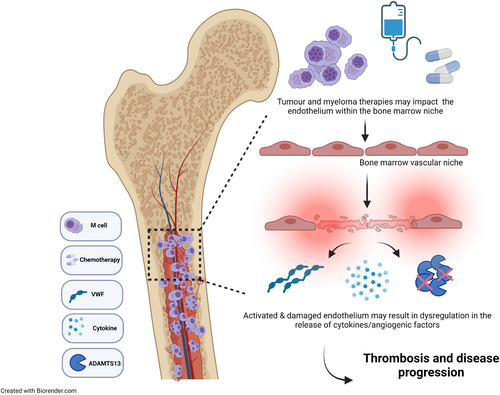
The role of VWF/FVIII in thrombosis and cancer progression in multiple myeloma and other hematological malignancies
Manuscript handled by: David Lillicrap
Final decision: David Lillicrap, 26 May 2022
Abstract
Cancer associated thrombosis (CAT) is associated with significant morbidity and mortality, highlighting an unmet clinical need to improve understanding of the pathophysiology of CAT. Multiple myeloma (MM) is associated with one of the highest rates of thrombosis despite widespread use of thromboprophylactic agents. The pathophysiology of thrombosis in MM is multifactorial and patients with MM appear to display a hypercoagulable phenotype with potential contributory factors including raised von Willebrand factor (VWF) levels, activated protein C resistance, impaired fibrinolysis, and abnormal thrombin generation. In addition, the toxic effect of anti-myeloma therapies on the endothelium and contribution to thrombosis has been widely described. Elevated VWF/factor VIII (FVIII) plasma levels have been reported in heterogeneous cohorts of patients with MM and other hematological malignancies. In specific studies, high plasma VWF levels have been shown to associate with VTE risk and reduced overall survival. While the mechanisms underpinning this remain unclear, dysregulation of the VWF and A Disintegrin And Metalloprotease Thrombospondin type 1, motif 13 (ADAMTS-13) axis is evident in certain solid organ malignancies and correlates with advanced disease and thrombosis. Furthermore, thrombotic microangiopathic conditions arising from deficiencies in ADAMTS-13 and thus an accumulation of prothrombotic VWF multimers have been reported in patients with MM, particularly in association with specific myeloma therapies. This review will discuss current evidence on the pathophysiological mechanisms underpinning thrombosis in MM and in particular summarize the role of VWF/FVIII in hematological malignancies with a focus on thrombotic risk and emerging evidence for contribution to disease progression.
1 INTRODUCTION
Cancer associated thrombosis (CAT) is associated with significant morbidity and mortality.1 It is reported across all cancer cohorts including both solid tumors and hematological malignancies. The risk of venous thromboembolism (VTE) is increased 5- to 7-fold in patients with cancer, such that 20% of those with malignancy will develop thrombosis during the course of their cancer journey.2 Importantly, patients with cancer who suffer VTE have a 3-fold increased risk of death at 1 year following diagnosis compared to those without thrombotic events.3 Although the biological mechanisms underlying CAT remain poorly understood, emerging data have highlighted critical roles for crosstalk among tumor cells, vessel walls, inflammation, and coagulation pathways.4, 5 To date, many of these studies, aimed at understanding the molecular mechanisms of CAT, have focused on solid tumor malignancies including pancreatic, lung, and brain cancers. However, significant interplay also exists between neoplastic cells and coagulation pathways in hematological malignancies. In fact, multiple myeloma (MM) is associated with one of the highest rates of thrombosis. A large population-based study reported that MM patients have a ~ 9-fold increased VTE risk compared to the general population.6 Unusually, thrombotic complications in MM occur not only in the venous circulation, but also within the arterial vasculature.7 Moreover, MM-related thrombosis is markedly increased by certain novel therapeutic agents.8, 9 Notwithstanding all of these findings, the mechanisms leading to such high rates of thrombosis in MM patients remain unclear but may also be clinically relevant in other hematological malignancies. A diverse array of mechanisms has been proposed to contribute to increased risk of thrombotic events in patients with cancer. These include tumor tissue factor expression, cancer-mediated pro-inflammatory changes, endothelial cell and platelet dysfunction, and procoagulant microparticle secretion.2 These mechanisms have been widely studied in high-risk solid tumors. However, the relative contribution of these mechanisms in mediating the hypercoagulable state in patients with hematological malignancies remains poorly defined. Previous studies have reported that plasma levels of glycoprotein von Willebrand factor (VWF) and coagulation factor VIII (FVIII) are elevated in patients with cancer, including MM.10, 11 In particular, mechanistic studies on solid tumor malignancies have shown that tumor cells directly stimulate endothelial cell activation, promoting the release of VWF multimers along the endothelium and correspondingly elevated plasma FVIII levels. While the mechanisms of this tumor-induced VWF secretion continue to be explored, it is proposed to contribute to both underlying risk of CAT as well as cancer metastasis.11-14 In this review we will discuss the pathological mechanisms underpinning thrombosis in MM, as an exemplar model of hematological malignancies, and in particular summarize the evidence to date on the role of VWF/FVIII on risk of thrombotic complications and emerging evidence for contribution to disease progression.
