Resolving DOAC interference on aPTT, PT, and lupus anticoagulant testing by the use of activated carbon
Abstract
Background
Direct oral anticoagulants (DOACs) affect laboratory coagulations tests. Activated carbon (AC) can be used for adsorption of DOACs during acute human intoxications.
Objectives
This study evaluates whether AC can also be used to resolve DOAC interference on in vitro clotting tests (prothrombin time [PT], activated partial thromboplastin time [aPTT], and lupus anticoagulant [LA] assays).
Patients/Methods
Interference on PT, aPTT, Liquid anti-FXa, DTI, and LA screening/confirmation (SCT and dRVVT) was determined by spiking citrated plasma from 5 adult controls with 0, 20, 40, 80, 120, or 160 mg/mL AC. DOAC concentrations, PT, and aPTT were compared before and after AC addition to citrated plasma from patients receiving DOACs (n = 29), low molecular weight heparin (n = 10), and coumarin (n = 10) therapy. Samples from 69 LA screened patients were compared before and after AC addition.
Results
A concentration of 20 mg/mL AC had the lowest interference and was selected for further experiments. After AC addition, all DOAC concentrations were below the limit of quantification in the 29 treated patients, except for 2 apixaban samples. AC removed DOAC interference on PT and aPTT but had no impact on results obtained during coumarin or low molecular weight heparin therapy. Of 15 LA samples with interference resulting from DOAC therapy, 14 samples became negative and 1 positive after AC addition. Interference from coumarin therapy was not resolved. All 19 LA negative samples remained negative. AC treatment of the negative pooled plasma was required to avoid false-negative LA results in 21 known LA-positive samples.
Conclusions
AC selectively removes DOAC interference on PT, aPTT, and LA assays.
Essentials
- Activated carbon (AC) can be used for adsorption of DOACs during acute human intoxications.
- This study evaluates whether AC can be used to resolve DOAC interference on coagulation tests.
- Our findings indicate that AC selectively removes DOAC interference on PT, aPTT, and LA assays.
- There was no significant interference of AC on the evaluated routine coagulation tests.
1 INTRODUCTION
Direct oral anticoagulants (DOACs) prolong PT, aPTT tests and affect other coagulations tests, including lupus anticoagulant (LA) screening.1, 2 The correct interpretation of these tests in patients taking DOACs is mandatory to prevent misclassification and subsequent clinical consequences.
Different reversing agents for individual DOACs have been developed for therapeutic use and display potential for in vitro laboratory testing. Idarucizumab (Boehringer-Ingelheim, Germany) neutralizes the effect of dabigatran in citrated plasma samples and has already been licensed in many countries.3, 4 Andexanet alpha (Portola Pharmaceuticals, CA) neutralizes factor Xa inhibitors in vivo and is under consideration by regulatory agencies.5 Ciraparantag (PER977, Perosphere, CT) is effective vs DOACs as well as other anticoagulants but is still undergoing phase III evaluation.6, 7 At the time of writing, these antidotes are not widely used for in vitro applications because of their high cost and limited availability. The latter two antidotes must also be accurately titrated into test plasmas to match the DOAC concentration as any excess could lead to interference.5, 6 Recently, DOAC-Stop (Haematex Research, Hornsby, Australia) and DOAC-Remove (5-Diagnostics, Basel, Switzerland) tablets have been marketed for laboratory use. They aim to remove all clinically relevant DOAC concentrations in vitro without significantly altering routine coagulation tests such as PT, activated partial thromboplastin time (aPTT), fibrinogen Clauss, and lupus anticoagulant (LA) assays.4, 8-11 The efficient adsorption of dabigatran by these tablets seems comparable to earlier experiments with activated carbon (AC).12-14 AC binds large amounts of dabigatran etexilate, the lipophilic prodrug of dabigatran, in water or human plasma in vitro.14 These findings were confirmed in vivo with apixaban and rivaroxaban. Administration of activated charcoal up to 6 hours after apixaban ingestion resulted in a reduced bioavailability and interruption of the enteroenteric recycling.12 AC administration up to 8 hours postdose resulted in a significant decrease in rivaroxaban exposure.15 The use of AC to reduce absorption of edoxaban has not been specifically studied but, based on the standard treatment of drug overdose and the data available from similar compounds, can be considered in case of an edoxaban overdose.13 In general, activated charcoal could be given within 1-2 hours after the ingestion of a DOAC overdose before the absorption of the drug is established by the intestine.13 From these data, it can thus be assumed that AC could also be used in vitro to overcome interference related to DOACs.
