Risk of thromboembolic and bleeding outcomes following hematological cancers: A Danish population-based cohort study
Abstract
Background
Therapeutic advances have improved survival after hematological cancers. In turn, patients may be at increased risk of thromboembolic and bleeding events.
Objectives
To examine the risks of myocardial infarction (MI), ischemic stroke, venous thromboembolism (VTE), and bleeding requiring hospital contact in patients with hematological cancers.
Methods
We conducted a Danish population-based cohort study (2000-2013). We identified all adult hematological cancer patients and sampled a general population comparison cohort in a 1:5 ratio matched by age, sex, previous thromboembolic events, bleeding, and solid cancer. Ten-year absolute risks of thromboembolism and bleeding were calculated and hazard ratios (HRs) were computed, controlling for matching factors.
Results
Among 32 141 hematological cancer patients, the 10-year absolute risk of any thromboembolic or bleeding complication following hematological cancer was 19%: 3.3% for MI, 3.5% for ischemic stroke, 5.2% for VTE, and 8.5% for bleeding. Except among patients with myeloid leukemia, acute lymphoid leukemia, or myelodysplastic syndrome, the risk of thromboembolic events surpassed that of bleeding. The hematological cancer cohort overall was at increased risk for MI [HR = 1.36, 95% confidence interval (CI): 1.25-1.49], ischemic stroke (HR = 1.22, 95% CI: 1.12-1.33), VTE (HR = 3.37, 95% CI: 3.13-3.64), and bleeding (HR = 2.39, 95% CI: 2.26-2.53) compared with the general population.
Conclusions
Approximately 2 of 10 hematological cancer patients experienced MI, ischemic stroke, VTE, or bleeding requiring hospital contact within 10 years. The hematological cancer cohort had higher hazards of MI, ischemic stroke, VTE, and bleeding requiring hospital contact than a general population comparison cohort.
Essentials
- The tradeoff between thrombosis and bleeding in hematological cancer patients is a challenge.
- We examined risks of cardiovascular disease (CVD) and bleeding in hematological cancer patients.
- In patients with hematological cancer, 10-year risk of CVD or bleeding was 19%.
- Both CVD and bleeding were more common in hematological cancer patients than in the general population.
1 INTRODUCTION
Hematological malignancies comprise a heterogeneous group of cancers, with different etiologies, treatments, and prognoses. Based on data from CONCORD-3 Programme, age-standardized 5-year survival is in the range of 30% to 50% for myeloid malignancies and 40% to 70% for lymphoid malignancies.1 Survival following most hematological cancers has improved during the past 50 years,2, 3 resulting in an expanding population of cancer survivors and a call for studies investigating long-term outcomes, such as thromboembolic and bleeding complications.
Several studies have suggested that some hematological cancers are associated with both arterial and venous thromboembolic events.4-11 These associations are presumably driven by factors such as specific treatment modalities, shared risk factors, infections, immobilization, cancer-associated prothrombotic states, clinicians’ reluctance to treat patients with optimal doses of antithrombotic drugs inherent to their elevated bleeding risks, and tumor compression of vessel walls.12
However, prior research focused on selected types of hematological cancers, were limited by small sample sizes,4, 9 assessed only venous or arterial thromboembolic events,5, 7-11 and did not include data on bleeding risk. Bleeding risk may be substantially elevated in patients with hematological cancers due to pathophysiological changes in the hemostatic system and presence of other risk factors for bleeding, e.g., advancing age and abnormal renal function.
The association of hematological cancer with thromboembolic and bleeding outcomes is of major public health importance and may guide clinicians in their choice of antineoplastic treatment and also antiplatelet and anticoagulant treatment. Thus, we examined the 10-year risk of myocardial infarction, ischemic stroke, VTE, and bleeding requiring hospital contact among patients with various types of hematological cancer. We also compared their risk with that of a matched comparison cohort from the general population.
2 METHODS
2.1 Design and setting
We conducted this population-based cohort study using linked data from medical databases covering all Danish hospitals. Denmark provides tax-funded health care to all its residents, and its registry databases contain extensive individual-level health care information.13 All databases have been linkable since 1968, when the Civil Registration System (CRS) began assigning a unique and permanent personal registration number to each Danish resident.13 The CRS also maintains up-to-date information on migration and on the civil and vital status of the population, allowing for nearly complete follow-up.13
2.2 Cohort of hematological cancer patients
We used the Danish Cancer Registry (DCR) to identify all patients ≥15 years old diagnosed with incident hematological cancers in Denmark between 1 January 2000 and 30 November 2013.14 We classified the cancers into the following subgroups: Hodgkin lymphoma, non-Hodgkin lymphoma, acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), chronic lymphocytic leukemia (CLL), multiple myeloma, myeloproliferative neoplasms, myelodysplastic syndrome, and “other” (Table S1). The index date was defined as the date of cancer diagnosis.
The DCR has recorded the diagnoses of all incident cancers in Denmark since 1943.14 Mandatory reporting to the DCR was first implemented in 1987. Completeness of cancers in the DCR has been consistently high, with 89% of tumors verified morphologically.15, 16 Cancers are coded according to the International Classification of Diseases, Tenth Revision (ICD-10). Registration is based on notification forms completed by pathology, forensic medicine, and other hospital departments. These manually recorded forms are supplemented by other cases identified by computerized links to death certificates and the Danish National Patient Registry (DNPR).17 The DNPR has recorded all non-psychiatric discharge diagnoses from inpatient admissions since 1977 and from outpatient clinics and emergency room contacts since 1995.17
Cancer therapy, administered in specialized hospital settings,16 has been registered in the DNPR since 1999.17 We used the DNPR to identify patients’ initial therapy, defined as cancer therapy provided during 180 days before and after registration in the DCR. Cancer therapy was ascertained using outpatient and inpatient records of chemotherapy, radiotherapy, bone marrow transplantation, and immunotherapy. Immunotherapy was defined as any treatment with antibody, immunomodulatory (IMID), or biologically modifying agents. Information on glucocorticoids is not recorded in the DNPR or DCR. We also obtained information on redeemed prescriptions of acetylsalicylic acid, non-aspirin non-steroidal antiinflammatory drugs (NSAIDs), platelet inhibitors, and oral anticoagulants provided during the 180 days before and after registration of cancer diagnoses. These data became available in the National Health Service Prescription Database starting from July 2004 onward.18 Cancer patients most often receive low-molecular-weight heparin directly from the hospitals; thus data on this agent are not captured by the Prescription Database.
2.3 General population comparison cohort
We constructed a comparison cohort from the general population using the CRS, the DNPR, and the DCR. For each hematological cancer patient, we randomly selected up to five individuals from the general population without hematological cancer as of the patient's cancer diagnosis date (defined as the index date). Each comparison cohort member was sampled with replacement,19 matched by sex, by birth year, and by hospital-based inpatient or outpatient history of myocardial infarction, ischemic stroke, VTE, bleeding requiring hospital contact, and prevalent underlying solid cancer.