2 THROMBOSIS IN MULTIPLE MYELOMA
Multiple myeloma is an incurable bone marrow cancer that is the second most common hematological malignancy.15 It has an incidence of approximately 2.1 per 100 000 people/year, with approximately 160 000 people being diagnosed with MM annually.15, 16 MM accounts for almost 100 000 deaths worldwide every year and, with an aging population, its incidence has continued to increase over the past number of decades.15 It is characterized by the unregulated expansion and accumulation of monoclonal plasma cells within the bone marrow compartment. MM is clinically characterized by the “CRAB” features: hypercalcemia, renal impairment, anemia, and lytic bone lesions. MM is consistently preceded by a precursor condition called monoclonal gammopathy of undetermined significance (MGUS), which progresses to smoldering multiple myeloma (SMM), and finally to symptomatic MM.17 In conjunction with the above clinical sequelae, MM is also associated with the highest risk of VTE among all hematological malignancies. MM patients with active disease have a ~ 9-fold increased VTE risk compared to the general population with up to 10% of patients suffering an episode of VTE.6, 7, 18 There are a multitude of risk factors for VTE in MM, which include both patient-related factors such as increased body mass index, advancing age, ethnicity, a personal history of VTE, as well as treatment-related factors, including use of erythropoietin stimulating agents, steroids, and immunomodulatory agents.19 Surprisingly, it has been shown that even in the precursor stages of MM disease, patients have an increased risk of VTE. Patients with MGUS exhibit an approximately 3-fold increased risk of VTE, highlighting the significant interplay between MM cell biology and coagulation pathways, even at an early disease stage, when the clonal plasma cell burden is relatively low.6 Arterial thrombotic risk also appears to be increased in patients with MM with rates of coronary artery disease and cerebrovascular events observed to be increased in MM cohorts and indeed, patients with MGUS have also been shown to manifest a higher risk of arterial disease.20, 21 Though some of this cardiovascular risk may be related to MM disease factors such as anemia and renal impairment, it does appear that certain anti-myeloma therapies exhibit significant arterial toxicity profiles, though the underlying mechanisms behind this are not fully elucidated.
Thrombosis in patients with MM not only significantly impacts quality of life and interrupts treatment regimens but it also increases the economic burden of the disease. Most critically, thrombosis may also be associated with reduced overall survival in MM. A recent review of nearly 10 000 MM patients demonstrated those with VTE had a higher mortality at 1, 5, and 10 years of follow-up compared to patients without VTE.22 Consequently, understanding the biology underpinning thrombosis is of direct clinical importance and may provide insights for development of novel therapeutic strategies to reduce VTE risk in MM. Due to these high rates of thrombosis in MM, thromboprophylaxis with agents such as low molecular weight heparin (LMWH) or aspirin is often employed. Recent studies have also shown some promise for the use of direct oral anticoagulants in this setting.23 Nonetheless, studies have shown that risk of thrombosis is not completely abrogated by any of these thromboprophylaxis strategies. In fact, high rates of both venous and arterial thrombosis have been reported in patients with MM receiving therapy with immunomodulatory agents despite thromboprophylaxis.24-26 Bradbury et al. reported on the thrombosis rates observed in the Myeloma IX and Myeloma XI phase 3 trials and disappointingly, thrombosis incidence rates at 6 months remained >10% in the later trial despite much higher rates of thromboprophylaxis (80.5% vs. 22.3%).25 The reasons behind this failure are not fully understood but are likely multifactorial.27 Some studies have proposed the possibility of “heparin resistance” in MM and have sought to evaluate the role of thrombin generation testing, a test of global hemostasis, to evaluate heparin efficacy in MM disease but results have been conflicting thus far.28, 29
A number of internationally validated and clinically utilized risk scores have been developed to stratify VTE risk in patients with solid tumors who may benefit from thromboprophylaxis with anticoagulant agents.30 These prediction tools are based upon a number of laboratory/clinical parameters. However, generic CAT risk assessment models do not always extend to patients with hematological malignancies, including MM, due to poor positive predictive values. Recently, newer models have been developed specifically for VTE risk in MM such as the “IMPEDE VTE” risk score, which is based on retrospective data from a large MM cohort and the “SAVED” score, which seeks to stratify VTE risk in patients specifically undergoing therapy with immunomodulatory agents.19, 31 While these scoring algorithms advance VTE risk discrimination in MM, incorporation of additional biomarkers is needed to improve clinical utility.32 Understanding the complex interplay between MM cells and coagulation biology will be essential to develop novel therapeutic strategies for thromboprophylaxis and to identify biomarkers to improve these risk assessment models.
