Prophylactic administration of glycoPEGylated factor IX provides protection and joint outcome superior to recombinant factor IX after induced joint bleeding
Abstract
Background
Following induced joint hemorrhage, hemophilia B results in the abnormal persistence of iron deposition, inflammation, and neovascularity of the synovial tissue, as well as deterioration of the bone articular surface and strength. Previously, we demonstrated that a factor IX (FIX) replacement protein with extended circulating FIX activity, glycoPEGylated FIX nonacog beta pegol (N9-GP), could improve synovial and osteochondral parameters in F9 knockout mice when administered after joint injury.
Objective
We explored the use of N9-GP prior to unilateral joint hemorrhage and compared to unmodified recombinant FIX (rFIX).
Methods
Pharmacodynamics, histology, and microcomputed tomography were used to assess the effects of prophylactic administration of glycoPEGylated FIX.
Results
In comparison to rFIX, N9-GP significantly improved soft tissue histological parameters, as well as bone outcome at 2 weeks post injury, while performing equally in reduction of blood present in the joint space assessed 1 day after injury.
Conclusions
These results indicate that, in comparison to rFIX, the prophylactic use of extended half-life FIX provides superior protection from bleeding-induced joint damage, manifested by improved correction of histologic parameters.
Essentials
- Bleeding-induced joint damage is the major morbidity of hemophilia.
- We compared recombinant factor IX (rFIX) and extended-activity factor IX (N9-GP) prophylaxis.
- Administration of N9-GP resulted in increased longevity of factor IX activity compared to rFIX.
- Prophylactic treatment with N9-GP improved joint outcome following injury compared to rFIX.
1 INTRODUCTION
Hemophilia is a rare genetic disorder resulting in excessive bleeding, caused by missing or defective factor VIII (FVIII) in hemophilia A or FIX in hemophilia B; these clotting factors are crucial in the ability to stop bleeding. In the clinical population, the most common sites of bleeding are musculoskeletal. Recurrent intraarticular bleeds result in chronic joint and bone degradation, which is the major morbidity of hemophilia. Traditionally, clinical hemophilia management entails treating bleeding episodes after they occur; however, the benefits of prophylactic maintenance are now substantiated by randomized clinical trials and recommended by the World Federation of Hemophilia.1-4
Several studies have shown the effect of rFIX in joint outcome models in F9 knockout (F9−/−) mice.5, 6 Nonacog beta pegol (N9-GP) is a recombinant FIX protein, modified by attaching a single 40-kDa polyethylene glycol (PEG) moiety for prolonged circulation of FIX zymogen, resulting in extended activity of FIX.7 Using a mouse model of hemophilic joint hemorrhage induced by a single puncture of the knee joint capsule, we previously showed that treatment of F9−/− mice with N9-GP following joint injury improved synovial and osteochondral outcome compared to treatment with unmodified rFIX.6 Here we hypothesized that protection from bleeding-induced joint sequelae would be superior after prophylactic administration of N9-GP compared to treatment with unmodified rFIX, since N9-GP maintains the hemostatic potential for a longer time, thus potentially improving outcome in the injured joint. To investigate this, we used the same histologic endpoints as in other joint bleeding studies; these endpoints have also been used in a cutaneous injury model in hemophilic mice.8-10 Furthermore, we examined the adjacent bone architecture.
2 METHODS
2.1 Animal care and study
Wild-type (WT) C57Bl/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). F9−/− mice were bred inhouse, backcrossed to the Jackson laboratory C57Bl/6J for 12 generations, and were 7 to 10 weeks old at the time of investigations. F9−/− mice were purchased from Taconic Biosciences Inc. (Borup, Denmark) for the pharmacokinetic study. Investigations were approved by the University of North Carolina Chapel Hill Institutional Animal Care and Use Committee and the internal Novo Nordisk ethical review committee. Mice were anesthetized using isoflurane/O2 for all procedures. Blood samples were collected from the retroorbital plexus into 1+9 parts 3.2% citrated sodium and the plasma was stored at −80°C. The intraarticular bleeding challenge was induced by puncture of the joint capsule using a Hamilton syringe with a 30.5-G needle as described.5, 6, 11-13 Following injury, all mice had access to Tylenol® gel for pain relief. Knee joints were collected by sectioning the femur and tibia/fibula 1 cm from the joint. Joints were fixed and decalcified using routine histologic procedures.
2.2 Recombinant factor IX proteins
Unmodified rFIX was BeneFIX® (Pfizer, Philadelphia, PA); N9-GP was supplied by Novo Nordisk A/S, Måløv, Denmark. Doses were 250 IU/kg except for rFIX in the pharmacokinetic study.
