Hepatitis D-associated hospitalizations in the United States: 2010–2018
Abstract
In the United States, hepatitis D is not a reportable condition, leading to gaps in epidemiological and clinical knowledge. We aim to estimate the incidence of hepatitis D-associated hospitalizations in the United States and describe the clinical, demographic and geographic characteristics of those hospitalizations. We utilized hospitalization data from the 2010–2018 National Inpatient Sample from the Healthcare Cost and Utilization Project. Hepatitis D and hepatitis B only (HBV only) hospitalizations were identified by International Classification of Diseases, Ninth Revision (ICD-9) and International Classification of Diseases, Tenth Revision (ICD-10) codes. We identified 3825 hepatitis D-associated hospitalizations. The hospitalization rate of hepatitis D was between 6.9 and 20.7 per 10,000,000 but did not change significantly over time. Compared to HBV only, the hepatitis D cohort had a greater proportion of males, Hispanics, hospitalizations in the Northeast region. The hepatitis D-associated hospitalizations also had significantly greater frequencies of liver failure, non-alcoholic cirrhosis, portal hypertension, ascites and thrombocytopenia. While mortality in hepatitis D was similar to that of HBV only, age >65 years (odds ratio [OR] = 3.79; p = .020) and having a diagnosis of alcoholic cirrhosis (OR = 3.37; p = .044) increased the odds of mortality within the hepatitis D cohort. Although the hepatitis D-associated hospitalizations were relatively uncommon, they were associated with severe complications.
Abbreviations
-
- CI
-
- confidence interval
-
- HBV
-
- hepatitis B virus
-
- HCUP
-
- Healthcare Cost and Utilization Project
-
- HCV
-
- hepatitis C virus
-
- HDV
-
- hepatitis D virus
-
- HIV
-
- human immunodeficiency virus
-
- ICD-9
-
- International Classification of Diseases, Ninth Revision
-
- ICD-10
-
- International Classification of Diseases, Tenth Revision
-
- IQR
-
- interquartile range
-
- IRR
-
- incidence rate ratio
-
- OR
-
- odds ratio
1 INTRODUCTION
Hepatitis D is a severe viral disease caused by the hepatitis delta virus (HDV), occurring only in populations with a simultaneous hepatitis B virus (HBV) infection. Globally, there are an estimated 291,992,000 individuals with chronic HBV infection.1 Of those infected with HBV, 4.5%–14.6% are also infected with HDV.2, 3 In the United States, the prevalence of HDV infections among hepatitis B surface antigen (HBsAg)-positive individuals is estimated to be between 5.9% and 7.2%.2, 3 Worldwide, hepatitis D is an important burden of liver disease. Hepatitis D is estimated to be responsible for 18% of cirrhosis and 20% of hepatocellular carcinoma among those with hepatitis B.2
Hepatitis delta virus is considered a satellite virus, an extremely rare class among human viruses, requiring HBV in order to propagate.4 Currently, eight genotypes of HDV have been identified, classified as HDV genotype 1 through 8.5 In the United States, HDV genotype 1 is the predominant genotype; however, the information about the circulation of other genotypes is lacking, and studies conducted in the past two decades have been limited in number and in geographic range.6-8 While studies are few, genotype-specific outcomes for hepatitis D have been demonstrated. For instance, patients infected with HDV genotype 5 have been shown to have better prognosis compared to those infected with HDV genotype 1.9
Infection by HDV results in severe complications, often causing fulminant hepatitis and causing cirrhosis in 70%–80% of cases.10 However, risk for HDV infection is not homogenous among the population infected with HBV. The prevalence of hepatitis D is higher in people who inject drugs and in people who are infected with hepatitis C virus (HCV) or human immunodeficiency virus (HIV).2 However, in pregnant persons and foetuses/neonates, the prevalence of HDV and its burden is not well known, particularly in the United States where hepatitis D is not routinely tested in pregnant persons with HBV and studies on this topic are lacking.11-13 It is known that in pregnant women with HBV there is an increased risk of preterm delivery,14 but knowledge of preterm delivery as well as knowledge of maternal and foetal/neonatal mortality in the setting of hepatitis D is sparse.