3 VWF/ADAMTS-13 AXIS AS A REGULATOR OF CANCER ASSOCIATED THROMBOSIS
von Willebrand factor is a large, multimeric plasma glycoprotein that is essential for normal hemostasis. It functions as a protective carrier molecule for FVIII and facilitates platelet aggregation at sites of vessel injury.33 In normal physiological conditions, VWF is synthesized in endothelial cells (ECs) and megakaryocytes. ECs store VWF in specialized organelles called Weibel Palade bodies (WPB) while megakaryocytes and their platelet progeny store VWF in alpha-granules with release upon platelet activation.34 The majority of ECs’ VWF is secreted constitutively, which contributes to circulating plasma VWF levels. Upon EC stimulation or damage, VWF is released from WPBs in a regulated fashion. Importantly, newly secreted VWF consists of hemostatically active ultra-large molecular weight multimers, capable of tethering platelets along the endothelium. Of note, within the general population plasma VWF antigen (VWF:Ag) and FVIII activity (FVIII:C) levels correlate with risk of VTE.35 In fact, Rietveld et al. reported that the observed relationship between coagulation factor levels and VTE risk can be largely explained by FVIII and VWF, which are associated with the highest risk of thrombosis.35 Furthermore, Nossent et al. reported a dose–response relationship between plasma levels of VWF propeptide, a marker of VWF secretion from ECs, and the risk of venous thrombosis.36 They concluded that high VWF levels associated with thrombosis are largely mediated by increased VWF secretion from ECs.
The multimeric composition, and thrombotic potential, of VWF is tightly regulated by a specific protease termed A Disintegrin And Metalloproteinase with Thrombospondin Type 1, Motif 13 (ADAMTS-13). ADAMTS-13 cleaves hemostatically active VWF high molecular weight multimers (HMWM) into smaller, less active forms. Deficiency and/or dysfunction in ADAMTS-13 can result in accumulation of prothrombotic VWF multimers leading to thrombotic microangiopathy.37 Interestingly, previous studies suggest that ADAMTS-13 activity levels are decreased in patients with cancer and they are also decreased further in patients with disseminated disease compared to localized disease.38 Thrombotic microangiopathy can also occur in association with cancer, and may be associated with ADAMTS-13 deficiency. Studies in patients with solid tumor malignancies have shown that a combination of elevated plasma VWF:Ag levels coupled with reduced ADAMTS-13 activity are associated with increased risk of CAT and decreased overall survival.39, 40 It has been hypothesized that, in malignant disease, the dysregulation of the VWF/ADAMTS-13 axis that is observed may in fact be caused by increased consumption of the ADAMTS-13 protease, similar to that which has been described in sepsis and other systemic inflammatory conditions.41 This raises the intriguing possibility of potential therapeutic benefit of recombinant ADAMTS-13, though clearly this would require extensive additional investigation.39 Importantly, tumor-induced local inhibition of ADAMTS-13 activity has also been observed in patients with melanoma, which directly contributed to increased platelet aggregates and microthrombi within the tissue vessels.12 Furthermore, Goertz et al. have described a significant pro-metastatic role of VWF multimers in malignant melanoma.42 In melanoma tumor-bearing mice, EC activation triggered the release of pro-thrombotic VWF in vitro resulting in the accumulation of VWF multimers in the tumor vasculature. Intriguingly, they also observed a marked increase in hematogenous lung metastasis in ADAMTS-13–deficient mice. In support of this, the VWF/ADAMTS-13 ratio has previously been described as a novel biomarker in solid tumors including hepatocellular carcinoma where the ratio correlated with tumor volume and stage.43 In the setting of ovarian cancer, an imbalance in the VWF/ADAMTS-13 ratio has been proposed to result from consumption of the protease due to excess ultra-large VWF multimer release from the activated endothelium.44 Taken together, these findings suggest that dysregulation of the VWF/ADAMTS-13 axis in cancer patients is likely to contribute to the pathological accumulation of VWF multimers and may promote thrombosis in the setting of malignancy. Given the high risk of both arterial and venous thrombosis seen in patients with MM, systematic assessment of the VWF/ADAMTS-13 axis may provide insights into the pathogenesis of thrombosis in patients with MM.
4 PATHOPHYSIOLOGY OF VTE IN MULTIPLE MYELOMA
4.1 Hypercoagulation in multiple myeloma
The pathophysiology of VTE in MM is likely multifactorial and appears to be both attributable to the disease process itself in addition to the agents used to treat it. Several groups have sought to define the hypercoagulable phenotype in patients with MM with common observations including significant elevations in FVIII:C and fibrinogen levels and reduced protein S levels in patients with active disease.10, 45-48 However, the relative effect and overall contribution of these abnormalities toward VTE occurrence in MM has not yet been fully determined. Significant evidence exists to support an association between VTE risk and activated protein C (APC) resistance in patients with MM. Under normal conditions, APC functions to proteolytically inactivate both factor Va and factor VIIIa, limiting coagulation activity and thrombin generation. Elice et al. characterized the incidence of acquired APC resistance in a large cohort of patients with MM and observed that almost 10% of patients displayed this hypercoagulation abnormality.49 Moreover, this subset of patients with APC resistance did indeed exhibit a higher rate of thrombosis. Interestingly, more than 20% of MM patients have been reported to display APC resistance in the absence of factor V Leiden mutation. Although predictive of VTE risk in MM patients, the molecular basis for this APC resistance in MM is unknown. Pertinently, APC resistance is attenuated upon treatment, suggesting it is likely to be a feature associated with active disease.50 M-protein levels, active disease, and C-reactive protein are all postulated mechanisms for APC resistance in MM patients but the specific role of these factors in modulating APC anticoagulant activity has not been determined.
