Comparison of the mitral valve morphologies of Cavalier King Charles Spaniels and dogs of other breeds using 3D transthoracic echocardiography
Abstract
Background
Myxomatous mitral valve disease (MMVD) is more prevalent in Cavalier King Charles Spaniels (CKCSs) compared to dogs of other breeds at a given age. Abnormal valvular stress is thought to contribute to the development and progression of MMVD, and a relationship exists between mitral valve (MV) morphology and stress acting on the valve.
Objectives
To determine whether the MV morphology of healthy adult CKCSs differs from the morphology of healthy adult dogs of other breeds determined by RT-3DTTE.
Animals
Thirty-five healthy CKCSs and 41 healthy dogs of other breeds.
Methods
Prospective cross-sectional study. Dogs underwent physical examination, conventional echocardiography, and RT-3DTTE. RT–3DTTE datasets were analyzed using dedicated software for MV morphologic analysis. Morphologic variables were compared between CKCSs and dogs of other breeds.
Results
The MV of healthy CKCSs had a smaller annulus height (0.46 ± 0.11 vs. 0.56 ± 0.17; P = .0021), tenting height (0.26 ± 0.12 vs. 0.42 ± 0.18; P < .001), tenting area (0.42 ± 0.15 vs. 0.79 ± 0.34; P < .001), normalized tenting volume (0.09 [0.05–0.13] vs. 0.14 [0.10–0.20]; P < .001), and normalized area of the posterior leaflet (0.57 ± 0.15 vs. 0.66 ± 0.18; P = .016) compared to healthy dogs of other breeds; this results in CKCSs having a flatter MV with reduced tenting, compared to the MV of other breeds.
Conclusions and Clinical Importance
These morphologic features could confer a mechanical disadvantage and play a role in the predisposition of CKCSs to the early development of MMVD.
Abbreviations
-
- 2DTTE
-
- two-dimensional transthoracic echocardiography
-
- AHCWR
-
- annulus height to commissural width ratio
-
- AnH
-
- annulus height
-
- BW
-
- body weight
-
- CKCS
-
- Cavalier King Charles Spaniel
-
- MMVD
-
- myxomatous mitral valve disease
-
- MR
-
- mitral regurgitation
-
- MV
-
- mitral valve
-
- nALA
-
- normalized anterior leaflet area
-
- nALL
-
- normalized anterior leaflet length
-
- nALPMD
-
- normalized anterolateral-posteromedial annulus diameter
-
- nAnA
-
- normalized annulus area
-
- nAnCirc
-
- normalized annulus circumference
-
- nAPD
-
- normalized antero-posterior annulus diameter
-
- nCmD
-
- normalized commissural diameter
-
- NPA
-
- nonplanar angle
-
- nPLA
-
- normalized posterior leaflet area
-
- nTnV
-
- normalized tenting volume
-
- PLL
-
- posterior leaflet length
-
- RT-3DTTE
-
- real-time transthoracic three-dimensional echocardiography
-
- SI
-
- annulus sphericity index
-
- TnA
-
- tenting area
-
- TnH
-
- tenting height.
