on ultrasound-guided flushing.
3.4 SUB migration
Nine migration events were diagnosed in 8 cats. One cat had 2 migration events diagnosed 59 days apart. Throughout these 9 migration events, 11 migrated SUB catheters were identified. The cat with 2 migration events had migration of both the right nephrostomy catheter (into the descending duodenum) and right cystotomy catheter (into the transverse colon) during the first event, and the left cystotomy catheter into the jejunum during the second event. Another cat had both the cystotomy and nephrostomy catheters migrate at the same time into the jejunum.
Abdominal ultrasound was performed in all cats and confirmed intestinal migration in 6/9 migration events. In 6 events, ultrasound identified with certainty the SUB catheter inside the intestinal lumen (Figure 1). Two cats underwent a focused urinary tract ultrasound and migration was missed. Catheters were incorrectly identified in the correct position on ultrasound evaluation in 2/9 migration events (Figure 2). Ultrasound missed catheter migration in 3 migration events.
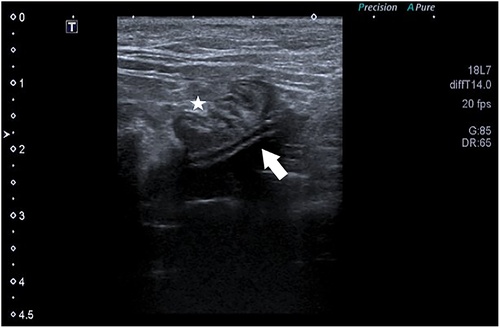
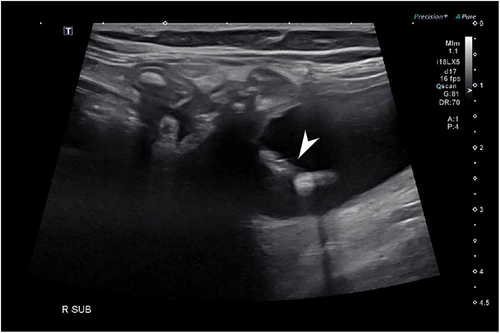
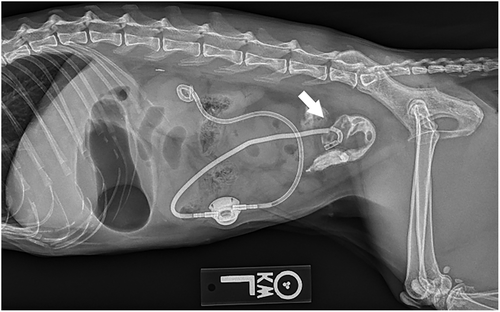
Radiographs were performed in 5/9 migration events. Radiographs confirmed catheter migration in 4/9 migration events. In all cases, migration or avulsion of the catheter was mentioned in the radiology report (Figure 3). Radiographs assisted in the diagnosis of other anomalies. A concomitant fracture/kink of the migrated cystostomy catheter was diagnosed in 1 cat (Figure 4). Another cat had a cystotomy catheter that was disconnected from the port. SUB device migration was missed in 1 event on radiographs (Figure 5), in this cat migration was confirmed on ultrasound.
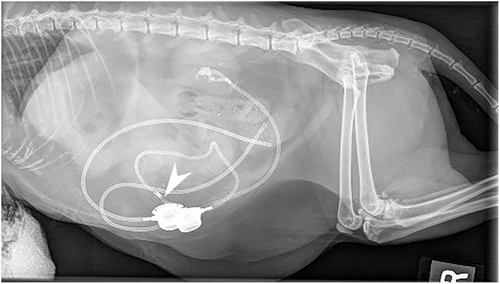
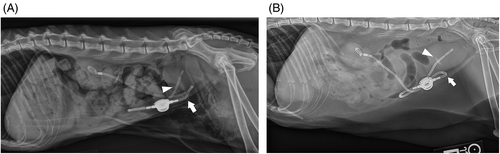
Contrast radiographs were performed in 2/9 migration events. They confirmed intestinal migration in the 2/2 events. In both cats, contrast radiographs showed contrast filling segments of intestine instead of the urinary bladder (Figure 4).
Migration was identified at time of laparotomy alone in 3/9 migration events.
3.5 SUB device migration: surgery
All animals underwent an exploratory ventral midline celiotomy.
