Differential effects of sleep position and sleep stage on the severity of obstructive sleep apnea
Soyun Lim and Hyun-Kyung Lee contributed equally to this work as the first author.
Summary
This study compared the effects of sleeping in the supine position and rapid eye movement sleep on the severity of obstructive sleep apnea, and investigated the effect of sleep stage on position-dependent obstructive sleep apnea, and of sleep position on rapid eye movement-dependent obstructive sleep apnea. We analysed epoch-labelled polysomnographic readouts of 3843 patients, and calculated the apnea–hypopnea index for each sleep position and sleep stage. Subgroup analyses were performed to evaluate whether the proportion of position-dependent obstructive sleep apnea patients changed during rapid eye movement and non-rapid eye movement sleep, and whether that of rapid eye movement-dependent obstructive sleep apnea patients changed during supine/lateral sleep. The apnea–hypopnea index was highest in the rapid eye movement-supine position (50.7 ± 22.6 events per hr), followed by non-rapid eye movement-supine, rapid eye movement-lateral and non-rapid eye movement-lateral (39.2 ± 25.3, 22.9 ± 24.4, 15.9 ± 21.9 events per hr, respectively; p < 0.001). Patients with position-dependent obstructive sleep apnea had a higher ratio of rapid eye movement sleep, and those with rapid eye movement-dependent obstructive sleep apnea had a higher ratio of sleep time in the supine position (p < 0.001). During rapid eye movement sleep, position-dependent obstructive sleep apnea was not observed in 21.1% of patients who otherwise had position-dependent obstructive sleep apnea. In the lateral position, 36.9% of patients with rapid eye movement-dependent obstructive sleep apnea did not retain rapid eye movement dependency. Although sleeping in the supine position and rapid eye movement sleep were both associated with more frequent respiratory events, this was the first study to demonstrate that the former had a stronger correlation with obstructive sleep apnea severity. Position dependency in patients with obstructive sleep apnea decreased during rapid eye movement sleep, and worsening of rapid eye movement dependency was alleviated in the lateral position, suggesting potential for personalized obstructive sleep apnea management.
1 INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by recurrent narrowing or collapse of the pharyngeal airway during sleep, and is associated with cardiovascular disease, metabolic syndrome and neurocognitive disease, resulting in increased morbidity and mortality (Parati et al., 2016; Uyama et al., 2018; Xu et al., 2019; Yaggi et al., 2005).
Previous studies have described the main pathophysiology of OSA (Eckert & Malhotra, 2008). Anatomically, patients with OSA have a narrower airway with more surrounding soft tissue, which makes the passageway more likely to collapse (Haponik et al., 1983; Schwab et al., 1995). Upper airway dilator muscle activity, mainly the genioglossus muscle, is reduced to a greater extent in patients with OSA (Mezzanotte et al., 1992, 1996; Worsnop et al., 1998). A higher arousal threshold in response to hypopnea or apnea and ventilatory control instability can aggravate OSA (Berry et al., 1996; White, 2006; Younes, 2004). In addition, OSA is a heterogeneous disorder. OSA phenotyping involves classifying the disease into groups based on clinical features, treatment response and health outcomes, which can help guide personalized treatments (Zinchuk et al., 2017).
Sleep position is an important phenotypic factor, and apneas and hypopneas are more frequently observed in the supine position than in the lateral position (Joosten et al., 2014). Patients with OSA whose respiratory events mostly occur in the supine position and who show improvement of symptoms in the lateral position are considered to have position-dependent OSA (POSA). Position dependency is explained by the anatomical structure of the pharyngeal airway. The distance between the anterior and posterior pharyngeal walls is shorter than that between the lateral walls, which renders the airway more susceptible to obstruction in the supine position (Ryu et al., 2024).
Sleep stage is another important factor associated with exacerbation of OSA, and rapid eye movement (REM)-dependent OSA (ROSA) refers to OSA that occurs predominantly during REM sleep. OSA can manifest during both REM and non-REM (NREM) sleep, but is more likely during REM due to decreased upper airway muscle activity (Fenik et al., 2005; Haba-Rubio et al., 2005; Koo, Dostal, et al., 2008; Koo, Patel, et al., 2008; Resta et al., 2005). REM sleep is associated with greater sympathetic activity and cardiovascular instability (Somers et al., 1993; Somers et al., 1995). Obstructive apneas and hypopneas during REM sleep result in more severe desaturation and increased sympathetic activity (Findley et al., 1985). However, the clinical significance of distinguishing between patients with ROSA and those with OSA throughout both REM and NREM sleep is not clear (Chami et al., 2010; Chervin & Aldrich, 1998), and whether ROSA has any influence on health outcomes is controversial (Punjabi et al., 2002).