In this study, we sought to evaluate whether AC could be useful in the setting of a clinical hemostasis laboratory to resolve DOAC interference on clotting tests. We were focused on PT, aPTT, and LA assays.
2 METHODS
2.1 Preparation of samples spiked with AC
AC powder (Norit Carbomix, KELA Pharma, Sint-Niklaas, Belgium) was added directly to 500 μL or 1 mL citrated plasma samples to reach the desired concentration. Samples were gently mixed for 5 minutes on a rocker platform after addition of AC. The samples were centrifuged (2000 × g, 5 minutes) and the supernatant was transferred to a new tube. To fully clarify the supernatant and remove any residual particles, another centrifugal cycle (2000 × g, 5 minutes) was performed and the supernatant was transferred to another clean tube for subsequent analysis.
2.2 Determining interference of AC on routine coagulation assays in healthy adult controls
To determine whether AC interferes on routine coagulation assays as well as on anti-FXa, DTI, and LA tests, 2 mL of fresh citrated plasma from five healthy adult controls were spiked with increasing AC concentrations (0, 20, 40, 80, 120, and 160 mg/mL). Samples spiked with AC were treated as previously described. Pre- and post-AC samples for PT and aPTT were treated and analyzed fresh (results obtained <4 hours after venipuncture). Pre- and post-AC samples for anti-FXa, DTI, and LA were stored at −80°C before analysis. Samples were thawed at 37°C (water bath) for 5 minutes, treated with AC and analyzed (results obtained <2 hours after thawing). Comparisons between pre- and post-AC results were only made for samples that received the same treatment and centrifugal cycles. Then, we calculated the difference between the results obtained with and without the addition of AC. The concentration of AC which induced no significant analytical differences (based on the between-run analytical variability (CVA), see Statistical analysis3.4) would be selected for further experiments.
PT (ReadiPlasTin, Werfen, Bedford, MA), aPTT (SynthASil, Werfen), anti-Xa activity (Liquid Anti-Xa, Werfen), anti-IIa activity (direct thrombin inhibitor-DTI, Werfen), TT (Thrombin Time, Werfen), SCT (HemosIL Silica Clotting Time, Werfen), and dRVVT (HemosIL dRVVT, Werfen) screen/mix/confirm assays were determined on each sample on the ACL TOP 550 (Werfen). Anti-Xa and anti-IIa activity assays were calibrated for apixaban (Werfen), rivaroxaban (Werfen), edoxaban (Hyphen BioMed, Neuville-sur-Oise, France), and dabigatran (Werfen), respectively. The limit of quantification (LoQ) of apixaban, rivaroxaban, edoxaban, and dabigatran assays were 15, 20, 20, and 20 ng/mL, respectively. Performance and evaluation of internal quality control (iQC) was done according to local procedures.
2.3 Quantification of DOAC concentrations in patient samples before and after addition of AC
To quantify DOAC concentrations before and after AC addition in plasma from patients receiving DOAC therapy, citrated plasma samples from 29 patients were stored at −80°C. Samples were gathered by query of the laboratory informatics system. Patients received the following DOACs: dabigatran (n = 4), rivaroxaban (n = 16), apixaban (n = 7), and edoxaban (n = 2). The anti-Xa activity (Liquid Anti-Xa, Werfen) and anti-II activity (DTI, Werfen) were performed for the respective DOAC before and after addition of 20 mg/mL AC. Ethical approval for residual sample collection and usage was obtained at Onze-Lieve-Vrouwziekenhuis Aalst-Asse-Ninove under study number B126201837413 (Onze-Lieve-Vrouwziekenhuis study number 2018/073).