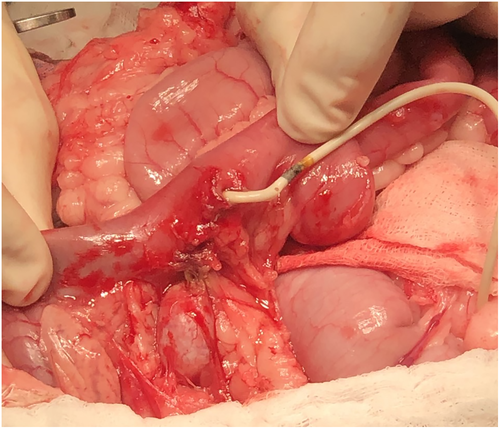
Four nephrostomy and 7 cystotomy catheters were confirmed to have migrated into the GI tract in 8 cats: 3 catheters entered the duodenum (Figure 6), 7 cats into the jejunum and 1 cat into the colon (Table 2).
| Duodenum | Jejunum | Colon | Total of catheters | |
|---|---|---|---|---|
| Nephrostomy catheter | 2 | 2 | 0 | 4 |
| Cystotomy catheter | 1 | 5 | 1 | 7 |
| Total of catheters | 3 | 7 | 1 | 11 |
For the 5 cats with bilateral SUB devices, the migrated catheters were as follows: right nephrostomy (3 catheters), right cystotomy, and left cystotomy (2 catheters each). For the 3 cats with unilateral SUB devices, the migrated catheters were: right cystotomy (2 catheters), right nephrostomy and left cystotomy (1 catheter each).
Surgical procedures performed were as follows: intestinal resection/anastomosis (7/8 cats), enterotomy (2/8 cats), migrated SUB device removal (7/8 cats, in 1 cat the contralateral non-migrated SUB was also removed), SUB device replacement (2/8 cats), partial cystectomy, nephrectomy, and jejunal and ileal biopsies (1/8 cat each). The intestinal sections resected were: duodenal-jejunal (n = 1), duodenal (n = 2), and jejunal (n = 4). There was no evidence of peritonitis at the time of surgery in any of the cats.
In 1 cat, the right SUB was removed, however, 7 days after surgery, the right renal pelvis remained dilated (1.2 cm). A right SUB device was placed and the left SUB device was exchanged, as the cat presented chronic bacterial UTIs and biofilm formation on the implant was suspected.
Histopathology of the renal and duodenal tissue resected during the first episode of the cat with 2 migrations revealed severe pyelonephritis, ureteritis, and transmural small intestinal foreign material with associated fibrosis and enteritis. The right nephrostomy catheter was completely encased within the lumen of the duodenum. The jejunal and ileal biopsies performed on the other cat revealed mild lymphoplasmacytic and eosinophilic mucosal infiltration of the jejunum with increased connective tissue in the lamina propria and mild lymphoplasmacytic mucosal infiltration with increased lamina propria connective tissue; possible thickening, tunica muscularis of the ileum.
3.6 Outcome
Of the 8 cats, 6 survived to discharge. Two cats vomited postoperatively, 1 had a cardiac arrest and the other suffered aspiration pneumonia and was euthanized.
One cat developed chronic UTI with Escherichia coli identified. This cat had 2 migration episodes. During the first episode, the cat had a right SUB device removed and a right nephrectomy. During the second episode, the left SUB device was replaced. The cat was presented for straining in the litter box and nausea 96 days after the migration surgery (creatinine: 792 μmol/L; 9 mg/dL). The cat was hospitalized for 3 days for administration of imipenem (IV) and tEDTA flushes because of suspected luminal mineralization and obstruction of the SUB device. The cat was later treated with monthly then every 6-week tEDTA infusions. Serum creatinine concentration progressively increased (1232 μmol/L; 14 mg/dL) and the cat was euthanized because of perceived decreased quality of life, 1 year after surgery to correct migration.
In 1 cat, during migration surgery, bilateral cystotomy tubes were replaced as well as a left SUB port. On follow-up, 2 weeks after surgery, Enterococcus faecium was cultured. Linezolid was initiated. Infection persisted despite antibiotics and SUB flushes with 4% tEDTA. Fifty days after the migration surgery, the cat developed pancytopenia suspected secondary to administration of linezolid and serum creatinine concentration increased to 880 μmol/L (10 mg/dL). Linezolid was discontinued and hematological abnormalities resolved, however azotemia persisted. The SUB device was removed after ureteral patency was confirmed by fluoroscopy 54 days after the migration surgery and continued to remain patent, until euthanasia 114 days later. Vancomycin was initiated 80 days after the migration surgery to treat Enterococcus faecium persistent infection. This cat was also euthanized because of perceived decreased quality of life, 168 days after surgery to correct migration.