Although many studies have investigated ROSA and position dependency, the effect of sleep stage and position on OSA have always been evaluated individually, without accounting for the effect one may have on the other as a confounding variable (Oksenberg et al., 2010; Steffen et al., 2017). Oksenberg et al. investigated how the apnea–hypopnea index (AHI) of patients with ROSA and nROSA changed in the supine and lateral body positions. However, the study population was limited to 100 patients, and the effect of sleep stage on patients with POSA and NPOSA remained unexplored (Oksenberg et al., 2010). Pevernagie et al. compared the degree of positional dependency during REM sleep and NREM sleep in 100 male patients with OSA. Although they reported that a large portion (40.9%) of patients with POSA during NREM sleep became non-positional during REM sleep and that patients with POSA had less supine sleep time, the study population was small and only included male patients, which limits the generalizability of the results (Steffen et al., 2017). In addition, they did not assess the effect of body position on ROSA and nROSA. Our study is the first to use epoch-labelled polysomnography (PSG) to address the effect of sleep stage and position simultaneously. AHI was calculated based on the patient's sleep stage and position: REM/NREM and supine/lateral. In addition, the effect of sleep stage on POSA and sleep position on ROSA were also analysed.
2 METHODS
2.1 Data collection
We retrospectively evaluated 7745 patients who visited the clinic with complaints of insomnia, snoring, sleep apnea or excessive daytime sleepiness, and underwent fully attended overnight PSG for the diagnosis of OSA from 2013 to 2020. PSG data were collected from four medical centres. The institutional review boards of Hallym University College of Medicine (Chuncheon, Republic of Korea: approval number 2020-03-022), Seoul National University Bundang Hospital (Seongnam, Republic of Korea: approval number C-2007-179-1143) and Seoul National University Hospital (Seoul, Republic of Korea: approval number B-2010/640-401) approved this study (Ahn et al., 2024; Soh et al., 2024). Written informed consent was not required because of the deidentified dataset used (Jeong et al., 2023).
We excluded patients aged < 18 years or > 90 years, and those whose total sleep time (TST) was < 180 min. To analyse the effect of sleep stage on OSA, participants whose REM sleep proportion was < 5% of TST were excluded. Patients with incomplete demographic information were also excluded. In addition, to analyse the differential effects of sleep position and sleep stage on the AHI, patients who slept in either position during either sleep state for < 10 min were excluded (Figure 1).

2.2 Diagnostic PSG
The diagnostic sleep studies performed in each case were overnight PSG tests including electroencephalogram, electro-oculogram, submental electromyogram, electrocardiogram, pressure transducer test, nasal thermistor, chest and abdominal respiratory analyses (using effort belts), snoring tests, leg electromyogram and pulse oximetry. Sleep positions (supine, left, right and prone) were defined based on video recordings (Choi et al., 2024).
Sleep stages, respiratory events, arousal, movements and sleep-related events were scored by one sleep specialist and two sleep technicians following the American Academy of Sleep Medicine Manual for the Scoring of Sleep and Related Events (version 2.4; Berry et al., 2017). Apnea was defined as a decrease in oronasal airflow ≥ 90% from baseline for ≥ 10 s. Hypopnea was defined as a decrease in oronasal flow ≥ 30% from baseline for ≥ 10 s resulting in a decrease in oxygen saturation ≥ 3%. We extracted and standardized data from the PSG results.
2.3 Sociodemographic characteristics
Sociodemographic information such as patient sex, age and body mass index (BMI) were collected by reviewing electronic medical records. Age data were collected at the time of full-night PSG. BMI was calculated by dividing weight in kilograms (kg) by height in meters squared (m2).
2.4 PSG parameters
Data on exam date, time in bed, TST, NREM time, Stage 1 time, Stage 2 time, Stage 3 time, REM time, sleep time in the supine position and sleep time in the lateral position were collected from PSG reports. PSG events included the start time and end time of total sleep, as well as the start time, end time, start epoch and end epoch of each sleep stage, each sleep position, and each respiratory event.
Patients with an AHI of < 5 events per hr were assigned to the control group, and patients with AHI ≥ 5 per hr were assigned to the OSA group. Patients with OSA were further classified into those with POSA, without POSA (NPOSA), with ROSA and without ROSA (nROSA).
2.5 AHI according to sleep position and sleep stage
The AHI according to sleep position and sleep stage was calculated using epoch-labelled PSG. If either factor changed during apnea or hypopnea, it was classified according to the position and stage of the epoch during which the respiratory event started. Using the Cartwright Index, a commonly used criterion for diagnosing POSA, POSA was defined as a ratio of AHI in the supine position to that in the lateral position ≥ 2 (Cartwright, 1984). ROSA was defined as a ratio of AHI during REM sleep to that during NREM sleep ≥ 2 (Cartwright, 1984; Conwell et al., 2012).
2.6 Statistical analyses
Time spent in each sleep stage-position was compared with multiple paired t-tests with Bonferroni correction. Individual t- and χ2-tests were performed to compare the demographic characteristics and PSG parameters of all groups. Univariate and multivariate logistic regression were performed to evaluate the predictive factors of POSA and ROSA.