2.4 Resolving DOAC interference on routine coagulation and LA assays by AC in patient samples
To determine whether AC can resolve DOAC interference on PT (ReadiPlasTin) and aPTT (SynthASil), fresh citrated plasma samples of 39 patients receiving DOACs were tested: dabigatran etexilate (n = 4), rivaroxaban (n = 23), apixaban (n = 10), and edoxaban (n = 2). A total of 20 mg AC was added to 1 mL citrate plasma (<4 hours after sampling) and results compared to the initial PT and aPTT result without AC. Samples were gathered by query of the laboratory informatics system. To evaluate whether AC selectively removes DOACs, fresh citrated plasma samples (<4 hours after sampling) from patients receiving low molecular weight heparins (LMWH) (n = 10) or coumarin (n = 10) therapy were also evaluated using the same procedure.
To determine whether AC can resolve DOAC interference on LA assays, platelet-free citrated plasma from 69 patients was stored at −80°C from patients with positive LA (n = 21), negative LA (n = 19), and false-positive LA resulting from interference with DOAC therapy (n = 26), and false-positive LA from interference with coumarin therapy (n = 3). However, as LA screening was never performed on the DOAC and coumarin samples before initiation of therapy, results of the LA assays were assumed to be false positive. After thawing at 37°C, 20 mg AC was added to 1 mL plasma and processed as previously described. Subsequent LA testing was performed on ACL TOP 550 according to International Society on Thrombosis and Haemostasis guidelines before and after addition of AC16: screening, mixing, and confirmation in silica clotting time (HemosIL SCT) and diluted Russell's viper venom test (HemosIL dRVVT). Cutoff screen, mix, and confirm ratios were determined using the 99th percentile of 50 healthy adult controls; under the age of 50 years, equal distribution of men and women, no medication, and normal inflammatory parameters.16 Results are expressed as ratios of patient-to-negative pooled plasma (NPP) results for screen, mix and confirm tests. The laboratory-specific cutoff ratio is 1:2 and 1:2 for SCT and dRVVT screening tests, respectively, and 1.09 and 1.08 for SCT and dRVVT mixing tests, respectively. Normalized ratios of screening over confirm are analyzed, with a laboratory-specific cutoff value of 1, 2 for both tests. In addition, the ratio ([screen ratio-confirm ratio]/screen ratio) × 100 is calculated with a cutoff of 10%.16 Samples were defined as LA positive when screening results were above the cutoff, and demonstrated a clear LA pattern in mixing and/or confirmation tests as described in the International Society on Thrombosis and Haemostasis guidelines.16 False-positive results are determined as screening results above the cutoff, but without a clear LA pattern in mixing and/or confirmation tests as described in the criteria. TT, aPTT, and PT are always performed for additional interpretation of the results.
2.5 Statistical analysis
Statistical testing, receiver operator characteristic (ROC)-curve analysis, and graphical representation of the data was done using GraphPad Prism (version 5.02, Windows). A nonparametric Wilcoxon matched pairs test was used for significance testing of paired data. The Westgard statistical process rule 1-3SD based on CVA was used to detect clinically significant changes before and after AC treatment.17 The long-term between-run CVA (%) derived from measurements of the normal level iQC material for each assay was used to describe the between-run analytical variability for the following assays: 2.0% for PT (mean 12.7 seconds, n = 100 measurements), 1.3% for aPTT (30 seconds, n = 70), 1.9% for SCT screen (44.8 seconds, n = 30), 2.0% for SCT mix (43.4 seconds, n = 26), 1.9% for SCT confirm (36.6 seconds, n = 29), 5.6% for dRVVT screen (36.4 seconds, n = 8), 6.6% for dRVVT mix (35.5 seconds, n = 8), 1.9% for dRVVT confirm (33.6 seconds, n = 8), and 4.3% for TT (26.2 seconds, n = 21). Because no long-term CVA data were available for the anti-Xa and DTI assays, the claimed between-run analytical CVAs from the manufacturer product insert were used: 1.9% for anti-Xa and 3.6% for DTI.