2.3 Pharmacokinetics
A total of 24 F9−/− mice received an intravenous (IV) dose of 353 IU/kg rFIX or 250 IU/kg N9-GP, and blood was sampled up to 7 days thereafter in a sparse sampling schedule. Factor IX activities in plasma samples were quantified by chromogenic BIOPHEN Factor IX(5) kit (HYPHEN Biomed SAS, Andrésy, France).
The FIX activity data were modeled by non-linear mixed-effects approach using Phoenix software (Phoenix NLME v6.4.0.768; Pharsight Corporation, St Louis, MO). Simulations were performed assuming that linear kinetics apply, using the model and estimated parameters: volume of distribution of both the central compartment (V1) and the periphery compartments (V2 and V3), clearance from the central compartment (CL1), intercompartment clearance (CL2), clearance from the periphery compartment (CL3), and interindividual variation (IIV).
2.4 Grading presence of blood in the joint
Sagittal knee joint sections obtained 24 h after injury were prepared and stained with hematoxylin and eosin (n = 5 or 6 mice per group). The joint space was outlined and quantified, as were the areas within the joint space containing free red blood cells, using Image J software.5, 14
2.5 Microscopic evaluation of synovium and cartilage
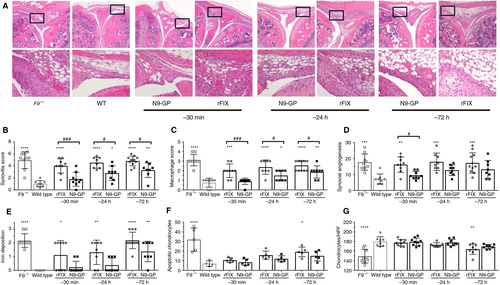
Sagittal knee joint sections obtained 14 days following injury were prepared and stained with hematoxylin and eosin. Hemophilic synovitis was graded 0 to 10 points for increasing pathology, according to the system validated by Valentino and colleagues,14 as previously described.5, 12 Additional sections were prepared with Prussian Blue stain for tissue iron and for immunohistochemical staining and grading for macrophages and endothelial cells, previously described by Hoffman et al.8 Joint cartilage pathology from Safranin O-stained sections was evaluated for total number of chondrocytes per high-powered field and TUNEL stain for number of chondrocytes undergoing apoptosis per high-powered field (n = 8-11 mice per group per stain).
2.6 Microcomputed tomography evaluation of bone
Mice were euthanized on day 14 following hemarthrosis and hind limbs were collected and fixed in formalin. Hind limbs were imaged using microcomputed tomography (μCT) at 10-μm resolution (μCT80: SCANCO Medical AG, Brüttisellen, Switzerland). Analysis of the injured limb was performed on the trabecular bone inferior to the growth plate of the proximal tibia and on the cortical bone nearing the midshaft of the femur. SCANCO analysis software was used to perform the histomorphometric analysis of trabecular morphology and bone mineral density, as previously described,15 and to derive a quantitative expression of the degree of disruption of the articular surface of the joint (n = 8-11 per group).
2.7 Statistics
The effect of extended rFIX was examined by one-way analysis of variance with Sidak's adjustment for multiple comparisons (10 pairwise comparisons; each compared to WT control, and N9-GP versus rFIX at each time point). An adjusted P value <.05 was considered a statistically significant difference. The analyses used Prism v6.00 for Windows, GraphPad Software, LaJolla, CA.
3 RESULTS AND DISCUSSION
Measured plasma FIX levels after administration of N9-GP or rFIX were comparable with previously published results.7 Pharmacokinetic modeling was performed to describe the levels of FIX activity at, and following, the time of injury: Evaluated by chromogenic activity assay, the pharmacokinetic profile of rFIX in mice showed multiexponential decline, best described by a three-compartment model. Lower in volume of distribution and clearance, the profile of N9-GP in mice was best described by a two-compartment model (Table 1). Levels of rFIX decreased to 5% of maximum plasma concentration (0.05 IU/mL) 18 h after dosing, whereas N9-GP reached the same level (5%) after 144 h (Figure 1A).
| Parameter | Estimate FIX (95% CI) | IIV FIX (%CV) | Estimate N9-GP (95% CI) | IIV N9-GP (%CV) |
|---|---|---|---|---|
| V (mL/kg) | 255 (221-288) | 70 | 164 (156-173) | 86 |
| V2 (mL/kg) | 362 (245-480) | 3898 (2260-5536) | ||
| V3 (mL/kg) | 186 (144-229) | |||
| CL (mL/[kg*h]) | 56.5 (51.6-61.4) | 30 | 1.24 (0.72-1.77) | 43 |
| CL2 (mL/[kg*h]) | 15.2 (12.0-18.4) | 2.87 (2.35-3.40) | ||
| CL3 (mL/[kg*h]) | 121 (69.4-171.5) |
- Abbreviations: CI, confidence interval; CL, CL2, and CL3, clearance from central, second and third compartment, respectively; CV, coefficient of variation; FIX, factor IX; IIV, interindividual variation; N9-GP, nonacog beta pegol; rFIX, unmodified recombinant factor IX; V1, volume of distribution of the central compartment; V2 and V3, volume of distribution in the second and third compartment, respectively.