Other potential contributors to the hypercoagulable profile seen in MM include both impaired fibrinolysis and abnormal thrombin generation. MM patient plasma has been reported to exhibit impaired fibrinolytic ability via direct M--protein interactions. Carr et al. observed that in MM fibrin clots appear to be composed of abnormally thin fibrin fibrils and moreover, that these clots appear to dissolve at a more protracted rate.51 Abnormalities in thrombin generation have been extensively studied in other malignancies and it has been observed that elevated peak thrombin generation may increase risk of development of VTE in particular cancer cohorts.52 Given the high rates of thrombosis in MM, several groups have sought to extrapolate these findings to the MM population with markedly conflicting results observed to date. For example, Leiba et al. demonstrated elevations in thrombin generation parameters in patients with MM who developed thrombosis while in direct contrast to this, others have observed reductions in endogenous thrombin potential in patients with MM.53-55 These reductions are hypothesized to be due to “exhausted thrombin generation” resulting from over-stimulation of the endothelium. Thus, it appears that dysregulation of thrombin production is markedly varied in this disease and the downstream effects of this on MM hypercoagulability are unclear as of now.
4.2 Multiple myeloma therapies contribute to VTE risk
The treatment of MM has shown rapid advancement over the past number of years with a multitude of therapeutic options now available. Triplet drug combinations are the initial standard of care for induction therapy. Younger, fitter patients who are eligible for autologous transplantation (ASCT) will usually receive a combination consisting of a proteasome inhibitor such as bortezomib and dexamethasone in conjunction with either lenalidomide, thalidomide, or cyclophosphamide.56 Moreover, there are many novel agents available for patients upon disease relapse such as carfilzomib, a second generation proteasome inhibitor, monoclonal anti-CD38 antibodies, and other immunomodulatory agents including pomalidomide.56, 57 Although the vast majority of patients with MM respond to first-line treatment, virtually all patients will suffer disease relapse and develop drug-resistant disease. Thus, understanding the critical factors involved in therapy refractoriness remains a major goal for MM researchers. However, since the widespread introduction of newer treatment options, there appears to be an increase in therapy-related cardiovascular complications including ischemic heart disease, accelerated hypertension, arterial thromboembolism, and VTE within the MM population.9, 58 Several studies have shown that the addition of high dose steroids, such as dexamethasone in MM, to a chemotherapy regimen is associated with a significantly increased thrombosis risk, with rates increased by up to eight times reported in certain studies.18, 59 The exact causative mechanism underlying this is unclear, though there is some in vitro evidence that dexamethasone can stimulate the endothelium to increase expression of both VWF and tissue factor.60 Given this, Jilma et al. sought to evaluate the effect of administration of intravenous dexamethasone to healthy individuals and interestingly, they observed an increase of approximately 15%–20% in VWF:Ag in those who were administered high dose dexamethasone.61 Dexamethasone is also routinely used in combination with immunomodulatory agents and appears to enhance their thrombogenic potential, above the well-documented increased risk of thrombosis associated with their use as single agents.18, 62, 63 Immunomodulatory agents constitute one of the therapeutic backbones of MM yet their use is strongly associated with increased risk of VTE, particularly when used in combination therapies, with VTE rates reported between 3%–31%.64 Increased inflammatory cytokine release and EC activation appear to be key factors in contributing to VTE occurrence with use of immunomodulatory agents.65 Johnson et al., noting the increased rates of VTE with thalidomide exposure, sought to examine inherited genetic variations associated with VTE in patients administered this agent. Intriguingly, they actually observed a protective effect of VWF single nucleotide polymorphisms in thalidomide-treated patients who did not suffer thrombosis.66
Proteasome inhibitors are also pivotal in the treatment of MM and carfilzomib, in particular, has been shown to be clearly associated with high rates of cardiotoxicity, poor cardiovascular outcomes, and increased risk of VTE (with rates of >15% reported in certain studies).8, 9, 67, 68 While some hypotheses for this increased cardiovascular risk include increased endothelial toxicity related to irreversible 26S proteasome inhibition, the pathophysiology of this major toxicity has not yet been fully determined.18 Given that MM typically affects older individuals, who are already more likely to suffer from cardiovascular co-morbidities, there is a compelling argument for enhanced understanding of the exact mechanisms underlying these toxicities in order to improve cardiovascular risk reduction approaches. It has been well described that the chemotherapy agent cyclophosphamide in particular is associated with increased release of VWF from ECs and increased plasma VWF:Ag and FVII levels in murine models. This effect may be mediated not only by cyclophosphamide-induced EC toxicity and subsequent secretion of stored VWF but also decreased circulatory clearance of VWF/FVIII via EC receptors.69, 70 Conversely, little is known of the effect of other anti-myeloma chemotherapy agents on EC function and VWF biology. Martinez-Sanchez et al. have recently reported that exposure of ECs to certain MM chemotherapy agents resulted in elevations in both VWF and intercellular adhesion molecule 1 expression as assessed by immunofluorescence staining on cultured endothelial cells, though changes at a gene level were not evaluated. Reduced V-cadherin expression was also noted, possibly indicating increased cell permeability.71 Interestingly, this effect appeared partially ameliorated by addition of the endothelial protectant agent defibrotide, perhaps highlighting the need for examination of the role of incorporating endothelial protection strategies into MM treatments.