The AHI according to sleep position and sleep stage was analysed to determine the effect of position and stage on OSA severity using multiple paired t-tests with Bonferroni correction. Subgroup analyses of POSA and ROSA were performed. PSG results of patients with OSA were separated into REM sleep and NREM sleep stages to determine the percentage of patients with POSA in whom it persisted across the different sleep stages and whether position dependency newly emerged after the separation of sleep stages in patients who did not have POSA.
Finally, the PSG results of patients with OSA were separated according to sleep position (supine or lateral). Statistical analyses were performed to determine the percentage of patients who were REM-dependent who retained ROSA in different sleep positions and whether REM dependency newly emerged after the separation of sleep positions in those without REM dependency.
Statistical estimates are given with 95% confidence intervals (CIs), and a p-value < 0.05 was considered to indicate a statistically significant difference. All statistical analyses were performed using Python version 3.11, an openly available programming language. Python libraries used are as follows: JSON was used to read the PSG results, which was recorded in the JSON format; NumPy, Datetime and Pandas were used for calculation of sleep time and AHI in each sleep stage and position; SciPy was used for comparison of statistical analyses; and Matplotlib was used for visualization of statistical analyses.
3 RESULTS
3.1 Participant characteristics
Ultimately, 3843 patients fulfilled the study inclusion criteria. The demographic characteristics and PSG parameters were summarized in Table 1. Overall, 86.3% of patients were diagnosed with OSA, while 13.7% were not (controls). The proportion of males was lower in the control group than in the OSA group. No differences in mean age were observed between the control and OSA groups. Patients with OSA had higher BMIs than controls.
| Characteristic | Control | OSA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Total | p (control versusOSA) | POSA | NPOSA | p (POSA versus NPOSA) | ROSA | nROSA | p (ROSA versus nROSA) | |
| All cases, n (%) | 526 | 3317 | 2343 (70.6) | 974 (29.4) | 754 (22.7) | 2563 (77.3) | |||
| Demographic characteristics | |||||||||
| Sex, n (%) | < 0.001 | 0.101 | < 0.001 | ||||||
| Male | 279 (53.0) | 2783 (83.9) | 1950 (83.2) | 833 (85.5) | 538 (71.4) | 3812 (87.6) | |||
| Female | 247 (47.0) | 534 (16.1) | 393 (16.8) | 141 (14.5) | 216 (28.6) | 318 (12.4) | |||
| Age, years, mean (SD) | 44.6 (15.5) | 45.1 (13.5) | 0.699 | 44.8 (13.4) | 45.8 (13.5) | 0.054 | 44.4 (14.0) | 45.3 (13.3) | 0.100 |
| BMI, kg m−2, mean (SD) | 22.4 (3.2) | 25.9 (3.9) | < 0.001 | 25.2 (3.4) | 27.6 (4.6) | < 0.001 | 25.6 (4.5) | 26.0 (3.7) | 0.062 |
| PSG parameters, mean (SD) | |||||||||
| Sleep efficiency, % | 84.5 (10.1) | 81.4 (10.0) | < 0.001 | 81.8 (10.0) | 80.6 (9.9) | 0.002 | 83.1 (10.3) | 81.0 (9.9) | < 0.001 |
| Sleep time | |||||||||
| TST, min | 336.4 (53.4) | 314.6 (56.2) | < 0.001 | 318.7 (56.5) | 304.9 (54.2) | < 0.001 | 324.1 (54.7) | 311.9 (56.3) | < 0.001 |
| Ratio of REM time, % | 22.7 (5.9) | 21.2 (6.2) | < 0.001 | 21.6 (6.2) | 20.4 (6.1) | < 0.001 | 22.4 (6.5) | 20.9 (6.1) | < 0.001 |
| Ratio of sleep time in supine position, % | 66.4 (12.7) | 64.7 (12.9) | 0.002 | 64.5 (13.0) | 64.9 (12.5) | 0.422 | 66.3 (13.1) | 64.2 (12.8) | < 0.001 |
| AHI | |||||||||
| AHItotal, events per hr | 2.1 (1.5) | 38.5 (24.2) | < 0.001 | 31.7 (18.6) | 54.9 (27.9) | < 0.001 | 18.8 (10.9) | 44.3 (24.0) | < 0.001 |
| AHIREM, events per hr | 4.6 (4.5) | 42.7 (23.8) | < 0.001 | 35.7 (20.2) | 59.4 (23.6) | < 0.001 | 40.5 (20.5) | 43.3 (24.7) | 0.005 |
| AHINREM, events per hr | 1.4 (1.4) | 37.3 (25.9) | < 0.001 | 30.6 (20.2) | 53.6 (30.7) | < 0.001 | 12.8 (9.1) | 44.6 (24.8) | < 0.001 |
| AHIsupine, events per hr | 2.8 (2.3) | 49.3 (27.9) | < 0.001 | 45.0 (25.5) | 59.6 (30.6) | < 0.001 | 24.7 (14.6) | 56.5 (26.8) | < 0.001 |
| AHIlateral, events per hr | 0.9 (1.7) | 20.5 (24.0) | < 0.001 | 9.4 (10.4) | 47.2 (26.2) | < 0.001 | 9.1 (11.7) | 23.8 (25.6) | < 0.001 |
| 3% ODI, events per hr | 1.7 (1.7) | 27.5 (23.6) | < 0.001 | 21.1 (16.9) | 43.1 (29.5) | < 0.001 | 11.4 (8.8) | 32.3 (24.5) | < 0.001 |
| Mean oxygen saturation, % | 96.6 (1.1) | 94.3 (2.1) | < 0.001 | 94.8 (1.6) | 93.1 (2.6) | < 0.001 | 95.2 (1.5) | 94.0 (2.2) | < 0.001 |
- Independent t- and χ2-test are used for continuous and categorical variables, respectively.
