Cortical hyperarousal in individuals with frequent nightmares
Klaus Junghanns and Ines Wilhelm contributed equally to this work.
Summary
Nightmares are common among the general population and psychiatric patients and have been associated with signs of nocturnal arousal such as increased heart rate or increased high-frequency electroencephalographic (EEG) activity. However, it is still unclear, whether these characteristics are more of a trait occurring in people with frequent nightmares or rather indicators of the nightmare state. We compared participants with frequent nightmares (NM group; n = 30) and healthy controls (controls; n = 27) who spent 4 nights in the sleep laboratory over the course of 8 weeks. The NM group received six sessions of imagery rehearsal therapy (IRT), the ‘gold standard’ of cognitive-behavioural therapy for nightmares, between the second and the third night. Sleep architecture and spectral power were compared between groups, and between nights of nightmare occurrence and nights without nightmare occurrence in the NM group. Additionally, changes before and after therapy were recorded. The NM group showed increased beta (16.25–31 Hz) and low gamma (31.25–35 Hz) power during the entire night compared to the controls, but not when comparing nights of nightmare occurrence to those without. Moreover, low gamma activity in rapid eye movement sleep was reduced after therapy in the NM group. Our findings indicate, cortical hyperarousal is more of a trait in people with frequent nightmares within a network of other symptoms, but also malleable by therapy. This is not only a new finding for IRT but could also lead to improved treatment options in the future that directly target high-frequency EEG activity.
1 INTRODUCTION
Nightmares are very common among the general population and even more so in psychiatric patients (Schredl, 2009). They are related to sleep disturbances (Lancee et al., 2010) and can contribute to impaired daytime functioning (Nadorff et al., 2019). Moreover, they are comorbid with many other disorders, e.g., depression (Agargun et al., 2007) and anxiety disorder (Nadorff et al., 2014), and have even been linked to increased suicide risk (Sjöström et al., 2007).
Much research on nightmares, their frequency and impact has been based on subjective reports. In addition to that, there has been a growing interest in finding physiological correlates of nightmares in the last two decades. One of the first potential ones to be examined was sleep macrostructure. Some studies have demonstrated differences in sleep continuity, with participants with frequent nightmares showing worse sleep efficiency and more wake after sleep onset (Woodward et al., 2000) and sleep architecture with longer Stage 1 sleep, longer Stage 2 sleep latency, and shorter slow-wave sleep duration (Simor et al., 2012). However, other studies failed to find these differences in sleep continuity (Blaskovich et al., 2020) or sleep architecture (Germain & Nielsen, 2003; Paul et al., 2015).
Sleep microstructure has also been studied regarding nightmare characteristics. For example, Simor et al. (2013) found differences in cyclic alternating patterns (CAP) with a significantly lower percentage of CAP A1 and higher rates of A2, as well as a trend of an increased number of A3 subtypes in people with frequent nightmares compared to healthy controls. Picard-Deland et al. (2018) found lower than normal density of slow spindles in most electroencephalography (EEG) derivations, a higher density of fast spindles in frontal derivations, and an elevated fast spindle oscillatory frequency of ‘faster fast’ spindles mainly in central derivations people with frequent nightmares compared to healthy controls. Additionally, slow-spindle density was positively correlated with dreamed fear and nightmare word count and negatively correlated with dreamed positive emotion. Most recently, Blaskovich et al. (2020) found increased cortical hyperarousal, that is, increased beta and gamma EEG activity, in participants with frequent nightmares, especially in the 10-min interval before rapid eye movement (REM) sleep.
Autonomous arousal has also been studied as a nightmare characteristic for a considerable period of time (Fisher et al., 1970). Elevated periodic limb movement indices have been found in people with frequent nightmares (Spoormaker et al., 2006), as well as differences in heart rate and heart rate variability (HRV; Paul et al., 2019) These parameters have been used both in association with actual nightmare occurrence (Phelps et al., 2018) and in comparing people with frequent nightmares to healthy controls (Paul et al., 2019).
The inconsistent findings on physiological correlates of nightmares, e.g., regarding differences in sleep architecture, may in part be explained by general methodological issues. More specifically, there is evidence for nightmares occurring less often in a sleep laboratory environment, thus study designs mostly focus on comparing people with frequent nightmares to healthy controls in more of a ‘trait approach’ while only a few (Paul et al., 2019; Phelps et al., 2018) include actual nightmare occurrence as an additional ‘state approach’.
Despite the inconsistencies in previous research on physiological correlates of nightmares, studying them in both trait and state approach is crucial to increase our understanding of nightmares. Physiological measures can be helpful in monitoring treatment success, as has been done with actigraphy in cognitive behavioural therapy for insomnia (Talbot et al., 2014). To the authors' knowledge, the outcome of one of the most common cognitive behavioural treatments for nightmares, imagery rehearsal therapy (IRT; Krakow et al., 2001), has not been monitored with physiological measures to date. In addition to monitoring outcome of already existing treatments, physiological correlates can also inform the development of new treatment options.