5 VWF/FVIII IN MULTIPLE MYELOMA: EVIDENCE TO DATE
Given the evidence base for elevated plasma VWF:Ag levels in a variety of solid organ malignancies, several groups have sought to explore VWF:Ag and activity levels in patients with clonal plasma cell disorders (Table 1). Perhaps the earliest study was carried out by Gomperts et al. in 1976; they evaluated a cohort of 19 patients with paraproteinemia (which included 15 patients with MM).46 They found that 7 patients had levels within normal ranges, 4 patients had levels that were mildly elevated, and 8 patients had markedly elevated levels (>200%). They also found that those with the most elevated levels also had the most severe disease, albeit severe disease was correlated with extent of disease on radiological skeletal survey without other clinical parameters being taken into consideration. Following on from this, Auwerda et al. conducted one of the larger investigations into coagulation abnormalities in this area and evaluated several coagulation parameters in 135 patients with newly diagnosed MM.48 They observed similarly increased levels of plasma VWF:Ag and also increased levels of FVIII:C prior to commencement of therapy. However, plasma levels were not evaluated at any further timepoints across the disease course or progression. Interestingly, they reported that the highest plasma levels of VWF:Ag and FVIII:C did in fact correlate with advanced stage of disease (as evaluated using the International Staging System for MM). Approximately 10% of the patients in this study subsequently suffered a VTE but they did not find a significant difference in pre-treatment coagulation variables in those who did/did not have a VTE. In contrast, Minnema et al. evaluated VWF:Ag and FVIII:C levels in a cohort of 20 patients with MM, all of whom were receiving thalidomide therapy.10 They reported markedly high levels of both plasma VWF:Ag (mean 374%) and FVIII:C (mean 352%) in their cohort. Interestingly, they did observe an association between high VWF:Ag levels and VTE occurrence, though high FVIII:C levels were not significantly associated with thrombotic events. However, given the low numbers of patients in this study and the high rates of VTE (35%), larger patient cohorts would be needed to further evaluate this correlation as it remains as yet unclear whether high VWF:Ag levels are associated with higher rates of VTE and indeed with poorer overall survival.10
| Patient cohort evaluated | Plasma VWF antigen (VWF:Ag) levels | Plasma FVIII:C levels and other coagulation findings | Association with VTE/advanced disease | |
|---|---|---|---|---|
| E. Gomperts et al., British Journal of Hematology, 1976 |
Multiple myeloma/MGUS (n = 19) |
12/19 patients had raised “FVIII -related antigen” i.e., VWF:Ag levels (4/12 = raised but <200% 8/12 = raised but >200%) |
Patients with MGUS (n = 3) all had normal VWF:Ag levels | Correlation between extent of disease and level of FVIII:C and VWF:Ag observed |
| M. Minnema et al., Journal of Thrombosis and Haemostasis, 2003 |
Multiple myeloma cohort with relapsed/refractory disease receiving thalidomide (n = 20) |
Raised VWF:Ag in overall group at 374% (mean) Significant increase in VWF:Ag in those with VTE vs. those without (375% vs. 235%) |
Raised FVIII:C levels in overall group (mean 311%) No variation seen in FVIII:C levels on sequential measurement |
Significantly higher VWF:Ag levels (but not FVIII:C) observed in patients on thalidomide with VTE vs. those without VTE occurrence |
| J. Auwerda et al., Haematologica, 2007 |
Multiple myeloma cohort undergoing treatment with thalidomide +/− dexamethasone (n = 31) |
Raised VWF:Ag vs. control (median 171% pre-treatment) |
Raised FVIII:C levels vs. control (median 235% pre-treatment) FVIII:C levels increased further after therapy with thalidomide + dexamethasone |
Correlation between FVIII:C and VWF:Ag levels and MM disease status (according to ISS criteria) |
| A. van Marion et al., Leukemia Research, 2008 |
Multiple myeloma cohort with newly diagnosed disease (n = 138) |
Baseline: raised VWF:Ag (median 1.95 U/ml) During induction therapy: further rise in VWF:Ag (median 2.96 U/ml) After intensification therapy: VWF:Ag levels fall (median 1.67 U/ml) |
Raised FVIII:C levels at baseline (median 2.26 U/ml) No relationship found between VTE risk and coagulation abnormalities |
No relationship observed between VWF:Ag levels and VTE Significant association seen between pre-treatment FVIII:C and VWF:Ag levels and mortality |
| E. Hatzipantelis et al., Acta Haematologica, 2011 |
Acute lymphoblastic leukemia pediatric cohort (n = 52 in acute phase) (n = 19 in ALL control group [1–10 years post therapy]) |
Raised VWF:Ag compared vs. ALL control group and also vs. healthy control group (mean 164.6% vs. 103.9% vs. 99.7%) |
Positive correlation between VWF:Ag levels and leucocyte count Raised serum thrombomodulin vs. ALL control (23.2 vs. 10.9 ng/ml) |
Correlation between VWF:Ag levels and leucocyte count before treatment Significantly higher thrombomodulin levels in patients who relapsed or died |
| Hagag et al., Journal of Oncology Pharmacy Practice, 2014 |