In 2020, bulevirtide, a drug for the treatment of HDV infection, was conditionally approved by the European Medicines Agency for use in the European Union.15 With approval for this drug by the U.S. Food and Drug Administration expected to occur in the near future, accurate and up-to-date epidemiological data are needed in order to target high-risk populations for diagnosis and treatment of HDV infection. Thus, we aim to estimate the incidence and describe trends of hepatitis D-associated hospitalizations in the United States as well as describe and analyse the clinical, demographic and geographic characteristics of patients and pregnant persons hospitalized with hepatitis D during the 2010–2015 and 2015–2018 time periods.
2 MATERIALS AND METHODS
2.1 Study population
After receiving approval from the institutional review board of the University of Florida, we utilized data from the National Inpatient Sample, a database on inpatient stays and hospital discharges in the United States from the Healthcare Cost and Utilization Project (HCUP). The National Inpatient Sample is a stratified sample of approximately 20% of hospitals participating in HCUP (2011 and prior) and a stratified sample of approximately 20% of more than 35 million discharges annually from all hospitals participating in HCUP (2012 and later).16, 17 An approximately six-year interval of 1 January 2010 to 1 September 2015 was selected for full analysis due to the consistent use of the International Classification of Diseases, Ninth Revision (ICD-9) diagnosis codes which was retired on 1 September 2015 and replaced with the International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes. The interval between September 1st of 2015 to the end of 2018 (the most recent National Inpatient Sample database available) was also analysed, but the analysis was separated from the 2010–2015 period and was limited to hospitalization rates and trends due to the large difference in ICD diagnosis code versions.
2.2 Data extraction
Data were extracted for analysis of two main groups of interest: hepatitis D and hepatitis B without HDV infection (HBV only). The hepatitis D group was defined by ICD-9 diagnosis codes 070.21, 070.23, 070.31, 070.33, 070.42 and 070.52, and by ICD-10 diagnosis codes B16.0, B16.1, B17.0 and B18.0. The HBV only group was defined by ICD-9 diagnosis codes 070.20, 070.22, 070.30 and 070.32, and by ICD-10 diagnosis code B16.2, B16.9, B18.1, B19.1, B19.10 and B19.11. For each of the three groups, data on in-hospital deaths were obtained.
For the 2010–2015 period, we extracted demographic and geographic data including age, sex, race/ethnicity, region of hospital and type of hospital (rural, urban non-teaching or urban teaching). The following clinical data were obtained: length of hospital stay, deaths, liver failure, acute kidney failure, other organ failure (i.e. heart, lung and brain), hepatic neoplasm, non-alcoholic cirrhosis, alcoholic cirrhosis, biliary cirrhosis, portal hypertension, hepatic encephalopathy, ascites, jaundice, chronic kidney disease, anorexia, haematemesis, thrombocytopenia, coagulopathy, HIV infection, HCV infection, history of injection drug use, diabetes, solid organ transplantation, pregnancy, maternal adverse events and foetal/neonatal adverse events. History of injection drug use was determined indirectly. Hospitalizations with the diagnosis of dependence of drugs commonly injected intravenously including opioids, sedatives, amphetamines, hallucinogens or combinations of these were classified as having a history of injection drug use.