2.4 Study outcomes
We used the DNPR to identify diagnoses of myocardial infarction, ischemic stroke, VTE, and bleeding requiring hospital contact, recorded after the index date (codes are provided in Table S1). Since 1994, all diagnoses in the DNPR have been coded using ICD-10. Myocardial infarction, ischemic stroke, and bleeding requiring hospital contact are managed in inpatient settings. In the DNPR, every inpatient discharge is recorded using one primary diagnosis, which represents the main reason for hospitalization. We thus used primary DNPR diagnoses to identify myocardial infarction, ischemic stroke, and bleeding requiring hospital contact. The admission date was used to define the date of these outcomes. Ischemic stroke included diagnoses of both ischemic and unspecified stroke, since more than two-thirds of unspecified stroke diagnoses are known to be ischemic.20
Venous thromboembolism are treated in hospital inpatient, outpatient, and emergency department settings. We ascertained VTE events in the DNPR by primary inpatient or outpatient clinic diagnoses of pulmonary embolism, deep venous thrombosis, or other VTE diagnoses. The first hospital admission/outpatient visit date was used to define the VTE date. We excluded VTEs registered only in emergency room departments because they frequently represent working diagnoses in that setting, with high rates of clinical misclassification.21
Bleeding requiring hospital contact was defined as the first inpatient diagnosis of cerebral bleeding (intracerebral and subarachnoid hemorrhage), gastrointestinal bleeding, anemia from acute bleeding, and bleeding from the respiratory tract. We also included diagnoses of hemorrhagic cystitis (bleeding from the urinary tract), due to its increased risk in patients receiving radiotherapy, cyclophosphamide, or bone marrow transplantation.
2.5 Comorbidity
We used inpatient and outpatient discharge records in the DNPR from 1977 until the index date, to identify comorbid conditions included in the Charlson Comorbidity Index (CCI) (with hematological cancers excluded from the index).22 We then defined the level of comorbidity for each patient as follows: CCI score of 0 (no comorbidity), CCI score of 1 to 2 (moderate comorbidity), or CCI core of 3+ (severe comorbidity). We also obtained information on underlying non-metastatic or metastatic cancer from the DNPR, but because these conditions are closely related to our study outcomes, we addressed non-hematological cancer comorbidity separately as well as part of the CCI score.
2.6 Statistical analyses
Follow-up started on the index date and continued until the date of first myocardial infarction, stroke, VTE, bleeding requiring hospital contact, emigration, 10 years of follow-up (main analysis), death, or 30 November 2013. Multiple study outcomes for the same individual were accepted, but only the first event for each outcome of interest was counted.
We computed absolute risks for all thromboembolic events and bleeding requiring hospital contact combined (any/none) and separately for myocardial infarction, ischemic stroke, VTE, and bleeding requiring a hospital contact, using the cumulative incidence risk function, treating death as a competing risk.23 For each study outcome, we also computed incidence rates per 1000 person-years and HRs with 95% confidence intervals (CIs), using stratified Cox proportional hazards regression within matching factors.24 Absolute risks, incidence rates, and HRs were calculated by type of hematological cancer. The main analysis provides 10-year estimates. Because the risk of most outcomes changes over time, we also conducted analyses for four separate follow-up periods (0 to 6 months, >6 to 12 months, >1 to 5 years, and 5 to 10 years).
As the risk of cardiovascular and bleeding outcomes may vary considerably for categories for a given cancer, we performed subgroup analyses for non-Hodgkin lymphoma patients with indolent versus aggressive lymphomas and for AML patients with or without acute promyelocytic (APL) type. We also stratified our main analysis by age groups (15 to 54 years, 55 to 69 years, and 70+ years) and index year categories (2000 to 2006 and 2007 to 2013). As initial chemotherapy and radiation therapy could modify the risk of study outcomes, we stratified the analyses by initial cancer treatment. To avoid conditioning on the future, we initiated follow-up 180 days after the hematological cancer diagnosis date and corresponding matching date for comparators. To assess first-time events only, in a sensitivity analysis, we excluded patients who had any record of thromboembolic or bleeding events in the DNPR before the index date.
All analyses were conducted using SAS version 9.4 (SAS Inst. Inc., Cary, NC). The project was approved by the Danish Data Protection Agency (record number 1-16-02-1-08). The data, analytic methods, and other study materials will not be made available to other researchers for purposes of reproducing the results or replicating the study. Such disclosure would conflict with regulations for use of Danish health care data.
3 RESULTS
We identified 32 141 patients in Denmark aged 15 years or older with incident hematological cancers (55% male), and 160 252 matched individuals from the general population (Table 1 and Table S2). Among the hematological cancer patients, 30% were diagnosed with non-Hodgkin lymphoma, 15% with CLL, 14% with myeloproliferative neoplasms, 13% with multiple myeloma, 9% with AML, 8% with myelodysplastic syndrome, while less frequent cancers were Hodgkin lymphoma (4%), ALL (1%), CML (3%), and others (3%). As expected, the age distribution varied greatly among cancer types. More than half of Hodgkin lymphoma and ALL patients were aged <55 years, while 66% to 91% of patients with other cancer types were aged 55 years or older. Initial cancer therapy was registered for 54% of hematological cancer patients. Approximately 39% to 45% of the overall hematological cancer cohort and matched members of the general population had hospital-based diagnoses of CCI comorbidities as of their index date. The most frequent comorbidity in the hematological cancer cohort was underlying cancer (non-metastatic solid tumor: 11%, metastatic disease: 1%). A history of thromboembolic and bleeding events before the index date was present in 13% and 11% to 12%, respectively, in both the hematological cancer and general population cohort (Table 1). The hematological cancer cohort was more likely to receive antiplatelet and anticoagulant treatment than the general comparison cohort: 30% versus 24% for acetylsalicylic acid, 6% versus 5% for platelet inhibitors, and 8% versus 6% for oral anticoagulants, respectively (Table S3).