3 RESULTS
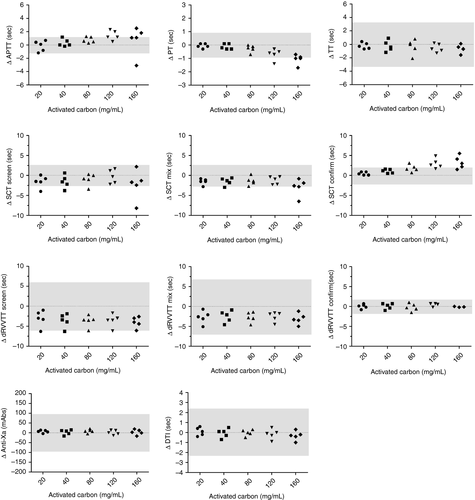
Increasing AC concentrations (>40 mg/mL) led to an increase in aPTT and SCT confirm results. PT results decreased after 80 mg/mL AC. A decrease was noted in SCT screen, SCT mix, dRVVT screen, and dRVVT mix results even with the lowest AC concentration. No significant drift or shift was noted in TT, anti-Xa, anti-II, and dRVVT confirm results (Figure 1). Differences only became significantly different vs baseline at higher AC concentrations (>80 mg/mL) based on the CVA with 1-3SD Westgard rule.17 One sample from the same healthy adult control was outside of the significance level for SCT screen, SCT mix, and dRVVT screen but had a normal cutoff ratio before and after AC addition on each assay, thus indicating no clinical impact. An AC concentration of 20 mg/mL therefore induced no clinically significant differences and led to the lowest Δ values before and after AC addition. This concentration was selected for further experiments.
To investigate the ability of a concentration of 20 mg/mL AC to suppress relevant DOACs concentrations, we quantified the DOACs before and after addition of 20 mg/mL AC in stored citrated plasma from 29 treated patients. Median and ranges for the different DOACs in the patients’ samples before and after addition of AC are displayed in Table 1. All DOAC concentrations were below the LoQ of the anti-Xa and DTI assays after AC addition, except for 2 apixaban samples. The resulting concentrations were only slightly above the LoQ (16.4 and 15.5 ng/mL vs LoQ 15 ng/mL). DTI and anti-Xa kit controls and calibrators were also compared before and after addition of AC (data not shown). All control and calibrator values dropped below the LoQ of the different DOACs, except the high calibrator for apixaban (pre-AC: 514.4 ng/mL; post-AC: 15.1 ng/mL; expected target value: 507 ng/mL).
| No activated carbon | +20 mg/mL activated carbon | |||||
|---|---|---|---|---|---|---|
| n | LoQ (mg/mL) | Median (mg/mL) | Range (mg/mL) | Median (mg/mL) | Range (mg/mL) | |
| Dabigatran | 4 | 20 | 131.0 | 122.3-139.7 | 0.0 | 0.0-0.4 |
| Rivaroxaban | 16 | 20 | 112.4 | 55.7-190.7 | 4.2 | 0.0-18.6 |
| Apixaban | 7 | 15 | 317.0 | 171.6-500.2 | 7.6 | 1.4-16.4 |
| Edoxaban | 2 | 20 | 131.0 | 122.3-139.7 | 11.4 | 10.3-12.4 |
- Abbreviation: LoQ, limit of quantification.