1 INTRODUCTION
Compared to other breeds of dogs, Cavalier King Charles Spaniels (CKCSs) have an earlier onset of myxomatous mitral valve disease (MMVD), and a higher prevalence of the disease at a given age.1-3 Fifty percent of CKCSs are affected by the age of 6-7 years, and almost 100% are affected by the age of 11.2-4 The development and severity of MMVD in CKCSs is heritable.5
The pathology of MMVD has been extensively described, but little is known of the cause or mechanisms that trigger the observed lesions. The mitral valve (MV) annulus is a hyperbolic paraboloid (commonly described as “saddle-shaped”).6, 7 This geometry, consistent across a number of mammalian species,8 confers a mechanical advantage and is important for optimizing leaflet curvature, reducing leaflet stress, and maintaining valve competency.8-11 Differences in MV apparatus morphology and presumably, the mechanical forces acting on the valve have been suggested as a stimulus for signaling pathways that contribute to myxomatous degeneration and its progression.12-14 Furthermore, abnormal stresses on the MV of dogs have been directly linked in vitro to an increased expression of myxomatous effector proteins.15 Therefore, a breed-specific difference in MV apparatus morphology might alter mechanical stresses on valve leaflets that can activate signaling pathways that contribute to myxomatous degeneration and its progression. Data from our laboratory show that the analysis of canine MV using RT–3DTTE is feasible and repeatable, and that healthy dogs have an elliptical, saddle-shaped MV, with parameters defining this shape similar to those described in healthy humans.7, 8, 16 In contrast, dogs affected by MMVD have a more circular and flatter MV,17 therefore lacking some of the aforementioned favorable geometric characteristics. Based on the results of these studies, we analyzed the MV morphology of healthy CKCSs and compared it to the MV morphology of healthy dogs of other breeds. We hypothesized that the MV of CKCSs would have morphologic differences compared to dogs of other breeds.
2 MATERIALS AND METHODS
2.1 Animals
In this prospective cross-sectional study, we enrolled dogs from two institutions: VA-MD College of Veterinary Medicine (VMCVM), and Swedish University of Agricultural Sciences (SLU). After interview of the pet-owner, each dog had a physical examination including comprehensive cardiac auscultation, conventional 2D/color Doppler echocardiography, and acquisition of a RT–3DTTE dataset. To be enrolled in the study, dogs had to be older than 1 year of age, and without a history of cardiac disease. Additional inclusion criteria were tolerance of a complete echocardiographic examination without need of sedation, absence of evidence of MMVD or other cardiac disease at 2DTTE and color Doppler examination, and acquisition of RT–3DTTE datasets free from significant stitching artifacts. Dogs with murmurs graded 1/6 or 2/6 were not excluded provided all other inclusion criteria were met.
2.2 Methods
The RT–3DTTE datasets were acquired using two different ultrasound units (Artida, Toshiba Medical Systems, Tokyo, Japan; IE33, Philips Medical Systems, Andover, MA) equipped with matrix probes (PST-25SX matrix-array transducer, Toshiba Medical Systems, Tokyo, Japan; X2-7, Philips Medical Systems, Andover, MA; X1-3, Philips Medical Systems, Andover, MA) from a left parasternal apical window, as previously described.7, 18 MMVD was identified when color Doppler echocardiography demonstrated a mitral regurgitant jet that was consistently evident in a right parasternal four-chamber view, and/or a left apical four chamber view, and associated with MV thickening and/or prolapse. All RT–3DTTE datasets were imported into a computer workstation equipped with a dedicated software for MV analysis (4D MV-Analysis 2.3, TomTec Imaging Systems, Unterschleissheim, Germany), and analyzed offline by a single operator (GM). Mitral models were created using a semi-automated procedure, and manually reviewed to identify and correct tracing errors, as previously described.7 Morphologic variables were automatically measured and calculated on the MV models, and variables known to be significantly related to body size were normalized using allometric equations reported elsewhere.7 Variables obtained were: normalized antero-posterior annulus diameter (nAPD), normalized anterolateral-posteromedial annulus diameter (nALPMD), normalized commissural diameter (nCmD), annulus sphericity index (SI) calculated as APD/ALPMD, annulus height (AnH), normalized annulus circumference (nAnCirc), normalized annulus area (nAnA), normalized anterior leaflet length (nALL), normalized anterior leaflet area (nALA), posterior leaflet length (PLL), normalized posterior leaflet area (nPLA), nonplanar angle (NPA), tenting height (TnH), tenting area (TnA), normalized tenting volume (nTnV), angle between aortic annulus and anterior leaflet (AAo-AP Angle). The ratio of AnH and CmD (AHCWR) was calculated as an index of nonplanarity of the mitral annulus.8 Detailed figures illustrating each variable are reported as supplemental material elsewhere.7
2.3 Data analysis
Data describing sex, age, body weight (BW), breed, institution of image acquisition, and the values of the MV morphologic variables, were entered into a spreadsheet and analyzed using commercially available software (JMP Pro 11, SAS Institute Inc., Cary, NC, 1989-2013). Dogs were divided in two groups: CKCSs and dogs of other breeds. Cavalier King Charles Spaniels were subdivided based on the institution at which images were acquired—VMCVM (VMCVM-CKCSs), or SLU (SLU-CKCSs). Proportions of males, age, BW, and the MV morphologic variables were compared between CKCSs and dogs of other breeds, and between VMCVM–CKCSs and SLU–CKCSs. Normal distribution of data was assessed by visual inspection of normal quantile plots. Homoscedasticity of data was assessed using a Levene's test. Normally distributed data are presented as mean ± standard deviation, while data that were not normally distributed are presented as median (25th-75th percentile).