Acute lymphoblastic leukemia pediatric cohort with newly diagnosed disease (n = 40) |
Raised VWF:Ag vs. control (mean 583.85 vs. 119.37 IU/dl) | Raised serum thrombomodulin vs. control (19.49 vs. 4.97 ng/ml) | Higher VWF:Ag and serum thrombomodulin levels seen in those with unfavorable outcome vs. favorable outcomes |
| K. Burley et al., Blood Cancer Journal, 2017 |
Acute lymphoblastic leukemia cohort (n = 35) |
Pre-treatment: raised VWF:Ag compared with controls (mean 1.84 vs. 1.01 μg/ml) During induction therapy: further rise in VWF:Ag (mean 2.22 μg/ml) |
Raised FVIII:C levels both pre-treatment (mean 2.18 μg/ml) and during induction therapy (2.00 μg/ml) | Higher pre-treatment VWF:Ag levels seen in those who developed VTE |
| M. Mohren et al., International Journal of Hematology, 2016 | Mixed lymphoma/leukemia cohort (lymphoma n = 35) (leukemia n = 10) | Raised VWF in both cohorts vs. normal control (median 321%) |
Raised VWF collagen binding activity (median 199%) Raised FVIII:C levels (median 150%) |
Not assessed (VTE occurrence = 2/45) |
| B. Hivert et al., Blood, 2012 |
Waldenstrom’s macroglobulinemia (n = 72) |
43/72 had raised VWF:Ag levels (>110 IU/dl) | 10 patients fulfilled criteria for acquired von Willebrand disease | High VWF:Ag was a significant adverse prognostic factor for survival after first-line therapy |
- Abbreviations: ALL, acute lymphoblastic leukemia; FVIII:C, factor VIII activity; MM, multiple myeloma; VTE, venous thromboembolism; VWF, von Willebrand factor; VWF:Ag, von Willebrand factor antigen.
Other groups have sought to sequentially track levels from diagnosis throughout the treatment course with van Marion et al. evaluating levels in 138 patients with newly diagnosed with MM before; during chemotherapy in the form of VAD (vincristine, adriamycin, dexamethasone), PAD (bortezomib, adriamycin, dexamethasone), and TAD (thalidomide, adriamycin, dexamethasone); and after intensification with high dose melphalan.72 As with prior studies, they observed increased levels in the newly diagnosed patients and, interestingly, found that, irrespective of treatment, the plasma levels of FVIII:C and VWF:Ag increased strongly during induction therapy, thus raising the question of the possible effects of MM chemotherapy agents on endothelial VWF synthesis and/or secretion. Furthermore, they observed that plasma levels of VWF:Ag and FVIII:C reverted toward normal levels approximately 3–6 months after autologous stem cell transplant indicating that perhaps the acute toxic effect of chemotherapy agents on the endothelium is transient and that normal endothelial function can be restored following a washout period. Given that the increased risk of VTE has been observed in those with early-stage disease including MGUS and SMM, a number of studies have sought to further understand this phenomenon and have assessed coagulation parameters in patients with these conditions. Crowley et al. compared coagulation profiles of eight patients with MM, eight patients with MGUS, and eight normal controls. Interestingly, they found that patients with MGUS appeared to have their own distinct coagulation profiles with modestly elevated VWF and FVIII:C levels, intermediate between those with MM and the normal controls.73 In contrast, Minnema et al. also evaluated coagulation parameters in patients with MGUS (n = 6) and did not find an increase in plasma VWF:Ag levels or FVIII levels, albeit again, this evaluation was limited to small numbers of patients.10 These differing reports, in small cohorts of patients, would suggest a systematic investigation with larger cohorts of patients with precursor MM conditions is warranted.
Thus, it appears that patients with MM do exhibit higher levels of VWF:Ag and FVIII:C but it is unclear if these raised levels are associated with VTE or indeed with overall survival. Furthermore, the biological mechanisms underlying these high levels is very poorly understood. Some of the hypotheses postulated by the above authors attribute changes in plasma levels to MM disease activity, increased bone marrow angiogenesis, and neovascularization while other groups counter that the chemotherapeutic agents have a more important causative role to play.10, 45 Given that patients with MM will often exhibit thrombocytopenia due to either bone marrow suppression or chemotherapy-induced thrombocytopenia, it is likely that the excess VWF seen in the plasma of patients with MM is of EC origin, though the effect of MM cells on EC VWF secretion has not been delineated thus far.74 It is likely multifaceted including a combination of chronic endothelial activation and dysfunction, increased bone marrow angiogenesis, and disturbances in the VWF/ADAMTS-13 axis, which may all contribute to elevated VWF/FVIII levels reported in patients with MM (Figure 1). However, additional robust studies, incorporating patients on the newer anti-myeloma treatments, will be required to fully evaluate these effects and provide better mechanistic insights. Given the high rates of thrombotic complications in this cohort and current sub-optimal thromboprophylaxis regimens, better insights into pathophysiology of thrombosis in MM will be clinically relevant.