| Hodgkin lymphoma (n = 11 194) | Non-Hodgkin lymphoma (n = 9511) | Acute myeloid leukemia (n = 2887) | Acute lymphoid leukemia (n = 419) | Chronic myeloid leukemia (n = 863) | Chronic lymphocytic leukemia (n = 4861) | Multiple myeloma (n = 4257) | Myeloproliferative neoplasms (n = 4380) | Myelodysplastic syndrome (n = 2695) | Others (n = 1074) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Age at cancer diagnosis (y) | ||||||||||
| 15-54 | 776 (65.0) | 1712 (18.0) | 619 (21.4) | 236 (56.3) | 293 (34.0) | 412 (8.5) | 432 (10.1) | 824 (18.8) | 236 (8.8) | 211 (19.6) |
| 55-69 | 240 (20.1) | 3675 (38.6) | 848 (29.4) | 80 (19.1) | 260 (30.1) | 1789 (36.8) | 1621 (38.1) | 1464 (33.4) | 648 (24.0) | 391 (36.4) |
| 70+ | 178 (14.9) | 4124 (43.4) | 1420 (49.2) | 103 (24.6) | 310 (35.9) | 2660 (54.7) | 2204 (51.8) | 2092 (47.8) | 1811 (67.2) | 472 (43.9) |
| Female | 509 (42.6) | 4231 (44.5) | 1301 (45.1) | 193 (46.1) | 379 (43.9) | 2014 (41.4) | 1890 (44.4) | 2332 (53.2) | 1129 (41.9) | 402 (37.4) |
| Cancer diagnosis year | ||||||||||
| 2000-2006 | 345 (28.9) | 2943 (30.9) | 1434 (49.7) | 167 (39.9) | 434 (50.3) | 2344 (48.2) | 1949 (45.8) | 1846 (42.1) | 1110 (41.2) | 464 (43.2) |
| 2007-2013 | 849 (71.1) | 6568 (69.1) | 1453 (50.3) | 252 (60.1) | 429 (49.7) | 2517 (51.8) | 2308 (54.4) | 2534 (57.9) | 1585 (58.8) | 610 (56.8) |
| CCI score | ||||||||||
| 0 | 882 (73.9) | 5415 (56.9) | 1698 (58.8) | 289 (69.0) | 541 (62.7) | 2826 (58.1) | 2375 (55.8) | 2139 (48.8) | 1176 (43.6) | 612 (57.0) |
| 1-2 | 232 (19.4) | 3066 (32.2) | 886 (30.7) | 104 (24.8) | 245 (28.4) | 1489 (30.6) | 1356 (31.9) | 1670 (38.1) | 1002 (37.2) | 332 (30.9) |
| 3+ | 80 (6.7) | 1030 (10.8) | 303 (10.5) | 26 (6.2) | 77 (8.9) | 546 (11.2) | 526 (12.4) | 571 (13.0) | 517 (19.2) | 130 (12.1) |
| Previous events | ||||||||||
| Myocardial infarction | 28 (2.3) | 503 (5.3) | 157 (5.4) | 14 (3.3) | 28 (3.2) | 286 (5.9) | 236 (5.5) | 322 (7.4) | 229 (8.5) | 56 (5.2) |
| Ischemic stroke | 33 (2.8) | 407 (4.3) | 135 (4.7) | 14 (3.3) | 29 (3.4) | 183 (3.8) | 219 (5.0) | 369 (8.4) | 182 (6.8) | 39 (3.6) |
| VTE | 23 (1.9) | 303 (3.2) | 94 (3.3) | 10 (2.4) | 19 (2.2) | 127 (2.6) | 143 (3.4) | 295 (6.7) | 124 (4.6) | 49 (4.6) |
| Bleeding requiring hospital contact | 65 (5.4) | 1018 (10.7) | 328 (11.4) | 20 (4.8) | 94 (10.9) | 456 (9.4) | 522 (12.3) | 578 (13.2) | 487 (18.1) | 116 (10.8) |
| Prevalent solid tumor | ||||||||||
| Non-metastatic solid tumor | 67 (5.6) | 1121 (11.8) | 359 (12.4) | 33 (7.9) | 82 (9.5) | 554 (11.4) | 479 (11.3) | 486 (11.1) | 407 (15.1) | 127 (11.8) |
| Metastatic solid tumor | 6 (0.5) | 130 (1.4) | 32 (1.1) | 1 (0.2) | 10 (1.2) | 45 (0.9) | 52 (1.2) | 29 (0.7) | 26 (1.0) | 10 (0.9) |
| Initial treatmenta | ||||||||||
| Chemotherapy | 1054 (88.3) | 5949 (62.5) | 1610 (55.8) | 319 (76.1) | 490 (56.8) | 611 (12.6) | 2451 (57.6) | 1836 (41.9) | 367 (13.6) | 427 (39.8) |
| Radiotherapy | 409 (34.3) | 1901 (20.0) | 139 (4.8) | 40 (9.5) | 11 (1.3) | 62 (1.3) | 664 (15.6) | 20 (0.5) | 52 (1.9) | 118 (11.0) |
| BMT | 2 (0.2) | 48 (0.5) | 63 (2.2) | 23 (5.5) | 1 (0.1) | 1 (0.0) | 194 (4.6) | 0 (0.0) | 17 (0.6) | 9 (0.8) |
| Immunotherapy | 123 (10.3) | 4435 (46.6) | 389 (13.5) | 109 (26.0) | 139 (16.1) | 348 (7.2) | 1974 (46.4) | 423 (9.7) | 194 (7.2) | 156 (14.5) |
- Abbreviations: BMT, bone marrow transplantation; CCI, Charlson Comorbidity Index; VTE, venous thromboembolism.
- a Includes multiple cancer treatment modalities per subject within 180 days before and after cancer registration in the Danish Cancer Registry.
3.1 Risks for thromboembolic events and bleeding requiring hospital contact
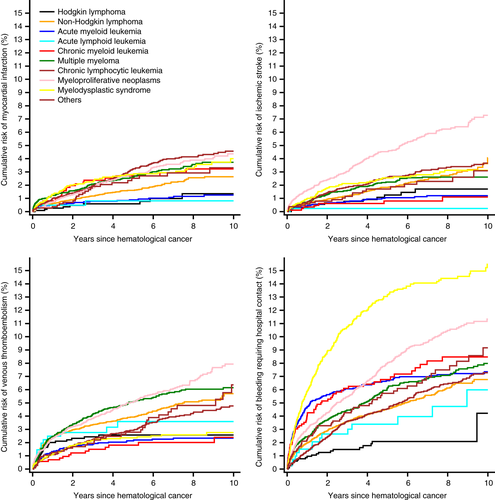
Bleeding requiring hospital contact and VTE accounted for most complications during follow-up (Figure 1, Tables 2 and 3). Except among patients with myeloid leukemia, acute lymphoid leukemia, and myelodysplastic syndrome, the 10-year risk of thromboembolic events surpassed that of bleeding (Table 4).
| Myocardial infarction | Ischemic stroke | |||||
|---|---|---|---|---|---|---|
| No. events/person-years | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | No. events/person-years | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | |
| Comparison cohort | 63/27 084 | 2.33 (1.82–2.98) | Reference | 55/27 133 | 2.03 (1.56–2.64) | Reference |
| Hodgkin lymphoma | 9/4807 | 1.87 (0.97–3.60) | 1.09 (0.53-2.25) | 13/4795 | 2.71 (1.58–4.67) | 1.64 (0.86-3.13) |
| Comparison cohort | 1001/206 131 | 4.86 (4.56–5.17) | Reference | 1187/205 729 | 5.77 (5.45–6.11) | Reference |
| Non-Hodgkin lymphoma | 153/31 500 | 4.86 (4.15–5.69) | 1.08 (0.90-1.29) | 172/31 464 | 5.47 (4.71–6.35) | 1.02 (0.86-1.21) |
| Comparison cohort | 401/75 006 | 5.35 (4.85–5.90) | Reference | 481/74 859 | 6.43 (5.88–7.03) | Reference |
| Acute myeloid leukemia | 28/4868 | 5.76 (3.98–8.34) | 1.63 (1.06-2.51) | 28/4880 | 5.74 (3.96–8.31) | 1.46 (0.95-2.24) |
| Comparison cohort | 34/10 577 | 3.21 (2.30–4.50) | Reference | 28/10 565 | 2.65 (1.83–3.84) | Reference |
| Acute lymphoid leukemia | 3/1171 | 2.55 (0.82–7.91) | 1.01 (0.29-3.51) | 1/1171 | 0.85 (0.12–6.04) | 1.00 (0.12-6.04) |
| Comparison cohort | 95/23 802 | 3.99 (3.26–4.88) | Reference | 133/23 741 | 5.60 (4.73–6.64) | Reference |
| Chronic myeloid leukemia | 23/3343 | 6.87 (4.57–10.34) | 2.65 (1.57-4.46) | 7/3379 | 2.07 (0.99–4.35) | 0.66 (0.30-1.47) |
| Comparison cohort | 792/124 062 | 6.38 (5.95–6.84) | Reference | 937/123 732 | 7.57 (7.10–8.07) | Reference |
| Chronic lymphocytic leukemia | 159/20 703 | 7.68 (6.57–8.97) | 1.31 (1.09-1.57) | 129/20 801 | 6.20 (5.22–7.37) | 0.95 (0.78-1.15) |
| Comparison cohort | 655/107 824 | 6.07 (5.63–6.56) | Reference | 735/107 567 | 6.83 (6.36–7.35) | Reference |
| Multiple myeloma | 116/11 871 | 9.77 (8.14–11.72) | 1.87 (1.50-2.33) | 88/11 895 | 7.40 (6.01–9.12) | 1.30 (1.02-1.65) |
| Comparison cohort | 579/105 969 | 5.46 (5.04–5.93) | Reference | 740/105 579 | 7.01 (6.52–7.53) | Reference |
| Myeloproliferative neoplasms | 126/18 947 | 6.65 (5.59–7.92) | 1.30 (1.06–1.59) | 209/18 629 | 11.22 (9.80–12.85) | 1.74 (1.47-2.04) |
| Comparison cohort | 455/59 194 | 7.69 (7.01-8.43) | Reference | 536/58 999 | 9.09 (8.35-9.89) | Reference |
| Myelodysplastic syndrome | 74/6625 | 11.17 (8.89-14.03) | 1.62 (1.24-2.12) | 64/6649 | 9.63 (7.54-12.31) | 1.28 (0.96-1.69) |
| Comparison cohort | 161/26 071 | 6.17 (5.29–7.21) | Reference | 170/26 108 | 6.51 (5.60–7.57) | Reference |
| Other hematological cancers | 24/3501 | 6.85 (4.59–10.22) | 1.22 (0.77-1.92) | 20/3514 | 5.70 (3.68–8.83) | 1.21 (0.73-1.98) |
- Abbreviations: CI, confidence interval.