2.3 Statistical analysis
Hospitalization rates were analysed for trends in the 2010 to 2015 period and the 2015 to 2018 period using Poisson regression and reported as incidence rate ratios (IRR) per year. Hospitalization rate calculations incorporated discharge weights provided by HCUP to provide the true number of hospitalizations, compensating for the 20% sampling of the database. Changes in discharge weights prior to 2012 due to database redesign were accounted for in the hospitalization rates. The denominator used in the calculation of hospitalization rates was the total United States population residing within the country on July 1st of the relevant year. These data were extracted from the database of the United States Census. Case-fatality rates were calculated as the percentage of deaths that resulted from all hospitalizations for a given disease. Quantitative data were tested for normality using the Kolmogorov–Smirnov test. The non-normal data were summarized using the median and interquartile range (IQR) and compared using the Mann–Whitney U test. Normally distributed data were analysed with the Student’s t-test. Frequencies were compared using the chi-squared test. Risk factors for mortality among hepatitis D hospitalizations were analysed by logistic regression in both univariate (crude) and multivariate (adjusted) models. The dichotomous outcomes were discharged alive versus death. Results of the logistic regression were reported by odds ratio (OR) with 95% confidence intervals (CIs).
All results reported were weighted, using discharge weights provided by HCUP in order to present the true number of hospitalizations in the United States. Results for a category that contain 10 or fewer hospitalizations but greater than zero hospitalizations were displayed as ≤10 due to the data use privacy policy of HCUP. p-values were calculated from weighted data using actual numbers regardless of whether ≤10 hospitalizations were reported.
All reported p-values were two-tailed p-values. Statistical significance was defined as p < .05. Statistical calculations were performed using STATA® software (StataCorp., 2013. Stata Statistical Software: Release 13; StataCorp LP.) and R program (R Core Team, 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/)18, 19.
3 RESULTS
Out of a total of 324,527,181 estimated hospitalizations in the United States between 2010 and 2018, we identified 3825 hospitalizations with a diagnosis of hepatitis D. In the same period, there were 413,355 hospitalizations with a diagnosis of hepatitis B without hepatitis D (HBV only). In the hepatitis D cohort, the median age was 53 years with an IQR of 45–61 years, and 2011 (66.3%) were male. The race/ethnic distribution was 1308 (43.1%) non-Hispanic White, 693 (22.8%) non-Hispanic Black, 440 (14.5%) Hispanic, 315 (10.4%) Asian or Pacific Islander, 29 (1.0%) Native American and 250 (8.2%) other/unknown. The majority of hepatitis D-associated hospitalizations occurred in the Northeast region (41.4%), and when examining the type of hospital, most (73.2%) occurred in urban teaching hospitals. The median age and distribution of hospital type were similar to the HBV only cohort (p = .554 and p = .094, respectively). However, compared to the HBV only cohort, the hepatitis D cohort had a greater proportion of males, 66.3% versus 61.3% (p = .012). Additionally, the hepatitis D cohort had a significantly different race/ethnicity distribution compared to the HBV only cohort (p < .001), with the greatest difference in the proportion of Hispanics, 14.5% versus 8.4% ; proportions of non-Hispanic Blacks and Asian or Pacific Islanders were significantly lower compared to the HBV only cohort. Furthermore, the regional distribution of hepatitis D-associated hospitalizations was significantly different from HBV only hospitalizations (p < .001), with a greater fraction of hepatitis D-associated hospitalizations in the Northeast region, 41.4% versus 24.9% (Figure S1). These results were summarized in Table 1.