- AHI, apnea–hypopnea index; BMI, body mass index; NPOSA, non-position-dependent obstructive sleep apnea; NREM, non-rapid eye movement sleep; nROSA, NREM-dependent obstructive sleep apnea; ODI, oxygen desaturation index; OSA, obstructive sleep apnea; POSA, position-dependent obstructive sleep apnea; PSG, polysomnography; REM, rapid eye movement sleep: ROSA, REM-dependent obstructive sleep apnea; SD, standard deviation; TST, total sleep time.
Demographic data of the 3750 excluded patients, who had less than 10 min of supine or REM sleep, is shown in Table S1.
Compared with controls, patients with OSA had lower sleep efficiency and a shorter TST. The PSG characteristics of the control group were all within the normal range. The proportion of REM sleep time was lower in the OSA group than in controls. Patients with OSA spent less TST in the supine position than did controls.
Of the 3317 patients with OSA, 612 (18.5%), 837 (25.2%) and 1868 (56.3%) had mild, moderate and severe disease, respectively. The prevalence of POSA was higher than that of NPOSA. Patients with NPOSA had a higher BMI than patients with POSA. No significant difference in sex or age was observed between patients with POSA and NPOSA.
Among patients with OSA, the proportion with nROSA was higher than that of those with ROSA. Patients with nROSA were more likely to be male than patients with ROSA. There were no significant differences in age or BMI between patients with ROSA and nROSA.
3.2 Risk factors of POSA
Compared with patients with POSA, patients with NPOSA had lower sleep efficiency, less TST, a lower ratio of REM sleep time (20.4 ± 6.1% versus 21.6 ± 6.2%; p < 0.001), higher AHI and 3% oxygen desaturation index (ODI), and lower mean oxygen saturation. There were no significant differences in the ratio of sleep time spent in the supine position between POSA and NPOSA groups.
Multivariate logistic regression analyses of the risk factors of POSA (Table 2) identified BMI, AHI in the supine position, and ratio of sleep time in the supine position as significant risk factors. Age, AHI during REM sleep and 3% ODI were associated with a decreased risk of POSA.
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Age | 1.00 | 0.99 | 1.00 | 0.041 | 0.99 | 0.99 | 1.00 | 0.022 |
| Male sex | 1.09 | 0.92 | 1.30 | 0.314 | 0.97 | 0.79 | 1.21 | 0.807 |
| BMI (kg m−2) | 0.88 | 0.89 | 0.90 | < 0.001 | 1.03 | 1.01 | 1.06 | 0.009 |
| PSG parameters | ||||||||
| AHI in supine position | 1.00 | 1.00 | 1.00 | < 0.001 | 1.10 | 1.09 | 1.11 | < 0.001 |
| AHI during REM sleep | 0.97 | 0.97 | 0.97 | < 0.001 | 0.95 | 0.94 | 0.96 | < 0.001 |
| TST | 1.00 | 1.00 | 1.01 | < 0.001 | 1.01 | 1.00 | 1.01 | < 0.001 |
| Sleep efficiency | 1.01 | 1.01 | 1.02 | 0.028 | 0.99 | 0.98 | 1.00 | 0.170 |
| Ratio of sleep time in supine position | 1.00 | 1.00 | 1.00 | 0.099 | 1.02 | 1.01 | 1.03 | < 0.001 |
| Ratio of REM time | 1.02 | 1.02 | 1.04 | < 0.001 | 0.99 | 0.98 | 1.01 | 0.427 |
| 3% ODI | 0.97 | 0.96 | 0.97 | < 0.001 | 0.90 | 0.89 | 0.91 | < 0.001 |
| Mean oxygen saturation | 1.40 | 1.35 | 1.46 | < 0.001 | 0.99 | 0.93 | 1.06 | 0.791 |
- Variables with a p-value < 0.05 in the univariate analysis were subjected to multivariate analysis. Values of p < 0.05 were considered statistically significant.