6 THROMBOTIC MICROANGIOPATHY IN MULTIPLE MYELOMA
Despite the lack of robust data available on the potential roles of VWF/FVIII in VTE occurrence and disease progression in MM, abnormalities of VWF function are well known to be associated with distinct clinical sequelae in MM. Thrombotic thrombocytopenia purpura (TTP) is a thrombotic microangiopathy (TMA) characterized by a pentad of hemolysis, thrombocytopenia, renal impairment, neurological dysfunction, and pyrexia. The pathophysiology of idiopathic TTP is well understood; under normal healthy conditions, ADAMTS-13 cleaves the ultra-large multimers of VWF that are secreted from endothelial cells under conditions of shear stress.75 In idiopathic TTP, autoantibodies against ADAMTS-13 develop with a resultant deficiency and/or dysfunction of this essential VWF protease.37 This in turn leads to an accumulation of ultra-large VWF multimers in the circulation, which form aggregates with platelets and can occlude vessels. However, secondary TTP, and in particular TTP in association with MM, is far less well understood. There are several possible causative factors for TMA/TTP in association with MM, including specific therapies, stem cell transplant, and indeed TMA induced by MM disease activity itself.76 One of the common culprits of drug-induced TMA in MM are proteasome inhibitors. These agents target the ubiquitin--proteasome pathway with resultant protection of pro-apoptotic factors from degradation. They also stabilize the nuclear factor kappa B (NFκB) complex, preventing its translocation to the nucleus and decreasing downstream signaling. Though not seen originally in clinical trials, TMA has now been described in association with use of proteasome inhibitors in case reports and case series.77-79 Carfilzomib carries the highest risk of TTP occurrence but it has also been described with ixazomib use.77, 79-83 In direct contrast to this, bortezomib has actually previously been reported to induce remission in idiopathic TTP.84 The pathophysiology of proteasome inhibitor-induced TMA has not been fully established, nor have optimal treatment strategies been ascertained; thus, improved insights into the biological processes underpinning this effect are of direct clinical relevance.1, 76, 85, 86 Others have suggested an immune mediated mechanism for proteasome inhibitor-induced TMA with Moore and Romeril describing a case of bortezomib-induced TMA/TTP where ADAMTS-13 levels were in fact found to be reduced at 12%.79 While an autoantibody to ADAMTS-13 was not present in this case, they speculated that the patient likely had high levels of pro-inflammatory cytokines such as IL-6 due to active disease and, when challenged with bortezomib, these levels were further increased, which may have provided the appropriate setting for inhibition of ADAMTS-13 activity.
Transplant-associated TMA in MM is a rare phenomenon which is likely partially due to the lower rates of TMA associated with autologous stem cell transplant.87 When it does occur, it appears to be associated with combined autografting-allografting and has a very high mortality rate.76, 88 It also appears that the MM disease process or burden can, in itself, cause TMAs with authors reporting TMAs as the presenting feature of MM, before introduction of possible offending drugs. Xiao et al. reported a case of MM that initially presented with TTP with a reduced ADAMTS-13 level of 29.9%.89 The TTP sequelae resolved with plasma exchange and intravenous dexamethasone leading authors to conclude that the increase in blood viscosity from circulating immunoglobulins in the microcirculation served as a key trigger for TMA.
Given its rarity, there are no optimal treatment strategy guidelines for TMA associated with MM. In proteasome inhibitor-induced MM, identification and avoidance of the offending drug is pivotal, in conjunction with supportive care. Certain cases appear to fully resolve with cessation of the proteasome inhibitor treatment, and reports of recurrence with reintroduction compound the temporal association.77 However, withdrawal of the proteasome inhibitor does not always result in resolution of the TMA. Other therapies that have been employed, with varying success, include eculizumab, a terminal complement inhibitor, and plasma exchange. Treatment of the underlying plasma cell dyscrasia in newly diagnosed cases may also be helpful but ultimately TMA is a dangerous and often fatal condition. It is thus imperative to further evaluate the VWF/ADAMTS-13 axis in MM and ascertain the biological processes underpinning MM-associated TMA.