- a Per 1000 person-years.
- b Controlled for matching factors by study design.
| Venous thromboembolism | Bleeding requiring hospital contact | |||||
|---|---|---|---|---|---|---|
| No. events/person-years | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | No. events/person-years | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | |
| Comparison cohort | 41/27 169 | 1.51 (1.11–2.05) | Reference | 98/27 047 | 3.62 (2.97–4.42) | Reference |
| Hodgkin lymphoma | 28/4721 | 5.93 (4.09–8.58) | 4.19 (2.50-7.01) | 21/4770 | 4.40 (2.87–6.75) | 1.44 (0.88-2.37) |
| Comparison cohort | 748/206 863 | 3.62 (3.37–3.88) | Reference | 1690/204 997 | 8.24 (7.86–8.65) | Reference |
| Non-Hodgkin lymphoma | 366/30 915 | 11.84 (10.69–13.12) | 3.79 (3.29-4.35) | 412/31 073 | 13.26 (12.04–14.60) | 1.87 (1.66-2.10) |
| Comparison cohort | 276/75 445 | 3.66 (3.25–4.12) | Reference | 719/74 505 | 9.65 (8.97–10.38) | Reference |
| Acute myeloid leukemia | 59/4758 | 12.40 (9.61–16.00) | 4.49 (3.14-6.42) | 187/4734 | 39.49 (34.21–45.57) | 7.79 (6.17-9.84) |
| Comparison cohort | 27/10 565 | 2.56 (1.75–3.73) | Reference | 45/10 553 | 4.27 (3.19–5.71) | Reference |
| Acute lymphoid leukemia | 13/1159 | 11.24 (6.52–19.35) | 7.42 (3.07-17.97) | 15/1147 | 13.13 (7.92–21.79) | 8.02 (3.51-18.35) |
| Comparison cohort | 74/23 851 | 3.10 (2.47–3.90) | Reference | 174/23 595 | 7.37 (6.36–8.55) | Reference |
| Chronic myeloid leukemia | 16/3355 | 4.77 (2.92–7.79) | 1.93 (1.07-3.51) | 61/3282 | 18.59 (14.46–23.89) | 4.19 (2.94-5.97) |
| Comparison cohort | 464/124 818 | 3.72 (3.39–4.07) | Reference | 1207/123 354 | 9.79 (9.25–10.35) | Reference |
| Chronic lymphocytic leukemia | 164/20 642 | 7.94 (6.82–9.26) | 2.18 (1.80-2.63) | 249/20 679 | 12.04 (10.64–13.63) | 1.29 (1.11-1.49) |
| Comparison cohort | 403/108 580 | 3.71 (3.37–4.09) | Reference | 1069/107 226 | 9.97 (9.39–10.59) | Reference |
| Multiple myeloma | 208/11 553 | 18.00 (15.71–20.61) | 5.53 (4.51-6.77) | 249/11 712 | 21.26 (18.78–24.08) | 2.72 (2.32-3.19) |
| Comparison cohort | 456/106 152 | 4.30 (3.92–4.71) | Reference | 1004/105 237 | 9.54 (8.97–10.15) | Reference |
| Myeloproliferative neoplasms | 235/18 568 | 12.66 (11.14–14.39) | 3.12 (2.63-3.70) | 349/18 520 | 18.84 (16.97–20.93) | 2.22 (1.94-2.53) |
| Comparison cohort | 264/59 621 | 4.43 (3.92-5.00) | Reference | 782/58 658 | 13.33 (12.43-14.30) | Reference |
| Myelodysplastic syndrome | 60/6612 | 9.08 (7.05-11.70) | 2.21 (1.62-3.02) | 323/6344 | 50.94 (45.67-56.80) | 4.71 (4.01-5.52) |
| Comparison cohort | 87/26 254 | 3.31 (2.69–4.09) | Reference | 240/25 949 | 9.25 (8.15–10.49) | Reference |
| Other hematological cancers | 38/3465 | 10.97 (7.99–15.08) | 3.48 (2.27-5.34) | 63/3440 | 18.30 (14.29–23.42) | 2.46 (1.80-3.35) |
- Abbreviations: CI, confidence interval.
- a Per 1000 person-years.
- b Controlled for matching factors by study design.