- AHI, apnea–hypopnea index; BMI, body mass index; CI, confidence interval; ODI, oxygen desaturation index; OR, odds ratio; PSG, polysomnography; REM, rapid eye movement sleep; TST, total sleep time.
3.3 Risk factors of ROSA
Compared with patients with ROSA, patients with nROSA had lower sleep efficiency, less TST, a lower ratio of REM sleep time (20.9 ± 6.1% versus 22.4 ± 6.5%; p < 0.001), a lower percentage of TST spent in the supine position (64.2 ± 12.8% versus 66.3 ± 13.1%; p < 0.001), higher AHI (total and in different sleep stages and positions), greater 3% ODI, and lower mean oxygen saturation.
Multivariate logistic regression analyses of the risk factors of ROSA (Table 3) identified female sex, higher BMI, higher AHI during REM sleep, higher ratio of REM sleep time and higher mean oxygen saturation as significant risk factors of ROSA. AHI in the supine position and the ratio of sleep time spent in the supine position were significantly associated with a decreased risk of ROSA.
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |||
| Age | 1.00 | 0.99 | 1.00 | 0.201 | 1.00 | 0.99 | 1.01 | 0.825 |
| Male sex | 3.37 | 2.86 | 3.97 | < 0.001 | 1.48 | 1.12 | 1.97 | 0.007 |
| BMI (kg m−2) | 0.94 | 0.92 | 0.96 | < 0.001 | 1.10 | 1.05 | 1.15 | < 0.001 |
| PSG parameters | ||||||||
| AHI in supine position | 0.94 | 0.94 | 0.95 | < 0.001 | 0.72 | 0.69 | 0.74 | < 0.001 |
| AHI during REM sleep | 0.98 | 0.98 | 0.99 | < 0.001 | 1.35 | 1.31 | 1.38 | < 0.001 |
| TST | 1.00 | 1.00 | 1.01 | < 0.001 | 1.01 | 1.00 | 1.01 | < 0.001 |
| Sleep efficiency | 1.03 | 1.02 | 1.03 | < 0.001 | 0.99 | 0.97 | 1.00 | 0.140 |
| Ratio of sleep time in supine position | 1.01 | 1.01 | 1.02 | < 0.001 | 0.93 | 0.92 | 0.95 | < 0.001 |
| Ratio of REM time | 1.04 | 1.03 | 1.05 | < 0.001 | 1.06 | 1.03 | 1.08 | < 0.001 |
| 3% ODI | 0.92 | 0.91 | 0.93 | < 0.001 | 0.88 | 0.86 | 0.91 | < 0.001 |
| Mean oxygen saturation | 1.57 | 1.50 | 165.00 | < 0.001 | 1.15 | 1.03 | 1.28 | 0.014 |
- Variables with a p-value < 0.05 in the univariate analysis were subjected to multivariate analysis. Values of p < 0.05 were considered statistically significant.
- AHI, apnea–hypopnea index; BMI, body mass index; CI, confidence interval; ODI, oxygen desaturation index; OR, odds ratio; PSG, polysomnography; REM, rapid eye movement sleep; TST, total sleep time.
3.4 Time spent in each sleep position and stage
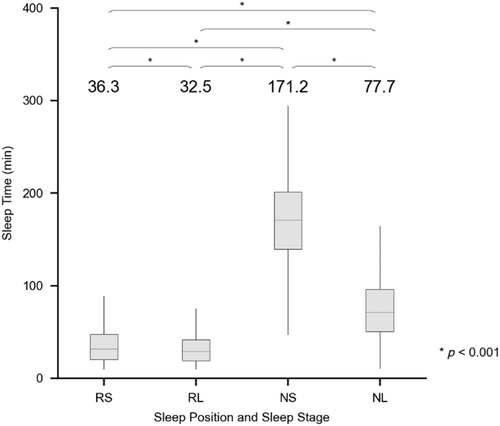
The time spent in each sleep position and stage (REM/supine [RS], REM/lateral [RL], NREM/supine [NS], NREM/lateral [NL]) was analysed (Figure 2). TST consisted mostly of NS sleep, followed by NL sleep, RS sleep and RL sleep.
The proportions of time spent in supine and lateral positions were also evaluated (Figure 3). The proportion of supine and lateral sleep time during total sleep, REM sleep only and NREM sleep only is shown in Figure 3(a). Chi-square test was performed to analyse whether the proportion of supine sleep during REM and NREM sleep differed significantly from that of total sleep. This step was necessary to determine whether a specific position was more likely during a specific sleep stage. If, for instance, supine position was more likely to be assumed during REM sleep, worsening of sleep apnea during REM sleep may actually be due to supine position, rather than REM sleep alone. However, the results of our study demonstrate that the percentage of supine sleep time, which accounted for 65% of TST, did not significantly change when analysed for REM or NREM sleep only (p = 0.051).