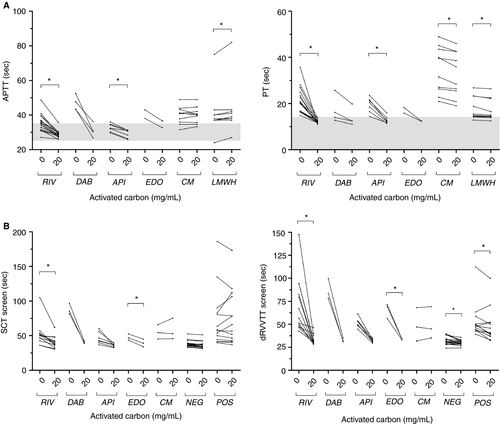
Considering that 20 mg/mL of AC efficiently neutralizes therapeutic DOACs concentrations, our next steps were: (a) to evaluate whether 20 mg/mL AC can overcome the effect of DOAC on PT/aPTT and (b) to analyze whether AC effect is selective toward DOACs. AC led to an important drop in PT (second) and aPTT (second) results of the 29 treated patients (Figure 2A). Results are displayed in Table 2. Differences were statistically significant for rivaroxaban and apixaban. Values for dabigatran and edoxaban were statistically not analyzable because of the limited sample size (Figure 2A). aPTT and PT results remained (slightly) elongated for four patients: three patients had increased inflammatory parameters suggesting interference from an acute-phase response and one patient was suspected to have an acquired vitamin K deficiency resulting from malnutrition. In addition, PT and aPTT results from both apixaban samples with post-AC concentrations above the LoQ (16.4 and 15.5 ng/mL), normalized after addition of AC. These results suggest that AC is able to remove DOAC interference on PT and aPTT. We further investigated the specificity and sensitivity for DOAC detection in conjunction with PT/aPTT (second) “normalization.” Based on ROC-curve analysis, a ratio ACtreated (second)/ACuntreated (second) <0.885 for PT and <0.945 for aPTT corresponds to a sensitivity/specificity of 0.96/0.96 and 0.93/0.96 for detection of DOAC presence, respectively. The AUC of the corresponding ROC-curve was 0.999 (95% confidence interval, 0.994-1.003) for PT and 0.995 (95% confidence interval, 0.985-1.006) for aPTT.
| aPTT (s, median and range) | PT (s, median and range) | ||||||
|---|---|---|---|---|---|---|---|
| n | Baseline | +20 mg/mL AC | P value | Baseline | +20 mg/mL AC | P value | |
| Dabigatran | 4 | 45.5 (43.3-52.5) | 30.6 (26.8-36.2) | 0.1250 | 15.1 (12.7-25.7) | 12.4 (11.0-19.8) | 0.1250 |
| Rivaroxaban | 16 | 35.3 (27.1-48.6) | 28.6 (26.0-35.7) | 0.0005a | 20.3 (14.8-35.7) | 12.0 (11.0-13.8) | 0.0005a |
| Apixaban | 7 | 32.3 (29.7-36.0) | 30.7 (25.8-31.3) | 0.0223a | 20.4 (14.4-23.5) | 12.4 (11.4-15.9) | 0.0156a |
| Edoxaban | 2 | 40.5 (38.0-43.0) | 34.7 (32.7-36.6) | 0.5000 | 17.0 (15.8-18.2) | 12.4 (12.3-12.4) | 0.5000 |
| Coumarin | 10 | 40.9 (31.5-49.0) | 40.2 (33.3-48.9) | 0.8948 | 35.6 (20.9-48.9) | 32.2 (19.0-46.0) | 0.0004a |
| Heparin | 10 | 37.5 (24.0-75.0) | 38.5 (27.0-82.0) | 0.0213a | 15.1 (12.8-26.8) | 14.8 (12.5-26.4) | 0.0058a |
- a P < 0.05.