| 0-6 months | >6-12 months | >1-5 years | >5-10 years | 0-10 years | |
|---|---|---|---|---|---|
| Myocardial infarction | |||||
| Hodgkin lymphoma | 0.09 (0.01–0.47) | 0.19 (0.04–0.67) | 0.36 (0.10–1.01) | 0.95 (0.25–2.69) | 1.35 (0.60–2.69) |
| Non-Hodgkin lymphoma | 0.46 (0.34–0.62) | 0.16 (0.09–0.28) | 1.28 (1.00–1.62) | 1.73 (1.17–2.47) | 2.63 (2.19–3.14) |
| Acute myeloid leukemia | 0.42 (0.23–0.72) | 0.14 (0.03–0.48) | 0.97 (0.48–1.79) | 1.94 (0.72–4.28) | 1.26 (0.84–1.82) |
| Acute lymphoid leukemia | 0.49 (0.10–1.65) | (.–.) | 0.49 (0.05–2.52) | (.–.) | 0.82 (0.23–2.26) |
| Chronic myeloid leukemia | 0.70 (0.30–1.47) | 0.14 (0.01–0.77) | 2.43 (1.39–3.95) | 0.95 (0.19–3.16) | 3.23 (2.07–4.80) |
| Chronic lymphocytic leukemia | 0.63 (0.43–0.88) | 0.40 (0.24–0.63) | 2.55 (2.05–3.15) | 2.20 (1.48–3.15) | 4.57 (3.86–5.35) |
| Multiple myeloma | 1.00 (0.73–1.34) | 0.28 (0.14–0.53) | 1.90 (1.41–2.52) | 3.34 (2.08–5.06) | 3.73 (3.06–4.49) |
| Myeloproliferative neoplasms | 0.37 (0.22–0.59) | 0.38 (0.22–0.62) | 2.28 (1.79–2.86) | 2.29 (1.46–3.42) | 4.37 (3.57–5.27) |
| Myelodysplastic syndrome | 0.72 (0.45–1.10) | 0.73 (0.43–1.17) | 2.16 (1.50–3.02) | 4.39 (2.03–8.16) | 4.00 (3.03–5.18) |
| Other hematological cancers | 0.28 (0.08–0.79) | 0.13 (0.01–0.69) | 2.91 (1.75–4.53) | 1.61 (0.41–4.51) | 3.31 (2.09–4.96) |
| Ischemic stroke | |||||
| Hodgkin lymphoma | 0.26 (0.07–0.72) | (.–.) | 1.12 (0.52–2.15) | 0.57 (0.12–1.91) | 1.71 (0.92–2.93) |
| Non-Hodgkin lymphoma | 0.41 (0.30–0.56) | 0.23 (0.14–0.36) | 1.57 (1.25–1.94) | 3.78 (2.14–6.14) | 4.06 (2.98–5.40) |
| Acute myeloid leukemia | 0.32 (0.16–0.59) | 0.21 (0.06–0.58) | 1.54 (0.88–2.52) | 0.75 (0.15–2.51) | 1.20 (0.81–1.73) |
| Acute lymphoid leukemia | 0.24 (0.02–1.29) | (.–.) | (.–.) | (.–.) | 0.24 (0.02–1.29) |
| Chronic myeloid leukemia | 0.12 (0.01–0.63) | 0.29 (0.06–0.98) | 0.57 (0.16–1.60) | 0.53 (0.05–2.72) | 1.10 (0.46–2.28) |
| Chronic lymphocytic leukemia | 0.48 (0.31–0.71) | 0.26 (0.14–0.45) | 2.13 (1.68–2.66) | 1.75 (1.10–2.64) | 3.66 (3.03–4.38) |
| Multiple myeloma | 0.48 (0.30–0.73) | 0.28 (0.14–0.52) | 2.11 (1.59–2.75) | 1.12 (0.53–2.13) | 2.62 (2.10–3.21) |
| Myeloproliferative neoplasms | 1.14 (0.85–1.49) | 0.51 (0.33–0.78) | 3.20 (2.61–3.88) | 4.19 (2.98–5.69) | 7.28 (6.23–8.45) |
| Myelodysplastic syndrome | 0.60 (0.36–0.96) | 0.44 (0.22–0.81) | 2.31 (1.61–3.20) | 2.09 (0.85–4.35) | 3.13 (2.40–4.01) |
| Other hematological cancers | 0.38 (0.13–0.92) | 0.25 (0.05–0.86) | 1.54 (0.76–2.82) | 2.79 (0.95–6.35) | 3.09 (1.80–4.93) |
| Venous thromboembolism | |||||
| Hodgkin lymphoma | 1.38 (0.82–2.19) | 0.69 (0.31–1.37) | 0.63 (0.24–1.41) | (.–.) | 2.57 (1.74–3.65) |
| Non-Hodgkin lymphoma | 1.79 (1.54–2.08) | 0.76 (0.58–0.98) | 1.94 (1.59–2.35) | 3.08 (2.04–4.44) | 5.70 (4.93–6.55) |
| Acute myeloid leukemia | 0.84 (0.56–1.24) | 0.90 (0.51–1.50) | 2.02 (1.25–3.08) | 1.07 (0.30–2.92) | 2.33 (1.79–2.99) |
| Acute lymphoid leukemia | 1.96 (0.93–3.68) | 0.68 (0.14–2.27) | 1.68 (0.45–4.54) | (.–.) | 3.59 (1.98–5.93) |
| Chronic myeloid leukemia | 0.58 (0.22–1.30) | 0.14 (0.01–0.79) | 1.44 (0.68–2.72) | 1.06 (0.19–3.65) | 2.40 (1.36–3.93) |
| Chronic lymphocytic leukemia | 0.73 (0.52–1.00) | 0.40 (0.24–0.63) | 2.09 (1.65–2.61) | 3.04 (2.17–4.13) | 4.77 (4.04–5.60) |
| Multiple myeloma | 1.58 (1.24–2.00) | 1.14 (0.81–1.56) | 3.65 (2.94–4.47) | 3.38 (2.09–5.13) | 6.15 (5.33–7.04) |
| Myeloproliferative neoplasms | 1.46 (1.13–1.85) | 0.93 (0.66–1.27) | 3.00 (2.43–3.66) | 4.41 (3.20–5.91) | 7.94 (6.86–9.11) |
| Myelodysplastic syndrome | 0.75 (0.48–1.14) | 0.44 (0.22–0.80) | 1.83 (1.23–2.61) | 1.46 (0.44–3.72) | 2.76 (2.09–3.58) |
| Other hematological cancers | 0.95 (0.49–1.70) | 0.38 (0.11–1.05) | 2.10 (1.15–3.55) | 7.40 (3.82–12.53) | 6.37 (4.27–9.02) |
| Bleeding requiring hospital contact | |||||
| Hodgkin lymphoma | 0.60 (0.27–1.19) | 0.39 (0.14–0.97) | 1.25 (0.61–2.31) | 2.70 (0.21–12.05) | 4.22 (1.30–9.96) |
| Non-Hodgkin lymphoma | 1.39 (1.16–1.64) | 0.70 (0.53–0.92) | 3.28 (2.82–3.79) | 3.84 (2.83–5.09) | 6.76 (5.99–7.59) |
| Acute myeloid leukemia | 3.41 (2.79–4.12) | 2.46 (1.75–3.37) | 4.98 (3.70–6.53) | 3.65 (1.73–6.74) | 7.35 (6.35–8.45) |
| Acute lymphoid leukemia | 1.24 (0.47–2.73) | 0.34 (0.03–1.76) | 2.83 (1.16–5.77) | 5.98 (1.43–15.46) | 5.98 (3.09–10.21) |
| Chronic myeloid leukemia | 3.04 (2.04–4.35) | 1.33 (0.66–2.44) | 3.49 (2.17–5.30) | 3.11 (1.37–6.04) | 8.48 (6.50–10.77) |
| Chronic lymphocytic leukemia | 0.75 (0.54–1.03) | 0.66 (0.45–0.94) | 3.67 (3.07–4.33) | 4.45 (3.38–5.74) | 7.28 (6.37–8.27) |
| Multiple myeloma | 1.86 (1.49–2.31) | 0.72 (0.47–1.07) | 5.12 (4.27–6.08) | 5.71 (3.83–8.10) | 7.99 (6.98–9.08) |
| Myeloproliferative neoplasms | 2.14 (1.74–2.61) | 0.96 (0.69–1.30) | 5.53 (4.74–6.40) | 5.08 (3.80–6.62) | 11.34 (10.12–12.64) |
| Myelodysplastic syndrome | 3.75 (3.07–4.52) | 2.62 (1.99–3.39) | 10.94 (9.34–12.67) | 9.06 (5.33–13.98) | 15.48 (13.76–17.28) |
| Other hematological cancers | 1.52 (0.91–2.40) | 0.88 (0.39–1.73) | 4.83 (3.27–6.82) | 7.08 (3.65–12.03) | 9.17 (6.84–11.90) |
- (.–.) Insufficient for estimates.