Similarly, the proportion of REM and NREM time for each patient was analysed (Figure 3b). Chi-square test was performed to analyse whether the proportion of REM sleep time changed significantly during REM and NREM sleep only, compared with TST. This was done to ascertain whether REM sleep was more prevalent in a specific sleep position. The proportion of REM sleep, when assessed during the supine/lateral position only, did not significantly differ from that of TST (p = 0.122).
3.5 Comparison of AHI based on sleep position and sleep stage
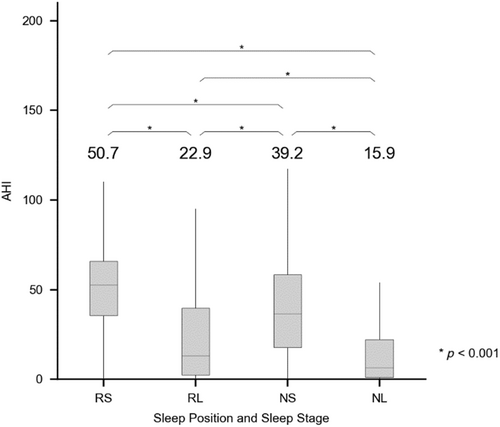
Multiple paired t-tests were performed to analyse the AHI according to sleep position and stage, to assess the effect of sleep position and stage on OSA severity. In all paired t-tests, there was a significant difference in the AHI among the four position and stage combinations (p < 0.001), with the value being highest for RS (AHIRS) followed by RL (AHIRL), NS (AHINS) and NL (AHINL) (50.7 ± 22.6, 39.2 ± 25.3, 22.9 ± 24.4 and 15.9 ± 21.9 events per hr, respectively; Figure 4).
3.6 Effect of sleep stage on position dependency
Subgroup analyses were performed to evaluate the effect of sleep stage on position dependency. Patients who had an AHIRS/AHIRL ratio ≥ 2 were considered position-dependent during REM sleep. Patients who had an AHINS/AHINL ratio ≥ 2 were considered position-dependent during NREM sleep. Patients with POSA during total sleep were assessed during REM sleep (Figure 5a) and NREM sleep (Figure 5c) only. During REM sleep, of the 2343 patients with POSA, 1849 (78.9%) had POSA and 494 (21.1%) had NPOSA. The percentage of patients who retained POSA during REM sleep significantly decreased in patients with severe OSA (Figure 5a). Most (93.5%) patients with POSA stayed position-dependent during NREM, and the percentage of patients with POSA who became NPOSA did not significantly change as OSA became more severe (Figure 5c).
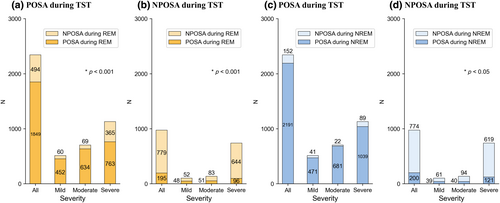
Patients with NPOSA during total sleep were assessed during REM sleep (Figure 5b) and NREM sleep (Figure 5d) only. During REM sleep, among the 974 patients who had NPOSA during TST, POSA newly emerged in 195 (20.0%) patients, and 779 (80.0%) remained NPOSA. The percentage of patients with NPOSA who had POSA during only REM sleep decreased as OSA became more severe (Figure 5b). During NREM sleep only, 20.5% of patients with NPOSA during total sleep had POSA, and patients with severe OSA were more likely to remain NPOSA (Figure 5d).
3.7 Effect of sleep position on REM dependency
Subgroup analyses were performed to evaluate the effect of sleep position on REM dependency. Patients who had an AHIRS/AHINS ratio ≥ 2 were considered to have ROSA while sleeping in the supine position. Those with an AHIRL/AHINL ratio ≥ 2 were considered to have ROSA while sleeping in the lateral position. Patients with ROSA during total sleep were assessed in the supine position (Figure 6a) and lateral position (Figure 6c) only. Overall, in the supine position, of the 754 patients who had ROSA during TST, 667 (88.5%) retained ROSA; and in 87 (11.5%) patients, ROSA disappeared. The percentage of patients who retained ROSA in the supine position significantly decreased as OSA became more severe (Figure 6a). Among the 754 patients who had ROSA during TST, 476 (63.1%) patients remained REM-dependent in the lateral position, and the percentage of patients with ROSA who retained ROSA decreased with increasing severity of OSA (Figure 6c).