In patients receiving coumarin therapy (n = 10), no statistical decrease was noted on aPTT before and after addition of AC. A significant but clinically unimportant decrease was found on PT before and after addition of 20 mg/mL AC (Figure 2A). In patients receiving LMWH treatment (n = 10), a significant but clinically unimportant decrease was noted on PT. In the same patients, a significant but clinically unimportant increase was noted on aPTT (Figure 2A). Median values and ranges are displayed in Table 2. These results indicate that AC selectively removes DOAC interference on PT and aPTT assays. Interestingly, of the 10 patients receiving heparin therapy that were treated with AC, 9 had higher levels than the PT cutoff (>14 seconds). We investigated whether these patients had underlying pathological conditions: six patients had an inflammatory condition (including one with associated impaired liver function), one patient had impaired liver function, and one patient received simultaneous coumarin therapy. Only in one of the nine patients (PT = 14.4 seconds), we could not determine an underlying pathological condition that could explain this increase.
Because DOAC therapy leads to false-positive LA results, we decided to explore the ability of AC to resolve the interference. The influence on the SCT and dRVVT screen assays is displayed in Figure 2B. A significant decrease was found for both assays in rivaroxaban and apixaban treated patients. Results for positive and negative LA samples were also significantly decreased with dRVVT screen. Interestingly, of the 26 samples with interference because of DOAC therapy and who were originally assumed to be false positive, 25 samples were reclassified as LA negative and 1 LA positive after AC addition (Table 3). The latter patient was suspected to have transient LA because she had no prior history of thrombosis and took no anticoagulant medication. Unfortunately, this could not be confirmed because no follow-up sample was received and other antibodies (anti-cardiolipin and anti-β2 microglobulin) were not evaluated. Interference resulting from coumarin therapy was not resolved by AC addition and, consequently, the three included samples remained LA false positive. Of note, as LA screening was never performed on these three samples before initiation of coumarin therapy, results of the LA assays were assumed to be false positive. All 19 LA negative samples remained negative. Of the 21 LA positive samples, the first 9 samples were analyzed without treatment of the NPP used to calculate cutoff ratios. Of the nine LA positive samples, three became LA false negative after AC addition. These three samples were weak LA positive results approaching the cutoff ratio, indicating that treatment of the NPP may be important for interpretation of the results. An additional 12 LA positive samples were tested with and without a pretreated NPP; no false-negative results were obtained with pretreated NPP, whereas two false-negative results were found without pretreatment. Of the 26 evaluated DOAC samples, 11 could be retested using treated NPP. The classification did not change vs untreated NPP: 11 LA false-positive-results were reclassified as LA negative (Table 3).
| Interpretation before AC addition | NPP treated? | Interpretation after AC addition | Total | ||
|---|---|---|---|---|---|
| LA positive | LA negative | LA interference | |||
| LA positive | No | 16 | 5 | 0 | 21 |
| LA positive | Yes | 12 | 0 | 0 | 12 |
| LA negative | No | 0 | 19 | 0 | 19 |
| LA interference from DOAC therapy | No | 1 | 25 | 0 | 26 |
| LA interference from DOAC therapy | Yes | 0 | 11 | 0 | 11 |
| LA interference from coumarin therapy | No | 0 | 0 | 3 | 3 |
- Abbreviation: NPP, negative pooled plasma.