One cat died 1113 days after corrective surgery. A resistant Escherichia coli was cultured from the urine 484 days after surgery for migration, and meropenem was administered. The cat was also receiving fluconazole as Candida albicans was cultured at the time of diagnosis of migration. The cat reobstructed its right SUB 1108 days after migration surgery. Surgery was performed to relieve the obstruction however the cat died of cardiac arrest 5 days later. At the time, the cat's serum creatinine concentration was 884 μmol/L (10 mg/dL) and urine culture was negative.
One cat was euthanized 568 days after surgery to correct catheter migration. The cat developed ureteral obstruction on the contralateral side and despite undergoing placement of a SUB device, serum creatinine concentration did not improve and the cat was euthanized 3 days after surgery.
Two cats were alive at the time of writing, 26 and 389 days after surgery to correct the migrated catheters. One cat had a negative urine culture 7 months after surgery with a stable serum creatinine concentration of 194 μmol/L (2.2 mg/dL). The other cat had a negative urine culture 16 days after the SUB migration surgery and a serum creatinine concentration of 114 μmol/L (1.3 mg/dL).
The median follow-up time from migration to the end of the study period was 365 days (mean: 403, range, 0-1114).
3.7 Incidence
A total of 838 SUB devices were placed in a total of 610 cats in all institutions participating in the study. SUB catheter migration occurred in 1.31% of cats and 1.07% of SUB devices.
4 DISCUSSION
This retrospective study documents 8 cats that experienced SUB catheter migration within the digestive tract. Complications reported with placement of SUB devices include luminal obstruction (5.3%-33.3%), UTI (8%-30.8%), intermittent dysuria (12.5%-38.5%), device leakage (3.5%), and kinking of the catheter(s; 4%-12.5%).4, 5, 13, 14, 21 Mineralization of the device is the most common long-term complication, recorded in 25% of cases in 1 study, with luminal occlusion of the device in about 12%.4 Occlusion with blood clots were more common as a postsurgical complication (8% of cases).4 Extrusion of a subcutaneous access port suspected secondary to chronic infection in a cat has also been reported.15 One cat with an enterovesicular fistula at the site of the cystotomy catheter was previously mentioned in Kulendra et al.'s retrospective study.14 The cat had a blocked nephrostomy catheter 18 months after the original surgery and developed dysuria. Enterococcus faecalis and Escherichia coli were cultured from the SUB device. The fistula was not identified on positive contrast radiography before the revision surgery. The SUB device was removed 23 months after the original surgery and a resection and anastomosis with a partial cystectomy was performed; the SUB device was not replaced and the infection did not reoccur. Recently, intestinal perforation of the nephrostomy catheter and dacron cuff into the duodenum without catheter migration was reported in 2 cats 14 and 36 months after SUB device placement.20 There is no mention of whether the cats had obstruction or infection at the time of diagnosis. The first cat was alive 1 month after resection-anastomosis surgery and SUB removal, the second was euthanized intraoperatively. Although rare, intestinal perforation with or without migration is a potential long-term complication that should be considered. Outcome for the 6 surviving cats in our study was deemed fair with a median follow-up time of 365 days.
Positive urine cultures can occur in cats after SUB device placement. The incidence of postoperative positive urine culture varies between 20.5% and 31%,5, 13, 14 with 25% of cats having at least 1 positive urine culture during their rechecks.4, 21 Chronic bacteriuria has been documented in 13% of cats, with 8% having signs suggestive of a UTI.4 In another study, clinical signs were present in 93% of cats with a positive culture.21 Enterococcus spp. was isolated most frequently followed by Escherichia coli and Staphylococcus spp.4, 13 In recent retrospective studies, Escherichia coli was the main bacteria isolated, representing up to 60% of cases,13, 14 followed by Klebsiella pneumoniae in 40% of cases in Vrijsen et al., and by Pseudomonas then Enterococcus in Kulendra et al.13, 14 In our study, 7/8 cats presented with persistent positive urine cultures. Of these 7 cats, 6 had signs compatible with a UTI. Escherichia coli and Enterococcus spp. were most frequently isolated in our cats, followed by Corynebacterium spp., Actinomyces spp., Pasteurella multocida, Enterobacter cloacae, and Candida albicans. Contamination of the SUB device by intestinal contents may explain the mixed bacteria cultured and can alert the clinician to a possible intestinal communication.