The aims of the present study were: (i) discerning which physiological correlates occurring in nightmares, especially cortical and autonomous hyperarousal, are more indicative of the nightmare state itself and which are rather a trait of people with frequent nightmares; and (ii) investigating whether any of these physiological measures change after successful treatment with IRT.
2 METHODS
2.1 Participants
Participants were recruited by advertisements in local newspapers and on the radio, postings in public places (e.g., supermarkets, libraries) and at the University of Luebeck. Participants with frequent nightmares were additionally approached via a mailing list of local psychotherapists and directly addressed by the study therapist at the outpatient clinic of the Universitätsklinikum Schleswig-Holstein. They were aged between 18 and 67 years and fluent in German. Participants in the frequent nightmare (NM) group and healthy control group were screened for psychiatric conditions, for depression, substance abuse, anxiety, obsessive–compulsive disorder and eating disorder symptoms, as well as retrospective subjective sleep quality in an in-person interview based on Diagnostic and Statistical Manual of Mental Disorders criteria. Presence of any psychiatric disorder was an exclusion criterion for the control group but not for the NM group. The participants in the NM group were required to: (i) have at least one nightmare a week, (ii) not take any benzodiazepines, antidepressants or other medication affecting sleep, and (iii) abstain from alcohol for at least a week. The control group were required to: (i) not have any somatic illness and (ii) not take any medication, smoke, or take drugs. All participants were asked to abstain from alcohol and caffeine on the days of polysomnography (PSG) measurements.
The study was approved by the university's ethics committee. All participants provided written informed consent after having been given a complete description of the study protocol. Participants were compensated financially for the nights spent in the laboratory.
The initial sample comprised 30 participants in the NM group and 16 age- and gender-matched healthy controls in the control group. Later, 11 further healthy, age-matched controls from other studies were included in the analyses. The sample consisted of 43 women and 14 men; the mean (SD) age was 36.3 (14.28) years. There were no differences in age (t[54] = 0.375, p > 0.7) or gender distribution (Χ2[1,57] = 1.011, p > 0.3) between the groups. The NM group had mostly had nightmares since childhood (n = 11) or for ≥10 years (n = six). On a 5-point Likert scale, 15 participants rated their degree of suffering from nightmares as ‘strong’ and five participants even reported a ‘very strong’ degree of suffering. The majority of the sample remembered nightmare content often or always. Half of the NM group reported an identical or almost identical repetition of the nightmare. Most of the NM group linked their nightmares to their biography (18 out of 30) and one was diagnosed with post-traumatic stress disorder (PTSD). We did not differentiate between idiopathic and traumatic nightmares. A total of 14 of the participants in the NM group reported having a nightmare in at least one of the sleep-laboratory nights allowing for a within-subject comparison of 19 nightmare and no nightmare nights.
2.2 Materials
2.2.1 Questionnaires
Participants completed questionnaires to assess current and childhood post-traumatic symptoms with the Impact of Event Scale–revised (IES-R; Weiss, 1997) and Childhood Trauma Questionnaire (CTQ; Bernstein & Fink, 1998), depressive symptoms with Beck's Depression Inventory (BDI-II; Beck & Steer, 1993), anxiety symptoms with the Beck's Anxiety Inventory (BAI; Beck et al., 1996), and general psychological symptom burden using the Symptom-Checklist-90-Standard (Franke, 2014). Nightmare experience was assessed by a customised questionnaire that assessed nightmare frequency, time of nightmare onset, nightmare content, ability to remember nightmares, and distress caused by nightmares (Appendix S1). Additionally, subjective sleep quality was measured with evening and morning protocols that assessed tiredness during daytime, concentration, mood, sleep quality and relaxation during sleep laboratory nights, and in retrospect for 2 weeks with the Pittsburgh Sleep Quality Index (PSQI; Buysse et al., 1989). Additionally, emotion regulation was assessed with the Emotion Regulation Questionnaire (ERQ; Gross & John, 2003) and EMOCheck (Berking & Znoj, 2008).
2.2.2 Polysomnography
Participants were fitted with six EEG electrodes (Fz, F4, C3, C4, Cz, O2, and Oz) according to the 10–20 system, referenced to linked mastoid (A1 and A2) electrodes. We used electromyography (EMG) placed on the chin, as well as electro-oculography (EOG) and electrocardiography (ECG) according to the American Academy of Sleep Medicine (AASM; Berry et al., 2020). During the adaptation night, periodic limb movements were measured with two EMG electrodes attached to the tibialis muscle and potential sleep apnea events were recorded via nasal airflow. None of the participants was subsequently diagnosed with periodic limb movements of sleep or sleep apnea and thus continued the further recordings of the study. Data was recorded with SOMNOscreen™ plus (Somnomedics, Randersacker, Germany). Impedances were < 4 kΩ. Sampling rate was 256 Hz. The device has inbuilt 0.2–35 Hz filters for EEG and EOG and 0.2–150 Hz filters for EMG and ECG channels.