4 DISCUSSION
The impact of DOACs on coagulation tests is a known issue and is dependent on type of DOAC, DOAC plasma concentration, and the type of coagulometer and reagent.2 Different reversing agents for individual DOACs have already been developed for therapeutic use but are not widely available for in vitro laboratory use.3-7 Recently, Exner et al8 demonstrated that the adsorbing agent DOAC-Stop is effective in removing DOACs from test plasmas with minimal effect on clotting tests such as aPTT and PT. These findings were confirmed by other authors.4, 9-11 The specificity for removing DOACs could be attributed to their molecular size. Almost every protein, clotting factor, antibody, and physiological inhibitor affecting coagulation tests have a molecular size above ±30 000 Da. The pore capacity of the DOAC-Stop adsorbing agent is ±5000 Da, which allows differentiation of lower molecular weight anticoagulant drugs such as DOACs.8 The experimental findings of DOAC-Stop are reminiscent of in vivo experiments with AC on DOAC intoxications.12-15 This led us to investigate whether AC could also be used to resolve in vitro DOAC interference on routine coagulation tests. AC adsorbs drugs present in the gastrointestinal tract, thereby reducing their systemic absorption and enhancing elimination by interruption of enteroenteric and hepatic recycling.18 Extensive clinical evidence proves AC's effectiveness in treating overdoses with a large variety of drugs (eg digoxin, phenytoin, and carbamazepine);18 however, no specific relationship between chemical drug characteristics and maximum AC adsorptive capacity has yet been identified.18
Our results indicate that 20 mg/mL AC in citrated plasma selectively removes DOAC interference on PT, aPTT, dRVVT, and SCT assays. At this dose, no clinically significant interference was observed on routine coagulation assays by spiking plasma from healthy adult controls. Because the imprecision of weighing and the knowledge that DOAC-Stop and DOAC-Remove contain 20 mg/mL active substance,4, 8-11 a concentration of 10 mg/mL AC was not evaluated in our study. This is a limitation of our study; further experiments could demonstrate whether this concentration is also sufficient to remove DOAC interference with less impact on the evaluated tests (e.g., on dRVVT); however, this concentration was sufficient to suppress relevant DOAC concentrations in citrated plasma from 29 treated patients. All DOAC concentrations were below the LoQ of the anti-Xa and DTI assays after AC addition, except for 2 apixaban samples with post-AC concentrations only slightly above the LoQ (16.4 and 15.5 ng/mL vs LoQ 15 ng/mL). In addition, DTI and anti-Xa kit controls and calibrators were quantified before and after addition of AC. These controls and calibrators were developed by spiking lyophilized human plasma (NPP) with apixaban, rivaroxaban, edoxaban, or dabigatran at various concentrations (personal communication; Werfen and Hyphen BioMed). Only the high calibrator for apixaban failed to drop below the LoQ. Based on previous findings in literature and on the validation studies performed by Werfen on PT (HemosIL ReadiPlasTin) and APTT (HemosIL SynthASil) reagents, ±15 ng/mL apixaban is unlikely to have a significant effect on routine coagulation assays (personal communication; Werfen). In addition, AC seems effective to remove interference from samples containing <500 ng/mL apixaban based on our experiments. Unfortunately, we did not test patient samples with ±15 and >500 ng/mL apixaban to evaluate whether both concentrations affect PT, aPTT, dRVVT, and SCT results before and after addition of AC.
AC had no clinically significant effect on plasma samples obtained from patients receiving heparin (LMWH) or coumarin therapy. The effect of AC on adsorption of LMWH remains largely unknown but some adsorption may be expected as LMWH formulations approach a molecular size of 5000 Da.19 This might be confirmed by the significant, yet slight and clinically unimportant, drop in aPTT results after AC addition to plasma from patients receiving LMWH therapy. AC can be used for in vivo gastro-intestinal absorption of acute coumarin intoxications.20 However, reminiscent of the pharmacological properties of vitamin K antagonists, in vivo inhibition of the hepatic synthesis of vitamin K-dependent clotting factors is not immediately reversible in plasma by an in vitro adsorbing reagent.