| Characteristics | Hepatitis D | Hepatitis B only | p-value |
|---|---|---|---|
| Number of hospitalizations, N | 3035 | 413355 | |
| Age, years, median (IQR) | 53 (45–61) | 53 (43–62) | 0.554 |
| Sex | 0.012 | ||
| Male, N (%) | 2011 (66.3) | 253358 (61.3) | |
| Female, N (%) | 1024 (33.7) | 159997 (38.7) | |
| Race/Ethnicity | <0.001 | ||
| Non-Hispanic White, N (%) | 1308 (43.1) | 171956 (41.6) | 0.787 |
| Non-Hispanic Black, N (%) | 693 (22.8) | 108899 (26.3) | 0.021 |
| Hispanic, N (%) | 440 (14.5) | 34597 (8.4) | <0.001 |
| Asian or Pacific Islander, N (%) | 315 (10.4) | 55997 (13.5) | 0.012 |
| Native American, N (%) | 29 (1.0) | 2212 (0.5) | 0.169 |
| Other/Unknown, N (%) | 250 (8.2) | 39694 (9.6) | 0.358 |
| Region of hospital | <0.001 | ||
| Northeast, N (%) | 1256 (41.4) | 102797 (24.9) | <0.001 |
| Midwest, N (%) | 360 (11.9) | 65336 (15.8) | 0.007 |
| South, N (%) | 802 (26.4) | 156097 (37.8) | <.001 |
| West, N (%) | 617 (20.3) | 89125 (21.6) | 0.459 |
| Type of hospital | 0.094 | ||
| Rural, N (%) | 105 (3.5) | 21343 (5.2) | 0.058 |
| Urban non-teaching, N (%) | 703 (23.2) | 101047 (24.4) | 0.432 |
| Urban Teaching, N (%) | 2222 (73.2) | 289249 (70.0) | 0.096 |
| Unknown, N (%) | ≤10 | 1716 (0.4) | N/A |
Note
- The time period did not include the full year of 2015 due to the transition from ICD-9 to ICD-10 codes on September 1st, 2015. This table included data from January 1st, 2010 to September 1st, 2015.
- p-values for subcategories of Race/Ethnicity, Region of Hospital, and Type of Hospital were provided in smaller font below the overall p-value for that category.
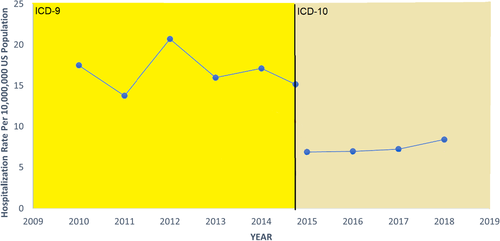
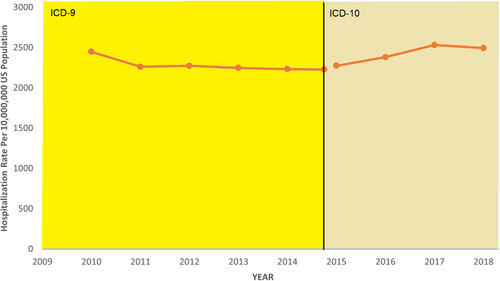
The hospitalization rate of hepatitis D did not change significantly over time during the 2010–2015 period (IRR = 1.01; 95% CI = 0.96–1.06; p = .679) nor in the 2015–2018 period (IRR = 1.09; 95% CI = 0.92–1.29; p = .306). The hospitalization rate of hepatitis D ranged from 13.8 per 10,000,000 to 20.7 per 10,000,000 in the 2010–2015 period and from 6.9 per 10,000,000 to 8.4 per 10,000,000 in the 2015–2018 period (Figure 1). The hospitalization rate of HBV only did not change significantly during the 2010–2015 period (IRR = 1.00; 95% CI = 1.00–1.21; p = .195); however, during the 2015–2018 period, the hospitalization rate increased significantly over time (IRR = 1.04; 95% CI = 1.03–1.05; p < .001). The hospitalization rate of HBV only ranged from 2229.0 per 10,000,000 to 2447.7 per 10,000,000 in the 2010–2015 period and from 2275.5 per 10,000,000 to 2494.7 per 10,000,000 in the 2015–2018 period (Figure 2). Figure S2 displays the change over time in ICD-9 and ICD-10 diagnosis codes used for the diagnosis of hepatitis D. Rates of mortality over time were also examined between 2010 and 2018. Figure S3 illustrates the case-fatality rate of the hepatitis D cohort as compared to the HBV only cohort. Case-fatality rates of the hepatitis D cohort varied greatly year to year but were similar to that of the HBV only cohort overall.