Differences in the proportion of males between two groups were assessed using Fisher's exact test. Differences in continuous variables between two groups were assessed using an unpaired Student's t-test when data had a normal distribution, Mann-Whitney U test when data were not normally distributed, and Welch's test when the homoscedasticity assumption was not met. Given the finding of an age difference between CKCSs and dogs of other breeds, a possible relationship between the MV morphologic variables and age was investigated using simple linear regression. A P-value Bonferroni-corrected for multiple comparisons ≤ .05 was considered significant for all tests.
3 RESULTS
3.1 Seventy-six dogs were enrolled in the study
Thirty-five CKCSs and 41 dogs of other breeds, of which the most represented (count ≥ 3) breeds were mixed breed (n = 17), Beagle (n = 4), and Border Collie (n = 3). Fourteen CKCSs and 33 other breed dogs were enrolled at the VMCVM, and twenty-one CKCSs and 8 other breed dogs were enrolled at the SLU. Of the 35 CKCS 28 were female and 7 were male. For the 41 other breed dogs, 28 were female and 16 were males.
3.2 CKCSs versus dogs of other breeds
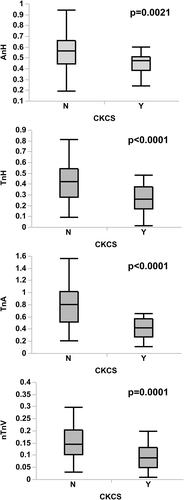
Differences between CKCSs and dogs of other breeds are summarized in Table 1. The median age for all dogs was 3.4 years (2.6-5.8 years), and CKCSs were significantly younger than dogs of other breeds. The median BW for all dogs was 10.1 kg (8.5-19.5 kg), and CKCSs weighed significantly less than dogs of other breeds. The AnH, nPLA, TnH, TnA, and nTnV were all smaller in CKCSs, compared to dogs of other breeds (Figure 1). The AAo-AP Angle was greater (less acute) in CKCS, compared to dogs of other breeds. No difference was found between CKCSs and dogs of other breeds regarding all the other study variables.