During median follow-up of 2.5 years [interquartile range (IQR): 0.7 to 5.4 years], 10-year risks were 3% for myocardial infarction and 4% for ischemic stroke among patients with hematological cancers. The risk of MI was highest among CLL patients (5%), while the risk of ischemic stroke was highest among patients with myeloproliferative neoplasms (7%). Absolute risks were lower following ALL (as was expected given the younger age of this population); 10-year risks were 0.8% for myocardial infarction and 0.2% for ischemic stroke (Figure 1).
During median follow-up of 2.4 years (IQR: 0.7 to 5.3 years), the 10-year risk of VTE was 5%. The VTE risk was highest after myeloproliferative neoplasms (8%) and multiple myeloma (6%) and lowest after AML (2. %). Particularly among ALL patients, VTE risk increased rapidly within the first year after cancer diagnosis (1-year absolute risk = 3%, Figure 1).
During median follow-up of 2.4 years (IQR: 0.7 to 5.3 years), the 10-year risk of bleeding requiring hospital contact was highest after myelodysplastic syndrome (15%), myeloproliferative neoplasms (12%), and CML (8%), while it was lowest after Hodgkin lymphoma (4%, Figure 1). Bleeding diagnoses mostly involved the gastrointestinal system (47%), followed by the respiratory tract (20%, of which epistaxis accounted for 88% of these diagnoses), urinary tract (16%), brain (12%), and anemia from acute bleeding (5%).
3.2 Relative risks for thromboembolic events and bleeding requiring hospital contact
Compared with the general population, hematological cancer patients were at increased risk for myocardial infarction (HR: 1.36, 95% CI: 1.25 to 1.49, Table 2), ischemic stroke (HR: 1.22, 95% CI: 1.12 to 1.33), VTE (HR: 3.37, 95% CI: 3.13 to 3.64, Table 3), and bleeding requiring hospital contact (HR: 2.39, 95% CI: 2.26 to 2.53). The magnitude of HRs varied considerably by type of malignancy.
3.3 Additional analyses
Cumulative risk and hazard ratios for different follow-up periods are reported in Tables 4 and 5. Although some estimates were relatively imprecise owing to paucity of events, risks of cardiovascular and bleeding events were generally higher in the short term than in the long term. Associations between specific types of hematological cancers and cardiovascular or bleeding events tapered off for some conditions (e.g., non-Hodgkin lymphoma was associated with an increased risk of myocardial infarction only during the first 6 months post diagnosis). Still, other hematological cancer patients remained at increased risk of an event (e.g., patients with myeloproliferative neoplasms were at increased risk of VTE in all follow-up periods).
| 0-6 months | >6-12 months | >1-5 years | >5-10 years | |
|---|---|---|---|---|
| Myocardial infarction | ||||
| Hodgkin lymphoma | 1.25 (0.14–11.18) | 3.33 (0.56–19.95) | 0.66 (0.20–2.23) | 1.28 (0.36–4.58) |
| Non-Hodgkin lymphoma | 1.95 (1.37–2.78) | 0.66 (0.36–1.22) | 0.92 (0.71–1.20) | 1.06 (0.69–1.63) |
| Acute myeloid leukemia | 1.79 (0.92–3.46) | 0.77 (0.17–3.46) | 1.99 (0.91–4.36) | 1.47 (0.52–4.17) |
| Acute lymphoid leukemia | 2.50 (0.46–13.65) | 0.00 (0.00–.) | 0.72 (0.09–6.00) | (.–.) |
| Chronic myeloid leukemia | 7.15 (2.01–25.41) | 1.49 (0.15–14.32) | 2.62 (1.34–5.12) | 1.05 (0.22–4.98) |
| Chronic lymphocytic leukemia | 2.04 (1.33–3.12) | 1.27 (0.74–2.17) | 1.19 (0.93–1.52) | 1.19 (0.76–1.86) |
| Multiple myeloma | 5.05 (3.29–7.77) | 0.83 (0.41–1.69) | 1.28 (0.91–1.79) | 2.44 (1.37–4.34) |
| Myeloproliferative neoplasms | 1.47 (0.84–2.56) | 1.25 (0.71–2.21) | 1.39 (1.06–1.82) | 1.02 (0.65–1.62) |
| Myelodysplastic syndrome | 2.63 (1.50–4.60) | 1.72 (0.95–3.12) | 1.26 (0.84–1.88) | 1.70 (0.76–3.80) |
| Other hematological cancers | 0.91 (0.27–3.14) | 0.38 (0.05–2.95) | 1.67 (0.94–2.97) | 0.85 (0.25–2.96) |
| Ischemic stroke | ||||
| Hodgkin lymphoma | 3.00 (0.72–12.55) | (.–.) | 2.38 (1.00–5.63) | 0.69 (0.15–3.23) |
| Non-Hodgkin lymphoma | 1.49 (1.03–2.14) | 0.94 (0.56–1.59) | 0.87 (0.68–1.11) | 1.12 (0.76–1.64) |
| Acute myeloid leukemia | 1.54 (0.73–3.26) | 0.71 (0.21–2.39) | 1.91 (1.01–3.60) | 1.19 (0.25–5.62) |
| Acute lymphoid leukemia | 2.50 (0.23–27.57) | (.–.) | (.–.) | (.–.) |
| Chronic myeloid leukemia | 0.56 (0.07–4.39) | 1.87 (0.36–9.67) | 0.56 (0.17–1.88) | 0.42 (0.05–3.31) |
| Chronic lymphocytic leukemia | 1.27 (0.80–2.00) | 0.72 (0.38–1.35) | 1.04 (0.80–1.34) | 0.68 (0.43–1.07) |
| Multiple myeloma | 1.53 (0.93–2.53) | 0.87 (0.43–1.77) | 1.42 (1.04–1.96) | 0.91 (0.40–2.06) |
| Myeloproliferative neoplasms | 3.42 (2.37–4.93) | 1.33 (0.81–2.20) | 1.37 (1.09–1.73) | 2.04 (1.39–2.99) |
| Myelodysplastic syndrome | 1.81 (1.02–3.21) | 0.93 (0.45–1.91) | 1.23 (0.83–1.81) | 1.27 (0.48–3.41) |
| Other hematological cancers | 2.86 (0.84–9.76) | 0.92 (0.20–4.19) | 0.91 (0.44–1.87) | 1.59 (0.56–4.51) |
| Venous thromboembolism | ||||
| Hodgkin lymphoma | 8.62 (3.80–19.54) | (.–.) | 1.23 (0.45–3.39) | (.–.) |
| Non-Hodgkin lymphoma | 9.74 (7.49–12.66) | 5.18 (3.55–7.56) | 1.99 (1.57–2.52) | 2.14 (1.47–3.11) |
| Acute myeloid leukemia | 11.63 (5.55–24.38) | 7.00 (2.99–16.39) | 3.21 (1.79–5.75) | 0.87 (0.25–3.01) |
| Acute lymphoid leukemia | 18.65 (3.94–88.31) | 4.73 (0.66–33.65) | 3.30 (0.74–14.77) | (.–.) |