2.2.3 Sleep macrostructure and spectral power analysis
Sleep stages were scored manually according to the AASM criteria (Berry et al., 2020) by two experienced sleep laboratory technicians. Further preparations and spectral power analysis were made with the FieldTrip (Oostenveld et al., 2011) toolbox. Recordings were visually inspected on a 30-s basis and channels with muscle- and technical-related artefacts were discarded. Artefact-free, 50% overlapping, 8.192-s epochs were Hanning-tapered and fast Fourier transformed (FFT) in order to calculate absolute power spectral densities for each frequency bin between 1.25 and 35 Hz for non-REM (NREM; Stage 2 and Stage 3) and REM sleep periods, separately. The specific window length was chosen as it is common procedure in FFT analysis (Ngo et al., 2013) that offers the ability to detect more transient phenomena, such as sleep spindles or slow oscillations, while offering a high resolution for long-lasting frequency bands. A 50% overlap and Hanning-tapered windows allowed for analysis of the entire dataset of interest without creating edge artefacts. Pre-REM periods were defined as 10-min intervals of NREM sleep directly before the onset of the first two REM periods. Accordingly, post-REM periods included similar 10-min NREM epochs following the end of the first two REM periods. The frequency bin boundaries were chosen as a replication of Blaskovich et al. (2020); however, due to inbuilt filters in the recording device recording of the gamma band was only possible up to 35 instead of 45 Hz, therefore findings on gamma activity will be referred to as ‘low gamma activity’. Band-wise spectral power was extracted by summing up bin-wise values into the traditional frequency ranges of delta (1.25–4 Hz), theta (4.25–8 Hz), alpha (8.25–13 Hz), sigma (13.25–16 Hz), beta (16.25–31 Hz) and low gamma (31.25–35 Hz) bands and averaged across all channels.
2.2.4 Heart rate variability
For HRV analysis, which was conducted with HRVtool (Vollmer, 2019), the last 5 min of REM phases were selected, following the approach of Paul et al. (2019). Artefacts were identified and corrected manually before extracting parameters of time (heart rate, mean interbeat interval between all successive heartbeats [MeanRRI], standard deviation of interbeat intervals from which artefacts have been remove [SDNN], root mean square of successive RR interval differences [RMSSD], and percentage of successive RR intervals that differ by >50 ms [pNN50]) and frequency domains (low frequency [LF]: 0.04–0.15 Hz, high frequency [HF]: 0.15–0.4 Hz, LF/HF ratio).
2.2.5 Intervention
The participants in the NM group attended six sessions of group IRT (Thünker & Pietrowsky, 2010). The IRT groups typically included six to eight participants at a time and were conducted by an experienced clinical psychologist. During therapy, the participants first analysed their nightmares for typical and negative elements with the help of the therapist. They were then instructed to find an alternative to the negative elements to make the dream less frightening or disgusting and then regularly imagine the alternative dream script before bedtime. Additionally, participants received psychoeducation on nightmares, information on sleep hygienic behaviour and learned a relaxation technique (progressive muscle relaxation or autogenic training).
2.3 Procedure
Participants spent 4 nights in the sleep laboratory, the first 2 prior to the NM group starting their 6 weeks of group IRT (Thünker & Pietrowsky, 2010). The first night served as an adaptation night and to test for medical conditions such as sleep apnea and restless legs syndrome. The third and fourth PSG nights took place ~8 weeks after the first measurements when the NM group had finished therapy. Participants completed questionnaires on the second and fourth PSG nights.
2.4 Statistical analyses
Statistical analyses were carried out with the Statistical Package for the Social Sciences (SPSS). For every test, we checked for violation of assumptions such as normality beforehand using Kolmogorov–Smirnov and Shapiro–Wilks tests. Differences in sleep architecture and psychometric measures between the NM group and controls on the second PSG night, that is before the NM group started IRT, were evaluated by independent-samples t tests, differences within the NM group (nightmare versus no nightmare nights and before versus after therapy) were evaluated by dependent-samples t tests. Šidák correction was used to correct for multiple comparisons. Band-wise spectral power differences between the NM group and controls, nightmare and no nightmare nights, and before and after therapy were examined by 2 (Group, Nightmare occurrence, or Time) × 3 (location) analyses of variance (ANOVA) for each frequency band separately. Electrode locations (frontal: F4 and Fz; central: C3, C4 and Cz; occipital: Oz and O2) were included to increase precision of results. To examine pre-REM and post-REM periods between the NM group and controls more closely, we tested a 2 × 3 × 2 ANOVA mixed model including Phase (pre-REM, post-REM) and Location (frontal, central, occipital) as within-subject factors, and Group (NM group, controls) as a between-subject factor. For heart rate analysis, an outlier correction of any values ±2 SD was applied. Then, one-way ANOVAs were calculated for the last 5 min of REM sleep, which were aggregated over as many sleep cycles as were available in the respective night.