Interestingly, despite the aforementioned studies observing raised VWF levels in MM, patients with paraproteinemia can in fact also suffer from acquired von Willebrand disease (aVWD). These patients usually present with mucocutaneous bleeding or are identified due to excessive intra-operative or peri-procedural bleeding or excessive bleeding post trauma.90, 91 However, aVWD is far rarer and is usually associated with an underlying disease process, including both MGUS and MM.92 The pathophysiology of aVWD is often unclear with various pathogenic mechanisms postulated including inhibitory autoantibodies to VWF, non-neutralizing autoantibodies to VWF, adsorption of VWF by tumor cells, decreased VWF synthesis, and increased VWF degradation and clearance.90, 91, 93, 94 In MGUS/MM auto-antibodies that are directed against specific VWF domains have been reported and it is believed that these antibodies either bind to VWF and form immune complexes that are subsequently cleared by the reticuloendothelial system or bind to functional domains of VWF with resultant VWF inactivation.90, 95 Eradication of the MM disease burden can resolve aVWD but naturally, given the difficulties often encountered with treating MM into a remission state, this is not always possible.92
7 VWF/FVIII IN OTHER HEMATOLOGICAL MALIGNANCIES
Aside from MM, markedly elevated plasma VWF:Ag levels have been reported across a spectrum of hematological diseases including lymphoma, Waldenstrom’s macroglobulinemia, and leukemia, summarized in Table 1. Mohren et al. studied VWF:Ag in patients with lymphoma (including both Hodgkin’s and non-Hodgkin’s lymphoma) and demonstrated both high plasma VWF:Ag and FVIII:C levels in addition to a sustained elevation in these levels at follow-up >/1 year later.96 Hivert et al. also evaluated VWF in lymphoid malignancy, focusing their investigation on VWF pathophysiology in 72 patients with Waldenstrom’s macroglobulinemia, and reported elevated levels in over half of these patients and, critically, these elevations appeared to be associated with poorer overall survival.97
VWF:Ag in acute lymphoblastic leukemia (ALL) has also been a focus of interest for several groups given that ALL is associated with high rates of VTE, particularly during induction therapy, which may include asparaginase which is known to increase the risk of thrombosis.98 Burley et al. reported their findings in 35 patients with ALL in which they evaluated a range of coagulation parameters, including VWF:Ag and FVIII:C levels.99 They observed that those who developed thrombosis during their treatment course exhibited higher VWF:Ag levels than those who did not. They thus concluded that it may be useful to combine markers such as VWF:Ag levels with known clinical risk factors for thrombosis in order to develop optimal targeted prevention strategies in patients with the highest VTE risk. VWF has also been assessed specifically in pediatric cohorts of ALL.100, 101 Hagag et al. observed significant increases in VWF:Ag levels during the acute phase of disease in their cohort of 40 pediatric patients with ALL, compared to healthy age-matched controls. Interestingly, they also found that VWF:Ag levels were significantly higher in those who had an unfavorable prognosis. Their findings were similar to Hatzipantelis et al., who also described significantly higher levels of VWF:Ag in children in the acute phase of ALL compared to either age matched controls or children previously treated for ALL.101 These findings are reinforced by the observation of markedly elevated plasma VWF in a number of solid organ cancer cohorts including esophageal, colorectal, and melanoma.12, 102-104 Crucially, higher plasma VWF plasma levels appear to be associated with advanced tumor stage and with worse clinical outcome.103, 104 In fact, multivariate analysis demonstrated that plasma VWF levels constituted an independent poor prognostic indicator in colorectal cancer patients and similarly, the ratio of VWF/ADAMTS-13 in lung cancer patients was identified as a significant independent predictor of patient mortality.105 It is thus evident that plasma VWF:Ag and FVIII:C levels do in fact appear to be increased across a spectrum of hematological conditions and that, in some cases, these elevations appear to correlate with adverse outcomes. However, further systematic evaluation of VWF:Ag and activity would be required to fully evaluate its prognostic predictive potential for either thrombosis or survival times.
8 CONCLUSION
Despite the high rates of both venous and arterial thrombotic complications observed in MM, little is known of the role of VWF/FVIII and regulatory protease ADAMTS-13 in this disease. Notwithstanding this, high levels of VWF:Ag and FVIII:C have been reported in patients with MM but evidence on their correlation with either VTE or overall survival is less well defined. The etiology of these high levels is also unclear, with possible causative mechanisms including endothelial toxicity from chemotherapeutic agents in conjunction with increased bone marrow angiogenesis. Similarly, the role of VWF in the bone marrow microenvironment and vascular niche of MM remains unexplored, but given the novel biological functions defined for VWF in angiogenesis and cancer metastasis this remains an intriguing area for further research. MM remains incurable despite novel therapeutic advances, with almost all patients progressing to develop relapsed refractory disease. Crucially, despite current thromboprophylaxis approaches, patients with MM remain at high risk for thrombotic complications. Consequently, improved understanding of the crosstalk between MM and coagulation pathways in contributing to thrombosis associated with MM may identify novel prognostic biomarkers and therapeutic strategies to not only reduce thrombotic risk but also attenuate disease progression.
AUTHOR CONTRIBUTIONS
C. C., S. G., J. Q., and J.M.O’S. were involved in writing and reviewing the manuscript.
ACKNOWLEDGEMENT
Open access funding provided by IREL. [Correction added on 5th July 2022 after first online publication: IREL funding statement has been added.]
CONFLICTS OF INTEREST
J.M.O’S. has received research grant funding awards from LEO Pharma. C.C. has received an educational grant funding from Janssen. J.Q. has received honoraria from Takeda and Janssen. S.G. has received research grant funding from Celgene and BMS, Abbvie, and Janssen.