Patients with nROSA during TST were assessed in the supine position (Figure 6b) and lateral position (Figure 6d) only. Of the 2563 patients who showed NPOSA during TST, when assessed in the supine position only, 2241 (87.4%) retained nROSA and 322 (12.6%) acquired ROSA (Figure 6b). Patients with severe OSA were more likely to become nROSA in the supine position. In the lateral position, of the 2563 patients who had nROSA during TST, 886 (34.6%) patients acquired ROSA and 1677 (65.4%) patients stayed nROSA (Figure 6d). The percentage of patients with nROSA during TST who newly developed ROSA in the lateral position stayed relatively unchanged across varying severities of OSA (Figure 6d).
4 DISCUSSION
Sleeping in the supine position and REM sleep are well-known factors that worsen airway obstruction in patients with OSA (Resta et al., 2005; Zinchuk et al., 2017). In this study, using epoch-labelled PSG data, we evaluated the combined effect of sleep position and sleep stage on OSA severity by calculating a new set of AHIs based on sleep position and stage. The supine position and REM sleep were associated with worsening of OSA symptoms, and when both were present there was a marked increase in the mean AHI. We also evaluated the effect of sleep stage on position dependency and the effect of sleep position on REM dependency.
Patients with NPOSA had worse PSG parameters than those with POSA, including lower sleep efficiency, less TST, reduced REM sleep ratio, higher AHI, 3% ODI and lower mean oxygen saturation. There were no significant differences in the time spent in the supine position between the two groups. Although a previous study suggested patients with NPOSA spent more time supine (Pevernagie & Shepard Jr., 1992), our study's exclusion of patients with insufficient supine and REM sleep time likely led to a more uniform supine sleep duration. Consistent with our study, Rissanen et al. found that patients with POSA had shorter total hypopnea and apnea–hypopnea times during REM sleep compared with patients with NPOSA (Rissanen et al., 2021). Of the patients with POSA, 78.9% retained it during REM sleep, while 21.1% did not. The proportion of patients with NPOSA during REM sleep increased with OSA severity. In contrast, during NREM sleep, 93.5% of patients with POSA retained it. Reduced upper airway muscle activity in REM sleep likely led to increased airway collapsibility, which supports previous studies showing greater positional dependency during NREM than REM sleep (George et al., 1988; Oksenberg et al., 2010).
Compared with patients with ROSA, patients with nROSA had worse PSG parameters, including lower sleep efficiency, less TST, reduced REM sleep ratio, less time spent supine, higher AHI, greater 3% ODI and lower mean oxygen saturation, consistent with findings by Oksenberg et al. (Oksenberg et al., 2010). In the supine position, most (88.5%) patients with ROSA during sleep in general retained ROSA, but the percentage decreased as OSA severity increased. Similarly, the percentage of patients with nROSA who had ROSA in the supine position decreased with an increase in OSA severity. The proportion of patients with ROSA who still had it in the lateral position decreased with increasing severity. The lateral position may counteract the worsening of OSA during REM sleep, as observed in previous studies in which REM-related breathing parameters were significantly worse in the supine position than in the lateral position for both patients with ROSA and nROSA (Isono et al., 2002; Oksenberg et al., 2010).
Previous studies have reported that OSA significantly worsens in the supine position compared with the lateral position (Joosten et al., 2014; Mokhlesi & Punjabi, 2012). In this study, AHI was greatest in RS, followed by NS, RL and NL, indicating that severity of OSA was more dependent on sleep position than sleep stage. These results are consistent with a previous study in which AHI was highest when both supine position and REM sleep was present (Oksenberg et al., 2010). Gillman et al. reported that supine-only OSA was more prevalent and had a higher AHI than REM-only OSA, and Pevernage et al. compared the PSG parameters of patients with POSA and NPOSA during both REM and NREM sleep (Gillman et al., 2012; Pevernagie & Shepard Jr., 1992). However, both studies were limited by small sample sizes and reliance on final PSG reports, which made it difficult to control for sleep stage when assessing POSA and body position when evaluating ROSA. Using JSON-format PSG data, which recorded patients' body position and sleep stage during each respiratory event, we demonstrated that the supine position has a stronger influence on AHI than REM sleep, especially in patients with mild-to-moderate OSA. While both supine position and REM sleep both increase AHI, we found no significant change in the amount of REM sleep time in the supine position compared with total sleep, suggesting that the supine position and REM sleep independently increase AHI. The results suggest that supine sleep time should be carefully monitored, as a change in supine sleep time may influence patients' clinical presentation or severity of OSA. Clinically, classification and analysis of OSA phenotypes are important as patients who are position-dependent may benefit from positional therapy alone (Ravesloot et al., 2013).