A prolonged PT and/or aPTT in a patient receiving DOAC therapy should be expected and is likely drug- and reagent-related.2 Nevertheless, PT or aPTT may be prolonged from other causes than DOAC treatment (e.g., factor deficiencies, von Willebrand disease, vitamin K deficiency, antibiotic use, lipoglycopeptide antibiotics use, lupus anticoagulant, compromised liver function, acute phase response, dys-/afibrinogenemia, or consumption coagulopathy, (such as disseminated intravascular coagulation).21 Our results indicate that AC could be used to selectively remove DOAC interference on PT and aPTT results. No significant clinical effect of AC on elongated PT and/or aPTT results by LMWH and coumarin therapy was found; however, we did not evaluate DOAC spiked negative patient samples or DOAC spiked QC materials for PT, aPTT, SCT, and dRVVT. We could therefore not assess the recovery on a larger DOAC concentration range for the evaluated tests. Nevertheless, we believe that AC might have broader applications for laboratory use. Especially in the setting of uncertain anticoagulation combinations (e.g., patient admitted to the emergency department), AC-treated patient plasma might offer a fast, easy, and unambiguous guide as to what agent might be present in a patient plasma with an abnormal elongated PT or aPTT.2 Although confirmation is needed in a larger prospective study, the high sensitivity and specificity values indicated that AC in conjunction with PT/aPTT testing could be used for accurate discrimination of DOAC presence in citrated plasma. Consequently, more rapid testing for underlying plasma defects, possibly contributing to an urgent bleeding or clotting problem, could be carried out.
DOACs lead to false-positive LA results due to high screen/confirmation assay ratios.2 A combination of Taipan snake venom time with ecarin clotting time, which are direct prothrombin activators unaffected by FXa inhibition, has therefore been proposed for the detection of LA in orally anticoagulated patients with vitamin K antagonists or rivaroxaban.16, 22, 23 Addition of AC, an easily obtainable and inexpensive pharmaceutical product, to citrated plasma might therefore seem an elegant and cost-efficient manner to resolve DOAC interference on LA testing. DOAC-Stop has already been evaluated on healthy adult controls and caused no false-positive results.8, 9 Our results with AC confirm these findings because no false-positive samples were detected in LA-negative samples. In addition, we evaluated citrated plasma from DOAC-treated, coumarin-treated, and LA-positive patients. DOAC interference was fully resolved, whereas this was not the case for plasma derived from coumarin-treated patients. Because determination of adequate cutoff ratios using NPP is of vital importance for accurate LA testing results,16 we noted some false negatives in LA-positive samples with untreated NPP. A similar finding was also reported by Platton et al,11 who reported a weakly positive LA sample that became false negative (calculated using the percentage correction between dRVVT screen and confirm) after treatment with DOAC-Stop. We confirmed the importance of NPP by performing additional experiments on LA-positive and DOAC interference samples using AC-treated and untreated NPP. These results demonstrated that AC treatment of the NPP is required to avoid false-negative results. Unfortunately, not all (assumedly) LA false-positive DOAC samples could be retested using treated NPP because of insufficient residual sample volume; therefore, the results of the 14 other DOAC samples that were reclassified to LA negative using untreated NPP must be interpreted with caution. When AC-treated and AC-untreated plasma/NPP are compared in the same run, we propose that both samples undergo the same centrifugation cycles to account for their influence on LA assays. Although the effect of freezing citrated plasma for coagulation testing is well known, the effect of multiple centrifugation cycles is not.8, 24 Ideally, AC should only be used for LA assays when the presence of a DOAC is known or suspected by the treating physician (eg noted on the laboratory request form or by query of the medical health records).
5 CONCLUSION
Our preliminary data are encouraging and suggest that AC can selectively resolve DOAC interference on PT, aPTT, and SCT/dRVVT LA assays. More experiments with AC are needed on different reagents, thrombophilia assays (e.g., activated protein-C resistance, protein S, protein C, antithrombin), and coagulometers to consolidate its use in thrombophilia testing.
AUTHOR CONTRIBUTIONS
G. Frans, P. Meeus, and E. Bailleul designed the study. G. Frans performed the experiments and wrote the manuscript. Analysis of the data was performed by all authors. The manuscript was critically reviewed and approved by all authors.
ACKNOWLEDGEMENTS
We thank Niels Devisch and Charlotte Bouckaert (Werfen Belgium, Instrumentation Laboratory International, Zaventem, Belgium) for their help in setting up the anti-Xa and DTI assays and their critical feedback on the performed experiments.
CONFLICT OF INTERESTS
G. Frans, P. Meeus and E. Bailleul declare that they have no conflict of interest.