| Chronic myeloid leukemia | 3.08 (0.95–9.95) | 1.14 (0.13–10.21) | 2.07 (0.90–4.77) | 1.03 (0.21–5.05) |
| Chronic lymphocytic leukemia | 3.59 (2.32–5.55) | 1.91 (1.09–3.35) | 1.71 (1.30–2.24) | 2.74 (1.82–4.13) |
| Multiple myeloma | 10.87 (7.06–16.75) | 5.89 (3.61–9.61) | 4.12 (3.06–5.54) | 3.93 (2.12–7.28) |
| Myeloproliferative neoplasms | 5.63 (3.89–8.16) | 3.39 (2.21–5.22) | 2.56 (1.97–3.33) | 2.41 (1.64–3.53) |
| Myelodysplastic syndrome | 2.77 (1.59–4.83) | 1.91 (0.87–4.19) | 2.36 (1.47–3.77) | 1.01 (0.33–3.08) |
| Other hematological cancers | 16.67 (4.59–60.56) | 1.57 (0.43–5.82) | 2.11 (1.05–4.24) | 4.57 (2.06–10.14) |
| Bleeding requiring hospital contact | ||||
| Hodgkin lymphoma | 3.27 (1.24–8.64) | 1.79 (0.57–5.62) | 1.14 (0.54–2.39) | 0.42 (0.05–3.28) |
| Non-Hodgkin lymphoma | 3.66 (2.92–4.60) | 1.93 (1.40–2.66) | 1.38 (1.16–1.65) | 1.60 (1.16–2.21) |
| Acute myeloid leukemia | 9.06 (6.48–12.66) | 11.89 (6.39–22.12) | 6.59 (4.17–10.41) | 2.53 (1.11–5.74) |
| Acute lymphoid leukemia | 24.14 (2.82–206.83) | 2.50 (0.23–27.57) | 5.62 (1.71–18.48) | 15.00 (1.56–144.20) |
| Chronic myeloid leukemia | 8.34 (4.41–15.78) | 3.85 (1.59–9.32) | 2.40 (1.35–4.28) | 4.75 (1.56–14.47) |
| Chronic lymphocytic leukemia | 1.42 (0.98–2.05) | 1.26 (0.83–1.91) | 1.25 (1.02–1.53) | 1.30 (0.95–1.78) |
| Multiple myeloma | 4.04 (2.99–5.47) | 1.71 (1.06–2.75) | 2.50 (1.99–3.13) | 2.57 (1.60–4.12) |
| Myeloproliferative neoplasms | 4.30 (3.24–5.71) | 2.53 (1.70–3.78) | 1.86 (1.54–2.25) | 1.57 (1.14–2.15) |
| Myelodysplastic syndrome | 6.09 (4.53–8.19) | 6.31 (4.18–9.52) | 3.90 (3.11–4.89) | 3.10 (1.64–5.84) |
| Other hematological cancers | 3.03 (1.62–5.64) | 3.45 (1.31–9.08) | 1.98 (1.26–3.10) | 2.85 (1.33–6.09) |
- (.–.) Insufficient for estimates.
For non-Hodgkin patients with indolent and aggressive lymphoma, there were no associations with myocardial infarction or ischemic stroke in either subgroup (Table S4). The risk of VTE and bleeding was pronounced in patients with aggressive lymphoma. Overall associations for AML were driven by patients with APL type.
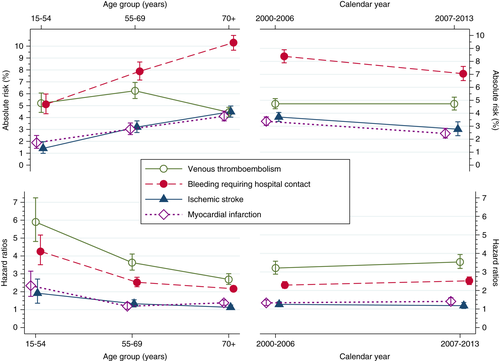
The absolute risk of myocardial infarction, ischemic stroke, and bleeding requiring hospital contact was higher in older age groups at the time of hematological cancer diagnosis. However, VTE risk was highest among patients aged 55 to 69 years (Figure 2). Conversely, the HRs of all types of complications of hematological cancer were lower among patients in younger age groups at diagnosis (Figure 2). Risks and HRs for other cardiovascular and bleeding complications remained rather stable during the two index periods (Figure 2).
The observed associations were present in subgroups of patients with and without chemotherapy as well as in patients who did and did not receive radiotherapy (Table 6). The risk of VTE was higher in patients who did receive chemotherapy than in those who did not receive this treatment. The risk of complications was virtually unchanged in patients without a history of a thromboembolic or bleeding event at the time of hematological cancer diagnosis (Table S5).
| Myocardial infarction | Ischemic stroke | |||||
|---|---|---|---|---|---|---|
| Treatment modality | No. events | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | No. events | Incidence ratea (95% CI) | Hazard ratiob (95% CI) |
| Chemotherapy | ||||||
| No | 429 | 7.41 (6.74-8.14) | 1.32 (1.18-1.47) | 452 | 7.82 (7.13-8.58) | 1.28 (1.15-1.43) |
| Yes | 286 | 5.79 (5.15-6.50) | 1.43 (1.25-1.64) | 279 | 5.65 (5.03-6.36) | 1.14 (0.99-1.30) |
| Radiation therapy | ||||||
| No | 660 | 6.99 (6.47-7.54) | 1.37 (1.26-1.50) | 683 | 7.24 (6.72-7.81) | 1.26 (1.15-1.37) |
| Yes | 55 | 4.27 (3.38-5.56) | 1.26 (0.93-1.71) | 48 | 3.72 (2.81-4.94) | 0.88 (0.64-1.21) |
| Venous thromboembolism | Bleeding requiring hospital contact | |||||
|---|---|---|---|---|---|---|
| Treatment modality | No. events | Incidence ratea (95% CI) | Hazard ratiob (95% CI) | No. events | Incidence ratea (95% CI) | Hazard ratiob (95% CI) |
| Chemotherapy | ||||||
| No | 559 | 9.74 (8.97-10.58) | 2.69 (2.42-2.99) | 1146 | 20.11 (18.98-21.31) | 2.31 (2.15-2.49) |
| Yes | 628 | 12.99 (12.01-14.04) | 4.32 (3.87-4.81) | 783 | 16.08 (14.99-17.24) | 2.51 (2.30-2.75) |
| Radiation therapy | ||||||
| No | 1054 | 11.32 (10.66-12.03) | 3.31 (3.06-3.59) | 1778 | 19.13 (18.26-20.04) | 2.42 (2.28-2.57) |
| Yes | 133 | 10.51 (8.87-12.45) | 3.97 (3.14-5.02) | 151 | 11.86 (10.11-13.91) | 2.09 (1.72-2.54) |
- a Per 1000 person-years. The incidence rates are for the hematological cancer cohort.
- b Controlled for matching factors by study design.
4 DISCUSSION
In this population-based study among patients with hematological malignancies, around 20% experienced either myocardial infarction, ischemic stroke, VTE, or bleeding requiring hospital contact within 10 years. Compared with a matched general population comparison cohort, hematological cancer patients had increased risks for myocardial infarction (1.4-fold), ischemic stroke (1.2-fold), VTE (3-fold), and bleeding requiring hospital contact (2-fold). The associations were generally stronger in the short term than in the long term.