The BMI was strongly associated with AHI, regardless of sleep position or stage. Patients with a higher BMI were more likely to have a higher AHI. This tendency was observed in comparisons between OSA and controls, patients with POSA and NPOSA, and patients with ROSA and nROSA. Obesity may be involved in the pathophysiology of OSA due to anatomical narrowing of the pharyngeal airway (Davies & Stradling, 1990); other factors include poor sleep quality and quantity and disruption of metabolism, involving leptin and ghrelin (Ozturk et al., 2003; Ulukavak Ciftci et al., 2005; Vgontzas et al., 2008). In this study, patients with NPOSA and nROSA had a higher mean BMI than patients with POSA and ROSA, respectively. While the supine position worsens OSA symptoms and increases the AHI, BMI's structural and metabolic changes have a greater impact on OSA severity, causing frequent obstructive events in all sleep positions. Although REM sleep exacerbates AHI by reducing upper airway muscle activity (Fenik et al., 2005), increased airway collapsibility due to higher BMI seems to override the effect of REM sleep across all sleep stages.
There were some limitations to our study. First, we were unable to associate our findings with clinical outcomes because all clinical information that could lead to the identification of patients was eliminated during data collection. Clinical data such as comorbidities, sleep history, sleep patterns and medications may provide useful insight into the associations among sleep position, sleep stage and clinical outcomes. Future studies with long-term follow-ups that consider OSA phenotype (ROSA/nROSA and POSA/NPOSA) are needed to enable personalized management of patients. Second, non-respiratory sleep disorders, such as REM sleep behaviour disorder and NREM parasomnia, have not been examined, which may have impacted sleep dynamics. Also, the presence of sleep disturbances in the “control” group could have introduced bias. Nonetheless, our large sample size would have reduced these confounding effects. Future prospective, multi-night studies on a general population would better identify true controls and patients with OSA. Third, strict exclusion criteria requiring at least 10 min of supine and REM sleep led to the exclusion of 3750 patients. However, these criteria were necessary to accurately assess how POSA and ROSA changed with different sleep stages and positions as little supine and REM sleep time would lead to overestimation of AHI. To ensure that the key findings of our study—that supine position exerts a stronger effect on AHI than REM sleep—can be applied to the excluded patients, we compared the AHI in different body position and sleep stages of the excluded patients (Figure S1). As seen in the included patients, AHI was highest in REM-supine, followed by NREM-supine, REM-lateral, NREM-lateral (44.3 ± 33.4, 37.4 ± 47.7, 23.0 ± 29.0, 19.8 ± 54.4 events per hr, respectively). However, excluding patients with REM sleep time, < 5% of TST may have left out patients with severe OSA who struggle to reach REM sleep (Romero-Corral et al., 2010). REM sleep typically starts 90 min after sleep onset and increases in duration with each cycle, often occurring in the latter part of the night. In-hospital sleep studies, which usually end before the patient's typical waking time, might underestimate REM sleep and contribute to the high exclusion rate. Fourth, the study population consisted mostly of male Korean patients. The amount of data on demographic characteristics and PSG parameters of female patients with OSA might have been insufficient to achieve statistical significance, and there might be bias when applying our findings to other ethnic groups. Fifth, differences in opinion between the two expert sleep technicians suggest the possibility of human error in the interpretations. Finally, different hardware and software were used to collect level 1 PSG data in the different sleep centres.
5 CONCLUSION
This was the first large-scale cohort study using epoch-labelled PSG data to evaluate how AHI may vary according to sleep position and sleep stage. The results provide valuable insight that could lead to personalized management of patients with OSA.
AUTHOR CONTRIBUTIONS
Soyun Lim: Methodology; software; data curation; writing – original draft; writing – review and editing. Hyun-Kyung Lee: Methodology; software; formal analysis; data curation; writing – original draft; writing – review and editing. Yun Jin Kang: Validation; formal analysis; writing – review and editing; writing – original draft; funding acquisition; project administration; supervision. Hyun-Woo Shin: Conceptualization; methodology; resources; writing – original draft; writing – review and editing; funding acquisition; project administration; supervision.
ACKNOWLEDGEMENTS
The results of this study were carried out based on the data established by the “2020 Artificial Intelligence Learning Data Construction Project (aihub.or.kr)” through National Information Agency of Korea (NIA) funded by Ministry of Science & ICT and National Information Society Agency, Republic of Korea.
FUNDING INFORMATION
This research was supported by grant no. 0320202090 from the SNUH Research Fund, the Soonchunhyang University Research Fund, and AI-Bio Research Grant through Seoul National University. The sponsors had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
H.W.S. is a founder of OUaR LaB, Inc., serves on the Board of Directors and as a chief executive officer for OUaR LaB, Inc., and owns OUaR LaB Stock, which are subject to certain restrictions under university policy. And other authors declare no conflicts of interest.
INFORMED CONSENT STATEMENT
Written informed consent was waived because the dataset was collected in a deidentified manner.
Open Research
DATA AVAILABILITY STATEMENT
Although the full dataset cannot be made publicly available because of legal restrictions imposed by the Korean government in relation to the Personal Information Protection Act, if some investigators wish to use these PSG data, they could access it after obtaining the relevant permit from the Korean National Information Society Agency (https://eng.nia.or.kr/site/nia_eng/main.do).