On the day of diagnosis, 7/8 cats were found to have a concurrent positive urine culture and obstruction of their SUB device. One cat was not flushed on the day of diagnosis, however Escherichia coli and Enterococcus faecalis were cultured in the urine. To the authors' knowledge, no specific link has been established between infection and SUB device obstruction. There is evidence that preoperative UTI or bacteriuria is not significantly associated with blood clot occlusion or device mineralization.4 The authors recommend that finding an obstructed SUB device with a concurrent positive urine culture should prompt further imaging such as full abdominal ultrasound, abdominal radiographs and/or contrast radiography.
Nonetheless, a seemingly patent catheter should not completely exclude migration. The attending clinicians in this study reported an obstructed SUB device in 8/9 migration events. This was based on lack of an observable ultrasound-guided flush in the kidney or bladder after catheter migration. However, the catheter was not necessarily obstructed as shown in cats undergoing contrast radiographs where flushing resulted in filling of the intestinal lumen (Figure 4).
Ultrasound-guided SUB device flushes are useful to monitor cats for obstructive complications.22, 23 However, ultrasound only identified migration in 6/9 migration events in our study. It is interesting to note that in 2 cases, catheters were seen correctly positioned on ultrasound. This is quite surprising and could be explained by the fact that the catheters visualized were actually nephroliths, mineralization, or debris deposited along the tract of the previous catheter. Given the lack of sensitivity of ultrasound to diagnose migration, the authors recommend combining it with other imaging modalities when migration is suspected. Radiographs were useful in 4 cats to confirm catheter migration and better image the position of the entire SUB device, thus identifying kinks, fractures, and confirming avulsion of catheters. However, radiographs missed migration in 1 cat (Figure 5). On these radiographs, the dacron cuff appeared clearly in the middle of the urinary bladder and not at the apex. Clinicians interpreting radiographs should be familiar with SUB device placement in order to correctly identify anomalies. A catheter visible in the appropriate general region also does not exclude migration. The cat whose migration was missed on radiographs was confirmed on ultrasound, further supporting the need to combine imaging studies to arrive at a diagnosis. Contrast radiography was performed in 2 cats and were diagnostic for migration in both. Contrast fluoroscopy has been shown to be useful in identifying the site of catheter obstruction, leaks and kinks, both static and positional.23 In our study, fluoroscopy was not used to diagnose migration. Contrast fluoroscopy could prove to be useful in cats with migrated SUB devices in which equivocal ultrasound results are obtained. CT with contrast could potentially be helpful in diagnosing migration however requires anesthesia and additional expense compared to a fluoroscopy guided contrast SUB device flush. No cat underwent CT imaging in this study.
Some clinicians have discussed SUB device removal to treat chronic infections if the native ureter is patent. In 1 cat, a SUB device was replaced 7 days after its removal because of persistent ureteral obstruction. Interestingly, the cats for which SUB devices were replaced during the procedure for migration continued to have positive urine cultures. The fact that the exchanged SUB continued to be infected with the same bacteria in most cases, suggests that biofilm may have been present in tissue, thus maintaining the infection. Whether the SUB should be changed or removed is yet unknown. The decision should be made according to the characteristics of the cat. In the case report by Johnston et al. (2021), the SUB devices were removed given the ongoing concerns around SUB systems being infected in both cases with risks of septic peritonitis and pyelonephritis; although no culture were performed.20 However, the authors recommend that ureteral patency be confirmed by an antegrade pyelogram before considering removal of a SUB device, otherwise replacing it should be considered.
No common factor for SUB device migration was clearly identified in our study. One of the hypotheses was that cats with intestinal tract disease will be at risk of developing migration. However, anomalies of the gastrointestinal tract were only seen on ultrasound in 1 cat at the time of initial SUB device placement. This cat was diagnosed with 3 catheter migrations over 2 episodes. Intestinal biopsies were only performed in another cat at the time of migration. Jejunal and ileal biopsies showed changes compatible with mild inflammatory bowel disease. No cat in the study showed chronic signs of gastrointestinal disease. A relationship between migration and concurrent gastrointestinal disease could not be established.
A common feature among these cats was the presence of a positive urine culture. At the time of diagnosis of migration, 7/8 cats presented a positive urine culture and 7/8 had a chronic history of positive urine culture(s), with 6 cats showing signs of lower urinary tract disease. In a previous report, chronic infection was suspected to be the underlying cause of extrusion of a subcutaneous access port in a cat.15 This suspicion was based on the identification of an Escherichia coli with the same resistance profile in the urine and the tissue surrounding the access port. An association between the wound and urine leakage was highly suspected. In our study, infection seems to be more a consequence of the migration than a cause. The absence of peritonitis in the reported cases seems to point to an absence of leakage of urine, digestive contents or both into the abdomen.