4.1 Arterial events
Earlier studies have suggested that hematological cancers are associated with increased risk of myocardial infarction,5-8, 4 but the existing literature has been contradictory for ischemic stroke.4-8 A Swedish population-based cohort study of 18 627 multiple myeloma patients diagnosed from 1958 to 2006 and 70 991 age-matched and sex-matched individuals from the general population computed the 10-year HRs for coronary artery disease (stable and unstable angina pectoris and myocardial infarction) and cerebrovascular complications (ischemic stroke, hemorrhagic stroke, and transient ischemic attack).6 Although there were disparities in outcome definitions between studies, the Swedish study HRs of 1.7 (95% CI: 1.6 to 1.8) for coronary artery disease and 1.2 (95% CI: 1.1 to 1.3) for cerebrovascular complications are similar to ours.
Two studies have assessed the risk of MI and ischemic stroke in patients with CML compared with non-cancer patients.4, 5 A study from the United States, based on linked Surveillance, Epidemiology, and End Results (SEER) cancer registry and Medicare Claims data for 1466 CML patients, reported an HR of 2.46 (P < .001) for myocardial infarction and 1.54 (P < .001) for ischemic and hemorrhagic stroke.5 Similar to our data, a more recent Swedish cohort study of CML patients during 2002-2012 and age-matched and sex-matched individuals from the general population found incidence rate ratios of 1.9 (95% CI: 1.3 to 2.7) for myocardial infarction and 0.9 (95% CI: 0.5 to 1.5) for ischemic stroke.4 The SEER cancer registry data also were used in another study of 15 669 non-Hodgkin lymphoma patients during 2002 to 2011.8 In this cohort of non-Hodgkin lymphoma patients, compared to a cohort of age-matched and sex-matched non-cancer patients, HRs during the first year after diagnosis were 9-fold to 1.4-fold increased for myocardial infarction, while associations were 5-fold to 1.2-fold elevated for ischemic stroke. It has been reported previously that patients with myeloproliferative neoplasms are at increased risk of arterial and venous thromboembolic events.25, 26 In addition, a previous report based on data from the SEER cancer registry of patients with myelodysplastic syndrome reported a 2.2-fold higher standardized mortality ratio for death due to cardiovascular disease relative to the risk observed in the general population.27 Our findings are largely confirmatory, highlighting the importance of integrating assessment of thrombotic risk into the management of patients with myeloproliferative neoplasms and myelodysplastic syndrome.
4.2 Venous thromboembolism
In line with existing literature, our study confirmed the increased risk of VTE associated with hematological cancers.6, 9-11, 4, 28 The magnitude of the association also was consistent with previous studies, which reported a two-fold or higher VTE risk, with particularly elevated risks in patients with multiple myeloma, myeloproliferative neoplasms, leukemia, and Hodgkin lymphoma.
4.3 Bleeding leading to hospitalization
Although the association between hematological cancers and bleeding is well established,29 our study builds on previous studies by quantifying the individual bleeding risk as well as the thromboembolic risk for all different types of hematological cancers. The risk of bleeding was markedly higher for some hematological cancer than for other cancers. Our data provided new data for the risk-benefit evaluation of anticoagulant treatment in both the short and long term, which are important to elucidate for clinical purposes, as anticoagulation often poses a challenge in these patients owing to high rates of bleeding and thromboembolic events. Our findings may guide thromboprophylaxis decision making in the future. However, more carefully designed randomized trials are needed to clarify the role of antithrombotic treatment in hematological cancer.
4.4 Mechanisms
The mechanisms through which patients with hematological cancer are at increased risk for arterial events and VTE are likely multifactorial and may vary according to type of hematologic cancer.30 Particularly among CML and multiple myeloma patients, the increased myocardial infarction risk may be linked to commonly used drugs such as IMIDs and tyrosine kinase inhibitors.30 Shared risk factors such as smoking also may account for the increased risk of cardiovascular events. However, they are unlikely to explain fully our findings because some hematological cancers we examined were not associated with ischemic stroke.
Interestingly, most studies have found that risks of myocardial infarction clearly exceed risks of ischemic stroke and persist for a longer time.4-8 This trend also is apparent in our analysis. This may be explained by the high incidence of type 2 myocardial infarction among patients with hematological cancers, an event rarely undetected owing to its acute onset, specific symptoms, and diagnostic criteria. Type 2 myocardial infraction is triggered by anemia, infections, circulatory instability, and hypoxemia inherent to the underlying hematological malignancy. Radiation therapy for the mediastinum and treatment with anthracycline may also contribute to the high risk of myocardial infarction.
Potential reasons for increased VTE risk may include patient-related risk factors (e.g., advanced age, high prevalence of VTE risk factors, and frequent immobility).28 Treatment-related risk factors such as central venous catheter implantation and other invasive procedures also may increase VTE risk.12, 30, 31 In addition, thrombogenic potential and interactions with hemostatic pathways of different drugs commonly employed in treating multiple myeloma (dexamethasone and IMIDs) and CML (tyrosine kinase inhibitors) may explain why the absolute and relative risks of VTE were particularly elevated in these patients.12, 30, 31 Although thromboprophylaxis with aspirin or anticoagulants is recommended for some hematological cancer patients in specific clinical situations (e.g., multiple myeloma patients who start therapy with IMID)32 and because many patients in our cohort were likely to receive thromboprophylactic treatment (e.g., more than 50% of patients with myeloproliferative neoplasm were treated with acetylsalicylic acid), we observed strong associations with most cardiovascular outcomes, particularly VTE. This suggests a high level of thrombogenicity among most hematological cancer patients. As their bleeding risk also was elevated, our findings underscore the need for individualized decisions about thromboprophylaxis based on each patient's risk factors for cardiovascular and bleeding events.
4.5 Strenghts and limitations
Several issues should be considered when interpreting our results. Study strengths include selection of a comparison cohort from the general population with a similar history of preexisting thromboembolic and bleeding events and of underlying solid cancer. This allowed us to control for prevalence bias. The positive predictive values of hematological cancer diagnoses, cardiovascular diagnoses, and bleeding diagnoses in the DCR and DNPR are high.17, 33-37 Several limitations also should be addressed. Underregistration of cancer therapies in the DNPR calls for cautious interpretation of data on complications associated with patient therapy regimen. We lacked data on in-hospital treatment with antithrombotic and anticoagulant treatment. In addition, we might have slightly underestimated study outcomes because of exclusion of secondary diagnoses.
5 CONCLUSION
Among patients with hematological cancer, approximately 20% experienced myocardial infarction, ischemic stroke, VTE, or bleeding requiring hospital contact within 10 years after diagnosis. Correspondingly, hematological cancer patients had substantially higher hazards of myocardial infarction, ischemic stroke, VTE, and bleeding requiring hospital contact, compared with risk in the general population.
ACKNOWLEDGMENTS
The study was supported by a grant from the Aarhus University Research Foundation and by the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation. The sponsors had no role in study design, data collection, analysis or interpretation of the data, writing of the manuscript, or the decision to submit the paper for publication. All authors had full access to the study data and had final responsibility for the decision to submit for publication.
CONFLICT OF INTERESTS
KA, PC, BD, HF, AO, EHP, MR, and HTS have no conflicts of interests
AUTHOR CONTRIBUTION
KA, PC, HF, MR, and HTS designed the study; BD, EHP and HTS obtained and assembled the data; KA, PC, HF, MR, EHP, BD, AO, and HTS analyzed and interpreted the data; KA and PC wrote the report; and all authors revised the report and approved the final version. HTS is the guarantor.