Detailed analysis was conducted on a six-year period (2010–2015), examining the clinical characteristics of the hepatitis D-associated hospitalizations comparison with HBV only hospitalizations. Compared to the HBV only cohort, the hepatitis D cohort had significantly greater frequencies of liver failure (6.5% vs. 4.5%; p = .018), non-alcoholic cirrhosis (20.5% vs. 15.2%; p < .001), portal hypertension (12.9% vs. 6.8%; p < .001), ascites (16.5% vs. 10.8%; p < .001) and thrombocytopenia (22.0% vs. 18.0%; p = .012). Additionally, history of injection drug use was significantly more common in the hepatitis D cohort compared to the HBV only cohort, 9.1% versus 7.0%, respectively (p = .044). However, there were no significant differences between the two cohorts in the age of patients, length of hospital stay and deaths (Table 2).
| Characteristics | Hepatitis D | Hepatitis B only | p-value |
|---|---|---|---|
| Number of hospitalizations, N | 3035 | 413,355 | |
| Age, years, median (IQR) | 53 (45–61) | 53 (43–62) | .554 |
| Length of hospital stay, days, median (IQR) | 4 (2–7) | 4 (2–7) | .585 |
| Deaths, N (%) | 112 (3.7) | 15,161 (3.7) | .995 |
| Hepatic complications | |||
| Liver failure, N (%) | 198 (6.5) | 18,779 (4.5) | .018 |
| Hepatic neoplasm, N (%) | 188 (6.2) | 20,508 (5.0) | .161 |
| Non-alcoholic cirrhosis, N (%) | 622 (20.5) | 62,643 (15.2) | <.001 |
| Biliary cirrhosis, N (%) | 0 (0.0) | 310 (0.1) | .497 |
| Portal hypertension, N (%) | 393 (12.9) | 28,303 (6.8) | <.001 |
| Hepatic encephalopathy, N (%) | 50 (1.6) | 4154 (1.0) | .115 |
| Ascites, N (%) | 500 (16.5) | 44,593 (10.8) | <.001 |
| Jaundice, N (%) | 60 (2.0) | 8139 (2.0) | .985 |
| Extra-hepatic complications | |||
| Acute kidney failure, N (%) | 522 (17.2) | 66,570 (16.1) | .461 |
| Chronic kidney disease, N (%) | 498 (16.4) | 76,855 (18.6) | .163 |
| Anorexia, N (%) | 25 (0.8) | 3518 (0.9) | .928 |
| Haematemesis HBV, N (%) | 30 (1.0) | 3781 (0.9) | .879 |
| Other organ failure, N (%) | 623 (20.5) | 88,800 (21.5) | .568 |
| Haematological complications | |||
| Thrombocytopenia, N (%) | 667 (22.0) | 74,534 (18.0) | .012 |
| Coagulopathy, N (%) | 153 (5.0) | 16,366 (4.0) | .171 |
| Co-infections | |||
| HIV, N (%) | 376 (12.4) | 57,974 (14.0) | .250 |
| HCV, N (%) | 959 (31.6) | 121,542 (29.4) | .235 |
| Risk factors | |||
| H/O of injection drug use, N (%) | 276 (9.1) | 28,892 (7.0) | .044 |
| Diabetes, N (%) | 667 (22.0) | 95,205 (23.0) | .535 |
| Solid organ transplantation, N (%) | 118 (3.9) | 11,122 (2.7) | .068 |
Note
- National Inpatient Sample, Healthcare Cost and Utilization Project (HCUP), (2010–2015).
- p-values were calculated for significant differences using chi-squared test, Fisher’s exact test and Mann–Whitney U test.
- The time period did not include the full year of 2015 due to the transition from ICD-9 to ICD-10 codes on 1 September 2015. This table included data from 1 January 2010 to 1 September 2015. Bold indicates statistically significant p-values p < .05.
- Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; H/O, history of; IQR, interquartile range.