| Variable | CKCSs | Other breeds | P-value |
|---|---|---|---|
| Agea | 2.83 (2.00-4.42) | 4.67 (3.04-6.33) | .0042 |
| BWb | 8.44 ± 1.19 | 17.70 ± 5.78 | <.001 |
| nAPD | 1.03 ± 0.12 | 1.05 ± 0.11 | .62 |
| nALPMD | 1.13 ± 0.12 | 1.18 ± 0.13 | .078 |
| nCmD | 1.07 ± 0.11 | 1.10 ± 0.13 | .27 |
| SIa | 0.95 (0.91-0.98) | 0.92 (0.88-0.95) | .073 |
| AnHb | 0.46 ± 0.11 | 0.56 ± 0.17 | .0021 |
| nAnnCirc | 3.63 ± 0.37 | 3.73 ± 0.36 | .26 |
| nAnA | 0.98 ± 0.19 | 1.03 ± 0.19 | .26 |
| nALL | 0.63 ± 0.11 | 0.62 ± 0.10 | .81 |
| nALA | 0.53 ± 0.13 | 0.55 ± 0.13 | .51 |
| PLLb | 1.06 ± 0.36 | 1.05 ± 0.27 | .84 |
| nPLA | 0.57 ± 0.15 | 0.66 ± 0.18 | .016 |
| NPA | 151.27 ± 11.26 | 151.71 ± 14.14 | .88 |
| TnHb | 0.26 ± 0.12 | 0.42 ± 0.18 | <.001 |
| TnAb | 0.42 ± 0.15 | 0.79 ± 0.34 | <.001 |
| nTnVa | 0.09 (0.05-0.13) | 0.14 (0.10-0.20) | <.001 |
| AAo-AP Angle | 133.34 ± 9.94 | 127.72 ± 11.33* | .026 |
| AHCWR | 0.22 ± 0.05 | 0.21 ± 0.05 | .34 |
- a Differences analyzed using the Mann-Whitney U test.
- b Differences analyzed using the Welch's test.
- * AAo-AP Angle from one dog had a value that exceeded the mean by more than 4SD and was therefore excluded.
- Normally distributed data are presented as mean ± SD, not normally distributed data are presented as median (25th-75th percentile). Groups were compared with a student's t-test unless indicated by a superscript letter beside the variable. AAo-AP angle, angle between aortic annulus and anterior leaflet; AHCWR, ratio of AnH and CmD; AnH, annulus height; BW, body weight; nAPD, normalized antero-posterior annulus diameter; nALPMD, normalized anterolateral-posteromedial annulus diameter; nCmD, normalized commissural diameter; nAnCirc, normalized annulus circumference; nAnA, normalized annulus area; nALL, normalized anterior leaflet length; nALA, normalized anterior leaflet area; NPA, nonplanar angle; nTnV, normalized tenting volume (nTnV); PLL, posterior leaflet length; nPLA, normalized posterior leaflet area; SI, annulus sphericity index calculated as APD/ALPMD; TnH, tenting height; TnA, tenting area.
3.3 VMCVM–CKCSs versus SLU–CKCSs
Differences between VMCVM–CKCSs and SLU–CKCSs are summarized in Table 2. The PLL was significantly greater in VMCVM–CKCSs than in SLU–CKCSs. Normalized tenting volume was significantly smaller in SLU–CKCSs compared to VMCVM–CKCSs. No difference was found between VMCVM–CKCSs and SLU–CKCSs regarding all the other study variables.
| Variable | VMCVM–CKCSs | SLU–CKCSs | P-value |
|---|---|---|---|
| Agea | 2.92 (1.27-4.46) | 2.83 (2.13-3.79) | .89 |
| BW | 8.49 ± 1.27 | 8.41 ± 1.17 | .86 |
| nAPD | 1.04 ± 0.14 | 1.03 ± 0.11 | .74 |
| nALPMD | 1.16 ± 0.13 | 1.11 ± 0.11 | .27 |
| nCmD | 1.09 ± 0.12 | 1.05 ± 0.10 | .24 |
| SI | 0.92 ± 0.06 | 0.95 ± 0.06 | .20 |
| AnH | 0.45 ± 0.09 | 0.47 ± 0.12 | .56 |
| nAnnCirc | 3.71 ± 0.40 | 3.58 ± 0.34 | .35 |
| nAnA | 1.02 ± 0.21 | 0.95 ± 0.18 | .34 |
| nALL | 0.66 ± 0.11 | 0.61 ± 0.11 | .21 |
| nALA | 0.56 ± 0.13 | 0.50 ± 0.13 | .19 |
| PLL | 1.43 ± 0.22 | 0.82 ± 0.19 | <.001 |
| nPLA | 0.58 ± 0.18 | 0.55 ± 0.13 | .59 |
| NPA | 152.05 ± 10.72 | 150.76 ± 11.84 | .74 |
| TnH | 0.30 ± 0.11 | 0.23 ± 0.12 | .080 |
| TnA | 0.47 ± 0.14 | 0.38 ± 0.15 | .058 |
| nTnV | 0.11 ± 0.05 | 0.08 ± 0.05 | .043 |
| AAo-AP Angle | 134.38 ± 12.33 | 132.64 ± 8.25 | .62 |
| AHCWR | 0.21 ± 0.04 | 0.23 ± 0.06 | .90 |
- a Differences analyzed using the Mann-Whitney U test.