All cats in this study underwent surgical correction of intestinal migration. No cat had evidence of peritonitis and 2 cats were treated by enterotomy. Migration might have occurred slowly and progressively resulting in minimal damage to the digestive tract and avoiding overt peritonitis. However, in the other cases, an enterectomy was necessary because of adhesions and digestive tract lesions caused by the presence of the catheter acting as a linear foreign body. In the report by Johnston et al. (2021), 1 of the 2 cats was noted to have a small volume of sero-sanguinous free abdominal fluid.20 A septic peritonitis was suspected, because of leakage of intestinal contents from the perforated duodenum however no cytology nor bacteriology was performed on the fluid.20 In the second cat, a scant volume of blood-tinged peritoneal fluid was seen and localized peritonitis was suspected.20 The duodenum and right nephrostomy catheters were affected in both cats.20 In our study, only 3 cystotomy catheters migrated on the left side, the other migrated catheters were all on the right side (4 nephrostomy and 4 cystotomy). The jejunum, followed by the duodenum, were the most commonly affected intestinal segments in our study. The colon was involved in only 1 cat. The anatomic vicinity of the duodenum and jejunum to the kidneys and urinary bladder may explain involvement of these segments and of the right-sided catheters, with cystotomy catheters mostly migrating to the jejunum and colon and nephrostomy catheters to the duodenum. The greater length and outer surface area of the duodenum and jejunum may also explain why these segments were most affected.18
Gossipyboma or textiloma is a rare but unfortunate surgical complication. Reports of migration of retained surgical material into bowel are scarce in human medicine and even more so in veterinary medicine.16-19 It has been suspected to occur as a result of inflammation of the intestinal wall that evolves to necrosis.16-19 The migration site closes after complete luminal migration of the foreign body, with peristaltic activity making it progress into the gastrointestinal tract until potential obstruction occurs.16-19 A similar mechanism might explain migration in our study. The dacron cuff, the catheter or both in proximity to the intestine could cause inflammation of the intestinal serosal wall resulting in progressive migration and eventually perforation. Peristalsis might pull the dacron and eventually the entire catheter out of the bladder or kidney. This slow incorporation can explain why no peritonitis was documented in these cats. Histopathology of tissue resected during the first surgery of the cat with 2 migrations appears to corroborate this hypothesis. The SUB device catheter was completely encased within the lumen of the small intestine. Given the hemi circumferential fibrosis with mucosal ulceration, it was likely that the catheter slowly embedded itself into the small intestinal lumen through mechanical transmural erosion and subsequently was encased by fibrous connective tissue. At some point during the transmural erosion, the small intestine was perforated. Small intestinal luminal contents may have tracked along the SUB tubing, seeding the renal pelvis. One cat was not included in this study as transmural migration was not present, however we believe it may represent the first steps in foreign body migration. In this cat, the digestive tract was wrapped around the right cystotomy catheter creating adhesions with the urinary bladder and digestive tract. It could represent the first step of migration of a SUB device, before inclusion into digestive tract. The cyanoacrylate glue reacting and adhering to the surrounding organs may also have played a role in migration.20 In Johnston et al., excessive glue during nephrostomy catheter placement was suspected to have acted as an abrasive resulting in an irregular surface on the renal capsule. This may have created areas of friction between tissue surfaces with intestinal peristalsis. Careful tissue glue application and subsequently covering the dacron cuff with omentum or perinephric adipose tissue was recommended. Moreover, the dacron cuff may not be essential in securing the device and eliminating or replacing it may decrease the risk of migration.
Our study has several limitations. Case enrollment relied on complications reported on the VIRIES list serve. Thus, our report might underestimate the number of intestinal migrations reported. The main limitations of our study come from its retrospective nature and the small study population. Complete case follow-up was not always available, and some data was incompletely documented in the medical records. The incidence of SUB device migration we calculated could be underestimated as some cats with a migrated SUB device could have gone unreported, the migration may have been diagnosed at another institution, the cat may have died or been euthanized without a necropsy or lost to follow-up. A prospective multicenter study including standardized long-term follow-ups of cats with SUB devices would allow for more accurate calculation of the incidence of migration.
ACKNOWLEDGMENT
No funding was received for this study. The study was presented in July 2021 at Vetmeet Summer Camp & VIRIES Meeting 2021.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Vancomycin, linezolid and meropenem were used off-label with multiresistant chronic bacteriuria and unrinary tract implants. These antibiotics were chosen based on culture and sensitivity testing.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.