Of the 3035 hepatitis D-associated hospitalizations in the 2010–2015 period, 59 (1.9%) involved pregnancy, and of those 59 pregnancies, 49 (83.1%) resulted in deliveries. The median age was 29 years (IQR: 27–31). The race/ethnic distribution was 15 (25.4%) non-Hispanic White, 20 (33.9%) non-Hispanic Black, 0 (0.0%) Hispanic, 20 (33.9%) Asian or Pacific Islander, 0 (0.0%) Native American and ≤10 other/unknown. The geographic distribution was 15 (25.4%) in the Northeast, ≤10 in the Midwest, 20 (33.9%) in the South and 15 (25.4%) in the West. Thirty-five (59.3%) were hospitalized at an urban teaching hospital, 20 (33.9%) at an urban non-teaching hospital and ≤10 at a rural hospital. Of the 59 pregnant persons, 25 (42.4%) had a history of caesarian delivery. There were no reported maternal deaths, but there were maternal morbidities including abnormal glucose tolerance and coagulation defects, as well as foetal/neonatal morbidities including abnormal heart rate/rhythm, preterm delivery and post-term delivery. The median length of hospital stay for these pregnant persons was 3 days (IQR: 2–3). However, the frequencies of these morbidities and the length of stay were not significantly greater than pregnancies in the HBV only cohort. Of note, the proportion of pregnancies in the hepatitis D cohort was found to be significantly smaller than in the HBV only cohort (p < .001) (Table S1). Upon close examination of each pregnancy hospitalization in the hepatitis D cohort, we found that each hospitalization had unique mothers; there were no cases of rehospitalizations in this subgroup.
During the 2010–2015 period, 112 (3.7%) of the 3,035 hepatitis D-associated hospitalizations resulted in death of the patient. Risk factors for mortality including demographics (age, sex and race/ethnicity), co-infections (HIV and HCV) and co-morbidities (alcoholic cirrhosis and diabetes) were examined. In the multivariate (adjusted) analysis, being over 65 years of age (OR = 3.79; 95% CI = 1.24–11.60; p = .020) and having a diagnosis of alcoholic cirrhosis (OR = 3.37; 95% CI = 1.04–10.92; p = .044) were both found to significantly increase the odds of mortality within the hepatitis D cohort. Other risk factors were not significantly associated with mortality (Table 3).
| Risk factors | Crude odds ratio (95% CI) | Crude p-value | Adjusted odds ratio (95% CI) | Adjusted p-value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | ≥65 (vs <65) | 2.71 (1.11–6.58) | 0.028 | 3.79 (1.24–11.60) | .020 |
| Sex | Female (vs. Male) | 0.87 (0.35–2.16) | 0.772 | 1.07 (0.44–2.63) | .878 |
| Race/Ethnicity | White (vs. Other) | 1.75 (0.73–4.16) | 0.209 | 1.39 (0.58–3.34) | .456 |
| Co-infection | |||||
| HIV | Yes/No | 0.28 (0.04–2.11) | 0.217 | 0.40 (0.05–3.08) | .379 |
| HCV | Yes/No | 1.39 (0.59–3.27) | 0.456 | 1.96 (0.77–4.96) | .158 |
| Co-morbidity | |||||
| Alcoholic cirrhosis | Yes/No | 2.81 (1.00–7.94) | 0.051 | 3.37 (1.04–10.92) | .044 |
| Diabetes | Yes/No | 1.59 (0.47–3.15) | 0.695 | 0.95 (0.33–2.77) | .924 |
Note
- National Inpatient Sample, Healthcare Cost and Utilization Project (HCUP), (2010–2015). Bold indicates statistically significant p-values p < .05.
- Abbreviations: CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus.
We conducted further analysis on liver transplantation within these cohorts during the 2010–2015 period. There were 73 (2.4%) liver transplantations in the hepatitis D cohort and 5365 (1.3%) liver transplantations in the HBV only cohort (p = .015). Of those with liver failure, 23 (11.6%) from the hepatitis D cohort and 3500 (18.6%) from the HBV only cohort had liver transplantation (p = .236). Of those with hepatic encephalopathy, 0 (0%) from the hepatitis D cohort and 79 (1.9%) from the HBV only cohort had liver transplantation (p = .657). Of those with jaundice, 0 (0%) from the hepatitis D cohort and 138 (1.7%) from the HBV only cohort had liver transplantation (p = .648). And of those with failure of other organs (non-liver and non-renal), ≤10 from the hepatitis D cohort and 1145 (1.3%) from the HBV only cohort had liver transplantation (p = .796).