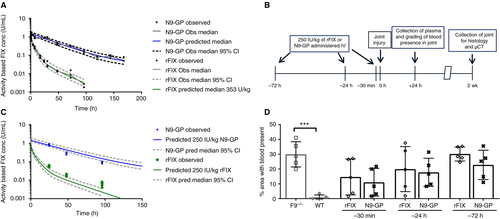
In order to assess the prophylactic use of N9-GP in comparison to rFIX in bleeding-induced injury, we performed joint hemorrhage in F9−/− mice or WT mice. At 30 min, 24 h, or 72 h prior to injury, the F9−/− mice were treated intravenously with either normal saline, 250 IU/kg of N9-GP, or 250 IU/kg of rFIX. Mice were euthanized at 24 h, for evaluation of plasma FIX levels and grading presence of blood in the joint space (Figure 1B). Plasma levels of N9-GP and rFIX were consistent with predicted values and showed quick decline in FIX activity for rFIX in contrast to N9-GP, where activity was preserved (Figure 1C).
Grading blood present in joints 24 h after injury showed significantly more blood in F9−/− animals compared to WT controls, where blood was minimal (P <.01). Though larger than in WT animals, the fraction of joint space occupied by blood in treated animals only reached statistical significance for later injuries (rFIX: P =.04 at 24 h; N9-GP: P =.01 at 72 h). Directly comparing N9-GP and rFIX treatment, no statistically significant difference was detected with the current group size (Figure 1D); extravascular stores of rFIX may contribute to hemostatic protection.16
In a second cohort, animals were administered IV N9-GP, rFIX, or saline prior to injury and euthanized 2 weeks later to evaluate the histopathologic and bone changes of the joint (Figure 1B), which returned a clearer picture of the differences between the rFIX and N9-GP therapeutic interventions. Antibodies against FIX were not detected in any animals (data not shown).
Histologic sections (Figure 2A) were evaluated and scored for synovitis, synovial angiogenesis, iron deposition, macrophage infiltration, chondrocyte death, and chondrocyte presence (Figure 2B-G). Consistent with previous publications, saline-treated WT animals consistently scored <2 points, while saline-treated F9−/− animals displayed scores ~5 points on the 0 to 10 Valentino hemophilic synovitis scale.5, 17 The N9-GP treatment was superior to rFIX in reducing synovitis scores at all treatment time points (P <.001 at 30 min; P <.05 at 24 h and 72 h). The N9-GP also provided superior protection from synovial angiogenesis (P <.05 at 30 min), and macrophage infiltration (P <.001 at 30 min, P <.05 at 24 h and 72 h). Iron deposition is considered related to the amount of blood and its duration in the joint space, potentially influenced by initial hemostasis as well as subsequent events. Iron deposition was not statistically different from WT levels in the N9-GP-treated F9−/− animals treated 30 min and 24 h prior to injury (Figure 2E), whereas rFIX-treated mice demonstrated significantly more iron deposition than WT mice at all treatment time points. As similar quantities of blood were found in the joint space 24 h after injury, the effect on iron deposition may reflect prevention of rebleeding events following injury. Alternatively, the similar low iron deposition in WT and N9-GP-treated mice may reflect iron clearance by macrophage phagocytosis.18 Total chondrocyte number and chondrocyte apoptosis approached WT levels with treatment of either compound at all three time points. In summary, in every histologic measurement, N9-GP treatment 30 min prior to injury normalized the histologic parameters to WT levels.
The dense cortical outer surface of the bone provides not only strength but a smooth articular surface for the joint. We used μCT analysis to evaluate the cortical bone thickness and the degree of disruption of the bone surface quantitatively. Two weeks after injury, untreated F9−/− mice display reduced cortical thickness and bone surface roughening (reduced smoothness ratio and increased cortical porosity) compared to untreated hemostatically normal mice (Figure 3A). The N9-GP treatment 72 h prior to injury significantly improved every measure of cortical bone health compared to rFIX treatment: smoothness ratio (P <.05), cortical thickness (P <.001), and cortical porosity (P <.001) (Figure 3B-D). Administered 30 min prior to injury, N9-GP treatment performed significantly better in terms of smoothness ratio (P <.05) and cortical thickness (P <.05). Furthermore, N9-GP treatment corrected cortical porosity to WT levels at every intervention time (Figure 3D).