- Normally distributed data are presented as mean ± SD, not normally distributed data are presented as median (25th-75th percentile). Groups were compared with a student's t-test unless indicated by a superscript letter beside the variable. AAo-AP angle, angle between aortic annulus and anterior leaflet; AHCWR, ratio of AnH and CmD; AnH, annulus height; BW, body weight; nAPD, normalized antero-posterior annulus diameter; nALPMD, normalized anterolateral-posteromedial annulus diameter; nCmD, normalized commissural diameter; SI, annulus sphericity index calculated as APD/ALPMD; nAnCirc, normalized annulus circumference; nAnA, normalized annulus area; nALL, normalized anterior leaflet length; nALA, normalized anterior leaflet area; PLL, posterior leaflet length; nPLA, normalized posterior leaflet area; NPA, nonplanar angle; nTnV, normalized tenting volume (nTnV); TnH, tenting height; TnA, tenting area.
There was no relationship between age and any MV morphologic variable in the entire study population (all P values > .16), nor among CKCSs (all P values > .13) and dogs of other breeds (all P values > .11).
4 DISCUSSION
This study demonstrated that the MV morphology of healthy CKCSs differs from that of healthy dogs of other breeds. Specifically, the MV of CKCSs was flatter than in dogs of other breeds, having a reduced AnH and reduced leaflet tenting, as demonstrated by reduced TnH, TnA, and nTnV (Figure 2), and, relative to dogs of other breeds, the AAo-AP Angle of CKCS was greater. The morphology of the MV has an effect on the stress acting on the leaflets and on the tensioning apparatus,8-11 and therefore an abnormal valvular shape and annular configuration could alter the forces applied on the MV during each cardiac cycle. Although most experimental evidence suggests that annular flattening is the source of increased valvular stress,8-11 it is implied that this increase in stress is mediated through changes in leaflet geometry. Indeed, finite element analysis models demonstrating increased valvular stress associated with annular flattening have tested hypotheses that relate to the degree of flatness of the leaflets, not to flatness of the annulus.8 Therefore, it can be speculated that a primary alteration in the spatial configuration of the leaflets would result in abnormal stresses that are independent of changes in annular configuration.
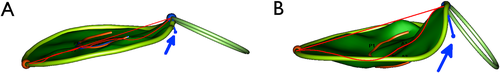
These abnormal stresses on canine MV are postulated to play a role in the pathogenesis and progression of MMVD in dogs, because they have been directly linked in-vitro to an increased expression of myxomatous effector proteins (such as α-smooth muscle actin and matrix metalloproteinases), chondrogenic markers (such as bone morphogenic protein and collagen type 2), and endogenous serotonin synthesis (up-regulation of tryptophan hydroxylase 1).15 In the present study, the differences between the MV morphology of CKCSs and dogs of other breeds did not include 3D indexes of annular nonplanarity, such as AHCWR or NPA, which have been associated with increased leaflet stress.8, 10 However, leaflets’ spatial configuration, is another fundamental characteristic that potentially minimizes valvular peak stress.8, 19 Interestingly, the four variables found to differ between healthy young adult CKCSs and healthy dogs of other breeds (AnH, TnH, TnA, nTnV), are the same four MV morphologic variables that our group found altered in a mixed population of dogs affected by MMVD without cardiac remodeling (ie, ACVIM Stage B1).17 This finding supports the hypothesis that morphologic alterations of the valve could represent factors that predispose to the development of the disease, rather than changes that result from disease status, as has been proposed in humans.20 However, a longitudinal study is needed to test this hypothesis.