4 DISCUSSION
This is the first study that attempts to quantify the burden of and risks for hepatitis D in a national sample of hospitalized patients in the United States. In comparison with the hospitalized HBV only cohort, hospitalized hepatitis D patients were more likely male, Hispanic and from the Northeast region of the United States. In general, males were more likely than females to be injection drug users.20 Injection drug use has been a major transmission route of hepatitis D,2, 3, 21 and in our study, we also found that injection drug use was more common in the hepatitis D cohort versus the HBV only cohort, potentially explaining the overrepresentation of males in the hepatitis D cohort. The disproportional distribution of hepatitis D in the Northeast may be due to an increased index of suspicion for HDV among clinicians in the Northeast region, resulting in increased screening of hepatitis B patients for HDV infection. Other possibilities were less clear; immigrants/foreign-born persons, who have been traditionally associated with having high HDV prevalence, were largely concentrated in the West region of the United States,22, 23 and syringe service programs were more likely to be available in the Northeastern states compared to many states in the South where they are illegal.24
While we found that hepatitis D constituted a small percentage of the total hepatitis B hospitalizations, hepatitis D hospitalizations rates remained relatively constant between 2010 and 2018. In fact, we report that HBV only hospitalizations actually increased in the 2015–2018 period. This is consistent with a disturbing trend in the United States. Since the advent of widespread hepatitis B vaccination, cases of acute hepatitis B in the United States, as reported by the Centers for Disease Control and Prevention (CDC), have decreased each year; however, after 2010, this has stagnated and has even begun to increase slightly in recent years.25, 26 Stockdale et al. and Chen et al. reported in their systematic review and meta-analysis that the prevalence of HDV infections among HBsAg-positive individuals in the United States is estimated to be 5.9% and 7.2%, respectively.2, 3 However, a major weakness of Stockdale et al. and Chen et al. was that they largely relied on retrospective studies which suffer from the lack of routine hepatitis D testing after a diagnosis of hepatitis B. In contrast, a study utilizing the 2011–2016 National Health and Nutrition Examination Survey (NHANES) database, found that 33% of native born and 46% of foreign-born HBsAg-positive individuals living in the United States had positive anti-HDV serology.22 Regardless of which of those prevalence values was referenced, they all starkly contrasted the 0.6% prevalence of hepatitis D hospitalizations among total hepatitis B hospitalizations in our study. This suggested that, in practice, hepatitis D appeared to be vastly underdiagnosed in this country. With the development of new HDV antibody tests in recent years that have demonstrated high sensitivity and specificity,27, 28 we believe that routine of hepatitis D in all individuals with confirmed chronic hepatitis B will be beneficial.
We found no differences in mortality between hospitalizations of hepatitis D versus HBV only hospitalizations. A few prior studies have reported increased rates of mortality in patients with hepatitis D compared to those with HBV only, though the evidence there is not strong.29, 30 Another potential reason could be that the number of HDV hospitalizations was not large enough to appreciate the difference in mortality rate. In Figure S3, the case-fatality rate for HDV versus the HBV only cohort demonstrates the large range of case-fatality rates in the HDV cohort compared to the HBV cohort, mainly due to very low total number of deaths each year in the HDV cohort. Still, while there were no differences in mortality in our study, there were several significant morbidities and complications in the hepatitis D cohort. Liver failure, non-alcoholic cirrhosis, portal hypertension, ascites and thrombocytopenia were all more common in the hepatitis D cohort. However, there did not appear to be an increase in extra-hepatic complications in the conditions explored. This was largely consistent with the literature.10, 13 Thrombocytopenia, however, did appear to have been associated as a complication of hepatitis D. This finding was likely related to the higher rates of liver failure and non-cirrhosis in the hepatitis D cohort.