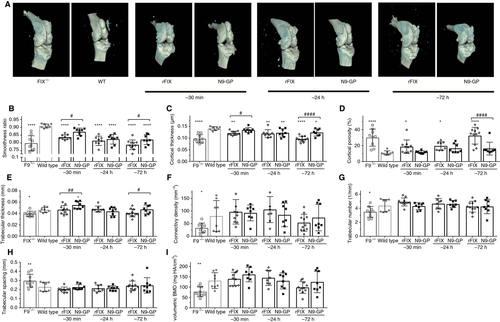
The trabecular structure on the interior of the bone imparts strength by dispersing the mechanical load through to the ends of the bone. Two weeks following injury, untreated F9−/− mice display reduced trabecular thickness, number, connectivity density, and increased trabecular spacing (Figure 3E-H). Prophylactic treatment with either N9-GP or rFIX at 30 min and 24 h before injury preserved trabecular bone structure approximately equally following joint hemorrhage. With pretreatment 72 h before injury, N9-GP showed slightly better protection of the trabecular thickness (P <.05); a similar trend was observed for connectivity density.
Untreated F9−/− mice in this study also experienced an acute loss of approximately 40% in volumetric bone mineral density (vBMD) of the proximal tibia of the injured joint compared with hemostatically normal mice (Figure 3I). The vBMD measures average mineral density over the entire trabecular compartment, including the space between the bone tissues, so this μCT measurement is similar to a clinical dual-energy X-ray absorptiometry (DEXA) bone density scan, but performed in 3-D space. Similar to other trabecular measurements, the 30-min and 24-h prophylactic treatments with either N9-GP or rFIX preserved vBMD. Albeit not statistically significant, in the 72-h pretreated group, there was a 22% reduction in vBMD in animals treated with rFIX, while only a 5% reduction in vBMD was observed in the N9-GP-treated group.
The prolonged period of high FIX exposure provided by N9-GP improved the treatment outcome over that seen with rFIX treatment in terms of soft tissue and cortical bone damage following injury. However, no difference was seen in the reduction of blood in the joint space 24 h following joint hemorrhage. Overall, in contrast to unmodified rFIX, N9-GP treatment prior to injury improved joint outcome to near WT levels when evaluated by iron staining, macrophage infiltration, and angiogenesis.
ACKNOWLEDGEMENTS
This study was supported in part by research funding from Novo Nordisk to T. A. Bateman and P. E. Monahan.
CONFLICTS OF INTEREST
P. E. Monahan during the conduct of these studies received research support through the University of North Carolina from Asklepios BioPharmaceutical and Novo Nordisk. He has received research support in the past from Baxter Healthcare, Novo Nordisk, Pfizer, and Prolor. He holds patents licensed to Asklepios, for which he receives royalties. He has received payment for consultation, services, and speaking for Asklepios, Chatham LLC, Baxter Healthcare, and Pfizer and has additionally consulted for Bayer, Novo Nordisk, and Biogen. Following the completion of these studies but during a portion of the time of manuscript preparation, he has been an employee of Baxalta (now Shire) and currently Spark Therapeutics. M. Ezban and C. D. Ley are employees and shareholders of Novo Nordisk. T. A. Bateman has received remuneration for consulting and speaker work for Baxter Health Care Corporation and research support from Novo Nordisk. S. Taves, M. L. Broberg, T. Knudsen, and P. B. Johansen were employees and shareholders of Novo Nordisk throughout the completion of these studies but have left Novo Nordisk during the process of manuscript preparation. The other authors state that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
J. Sun performed the studies, generated and adapted the methods, and reviewed the manuscript. M. L. Broberg performed the studies, adapted the methods, prepared the figures, and edited and reviewed the manuscript. E. W. Livingston performed the studies, adapted the methods, prepared the figures, and reviewed the manuscript. P. B. Johansen performed statistical analysis and reviewed the manuscript. C. D. Ley reviewed and interpreted data and edited and reviewed the manuscript and figures. T. Knudsen: provided a critical review of the study design and reviewed the manuscript. M. Ezban provided a critical review of the study design and edited and reviewed the manuscript. T. A. Bateman designed and oversaw the studies, reviewed and interpreted the data, and reviewed the manuscript. P. E. Monahan designed and oversaw all studies, reviewed and interpreted all data, and edited and reviewed the manuscript. S. Taves wrote and revised the manuscript, prepared the figures, performed statistical analysis, and reviewed and interpreted all data.