The nTnV and PLL were also significantly reduced in the CKCSs enrolled at SLU, when compared to the ones enrolled at VMCVM. This difference could arise from several sources. First, it might be that the lineages of the two study populations were different. However, evaluation of pedigrees was not part of this study, and therefore this is speculation. Also, time of development of MMVD among CKCSs is similar.2, 21 Second, the difference between these two populations of CKCSs could reflect interoperator variability, or differences in equipment used at the two centers.
The differences in age between CKCSs and dogs of other breeds should not have had a significant impact on the results of this study. In fact, simple linear regression analysis failed to identify a significant linear relationship between MV morphologic variables and the age of the dogs, both among the entire population, and in the four subpopulations used for data analysis. Interestingly, this might also suggest that the morphologic variables measured in this and previous studies7, 17 are intrinsic characteristics of the MV and of its apparatus that do not change throughout the life of the dogs, at least in dogs with ages comparable to the ones analyzed in this study. However, given the cross-sectional nature of the study, and the fact that dogs were selected based on disease status and therefore have a comparably narrow distribution in age, this is speculation.
4.1 Limitations
Datasets analyzed in this study were acquired in two different centers, equipped with two different ultrasound units, and by multiple operators. This could have introduced some variability in the data. Therefore, the differences in MV morphologies found between CKCSs examined in the two centers, could be attributed to a center bias. The influence of these factors however, should have been minimized by the nature of the three-dimensional technique, in which the operator has very limited influence on the final dataset. Moreover, one operator using the same software, analyzed all the datasets, which should have further minimized the variability. However, an influence of a parameter intrinsic to the two different acquisition platforms, such as the volume-rate of the acquisition, cannot be excluded. An assessment of the intercenter variation, would require the same dogs to be examined in both institutions, which was not feasible. Also, although the breed of the dog was not clearly identifiable by the operator at the time of dataset analysis, no attempt was made to anonymize the datasets; therefore, the dataset analysis was not conducted in a blind fashion. Furthermore, dog's pedigrees information was not acquired, therefore whether the CKCSs examined belong to different lineages is unknown. Lastly, BW and age differed significantly between CKCSs and dogs of other breeds. Some measurements of MV morphology have been reported to be significantly related to BW7; however, in the current study, the variables known to be significantly related to body size had been standardized using allometric indexes derived from a population of healthy dogs,7 minimizing the effect of body size on the morphologic variables. Furthermore, as mentioned in the previous section, regression analyses failed to identify any effect of age on MV morphologic variables. Therefore, we do not expect the age difference between groups to have affected the morphologic comparisons.
5 CONCLUSION
The MV of young adult CKCSs, as assessed by RT–3DTTE and offline dedicated analysis, was shorter, had reduced leaflet tenting, and had a smaller posterior leaflet, compared to dogs of other breeds. These morphologic differences could represent factors that predispose to early onset of MMVD in this breed, but a longitudinal investigation is required to confirm this hypothesis.
ACKNOWLEDGMENTS
The study was performed at the Department of Small Animal Clinical Sciences, Virginia-Maryland College of Veterinary Medicine, Blacksburg, VA 24061, USA, and at the Department of Clinical Sciences, Swedish University of Agricultural Science, Uppsala, Sweden. Part of this study was presented as an abstract at the 26th ECVIM-CA annual congress (2016), Goteborg, Sweden.
CONFLICT OF INTERESTS
Authors declare no conflict of interest.
OFF-LABEL ANTIMICROBIAL DECLARATION
Authors declare no off-label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
This study was approved by the IACUC at Virginia Tech, VA, U.S.A., and by the Ethical Committee for Animal Welfare in Uppsala, Sweden.