While there were no significant differences in complications and death among the pregnant persons and foetuses/neonates with hepatitis D versus HBV only, possibly due to a relatively small sample size, the proportion of pregnant persons in the hepatitis D cohort was less than one-third of those in the HBV only cohort. The age distribution between the two cohorts was similar, and although females were slightly less prevalent in the hepatitis D cohort, this difference was not proportional to the large difference in pregnancy proportions. This may suggest that hepatitis D could be adversely affecting fertility in women. Currently, there has been no published research on this question. In males, hepatitis B is known to increase the risk of infertility, reducing the quality of sperm and increasing the risk of varicoceles.31, 32 In females, Lao et al. reported increased frequencies of tubal and uterine factors involved in infertility in those with hepatitis B.33 It is possible that hepatitis D could be enhancing the effects of hepatitis B on infertility, or perhaps acting via an independent mechanism. On the other hand, the stark difference in pregnancy proportions between hepatitis D and HBV only cohorts may suggest that there were pregnant women in the HBV only cohort who have hepatitis D but have not been diagnosed with the disease. While hepatitis B is routinely screened in pregnant women, hepatitis D is not routinely tested for after a positive hepatitis B result. More research is needed to address this issue.
Although the rates of mortality were not significantly different between hepatitis D and HBV only cohorts, we further investigated the contributors of death within the hepatitis D cohort. As expected, older age (≥65 years) and alcoholic cirrhosis were both associated with mortality during hepatitis D-associated hospitalization. However, we did not expect that HIV and HCV co-infections would be insignificant in contributing to mortality. HIV infection in individuals with viral hepatitis, including hepatitis D, has typically been associated with severe complications such as increased rates of hepatocellular carcinoma and cirrhosis as well as increased mortality.34-36 This is similar in the case of HCV co-infection.37, 38
Due to the nature of the National Inpatient Sample database, a limitation of this study was the lack of standardization of diagnosis criteria. We rely on the professional judgement of clinicians in entering the most appropriate diagnostic codes. Another limitation is that method of diagnosis of HDV, whether anti-HDV antibody and/or HDV RNA was used, was not specified in the National Inpatient Sample, as such, the presence of HDV viremia could not be determined. However, while HDV viremia cannot be confirmed, there were clear increased clinical complications in the HDV cohort, suggesting that a large portion of the cohort may have had HDV viremia or have significant liver injury from past HDV infection. Additionally, there was the risk of miscoding as well. For this study, we made the assumption that any miscoded diagnoses and differing coding behaviours among clinicians would be randomly distributed and would not result in a statistical significance. Our study paves the way for further improved analysis in future with the next generation ICD-11 codes which better classify superinfection and co-infection of hepatitis D and make available options for diagnosis based on presence of HDV antibody versus detection of HDV RNA.
5 CONCLUSION
Hospitalizations of hepatitis D in the United States were uncommon with no significant changes in hospitalization rates overall in the 2010–2015 and 2015–2018 periods. Additionally, the burden of hepatitis D was disproportionately greater in certain demographics and geographic regions. Hepatitis D hospitalizations did not result in increased frequencies of mortality compared to HBV only hospitalizations; however, complications such as liver failure were significantly more frequent in the hepatitis D cohort. Nevertheless, we found that within the hepatitis D cohort, older age and the presence of alcoholic cirrhosis were both major risk factors of mortality. And while pregnant persons and their foetuses/neonates did not seem to be at increased risk for morbidity or mortality if they were infected with hepatitis D compared to HBV only, further research is needed with larger cohorts.
Hepatitis D appeared to be largely underdiagnosed in the United States. There is a need for routine hepatitis D testing after a confirmed chronic hepatitis B diagnosis, especially considering the severity of the disease as well as the future availability of hepatitis D-specific therapy.
ACKNOWLEDGEMENTS
We would like to thank Dr. Philip Spradling and Dr. Nelson Adekoya from the Centers for Disease Control and Prevention for reviewing and providing important feedback on this study. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTERESTS
The authors declare no financial or personal conflicts of interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.