3 RESULTS
3.1 Comparison between the NM group and healthy controls
3.1.1 Psychometric characteristics, sleep architecture and subjective sleep quality
The NM group had a higher level of depression, anxiety, hyperarousal, childhood trauma and negative affect as compared to the controls (all p ≤ 0.002, see Table 1 for descriptives). Group differences in traditional parameters of sleep architecture were calculated and are summarised in Table 2 together with measures of subjective sleep quality. The NM group reported to have worse mood on the first experimental night in the sleep laboratory (p = 0.002) and lower subjective sleep quality in the 2 weeks before the intervention as indicated by the PSQI (p < 0.001). Other differences did not remain significant after correction for multiple testing.
| Characteristic | NM, n = 25 | CTL, n = 16 | p |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Psychological Symptom Severity | |||
| BDI-II | 20.48(12.26) | 2.00(3.20) | <0.001a |
| BAI | 17.31(10.60) | 2.81(2.88) | <0.001a |
| SCL-90-S total | 58.88(12.30) | 38.60(9.09) | 0.194 |
| IES-R | |||
| Intrusions | 19.27(9.74) | 9.67(3.21) | 0.121 |
| Avoidance | 21.38(10.32) | 2.93(2.57) | 0.119 |
| Hyperarousal | 14.35(13.02) | 1.33(0.58) | 0.001a |
| CTQ | |||
| Emotional neglect | 16.63(7.86) | 5.00(0.00) | <0.001a |
| Sexual abuse | 9.08(5.18) | 7.47(1.55) | <0.001a |
| Physical abuse and neglect | 14.56(7.09) | 6.67(1.95) | 0.013b |
| Emotional abuse | 13.83(5.47) | 0.73(1.10) | <0.001a |
| Denial | 0.04(0.20) | 4.95(1.29) | <0.001a |
| Emotion regulation | |||
| EMOCheck | |||
| Positive affect | 1.78(0.82) | 2.98(0.54) | 0.021b |
| Negative affect | 1.28(0.78) | 0.33(0.28) | 0.002a |
| Emotional competence | 2.3(0.68) | 3.19(0.52) | 0.522 |
| ERQ | |||
| Reappraisal | 3.51(1.21) | 4.98(1.29) | 0.515 |
| Suppression | 3.77(1.47) | 3.02(1.06) | 0.081 |
- Note: Psychological symptoms and measures of emotion regulation in the nightmare (NM) and control group (CTL) at baseline. Bold indicates significant differences at the Šidák-corrected significance level.
- Abbreviations: BAI, Beck's Anxiety Inventory; BDI-II, Beck's Depression Inventory; CTL, control group; ERQ, Emotion Regulation Questionnaire; IES-R, Impact of Event Scale–revised; NM, nightmare group; SCL-90-S, Symptom-Checklist-90-Standard.
- a p < 0.003 (Šidák adjusted p).
- b p < 0.05.
| NM, n = 26 | CTL, n = 27 | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Polysomnography data | |||
| SE | 89.94(8.25) | 93.59(3.69) | 0.041b |
| TST | 442.61 (27.49) | 451.37(32.10) | 0.292 |
| WASO | 2.78 (6.57) | −6.25(56.57) | 0.423 |
| SL | 18.40(18.40) | 18.55(56.45) | 0.990 |
| MA total | 56.5(19.81) | 53.33(23.32) | 0.597 |
| MA REM | 11.62(6.69) | 11.74(5.68) | 0.942 |
| REM duration | 87.38(31.27) | 93.36(15.59) | 0.380 |
| N1 duration | 24.3(12.08) | 22.81(9.65) | 0.621 |
| N2 duration | 221.8(38.47) | 231.07(42.74) | 0.411 |
| N3 duration | 83.69(46.67) | 86.17(32.54) | 0.823 |
| REM density | 13.57(6.2) | 9.89(4.93) | 0.020b |
| Subjective sleep quality | |||
| Tiredness during daytime | 3.79(0.88) | 2.48(1.15) | 0.066 |
| Concentration | 3.34(0.73) | 2.5(0.78) | 0.004b |
| Mood | 2.87(1.01) | 2.1(0.58) | <0.001a |
| Sleep quality | 3.13 (1.2) | 2.24(0.94) | 0.009b |
| Relaxation | 3.35(0.83) | 2.18(0.86) | 0.018b |
| Retrospective subjective sleep quality | |||
| PSQI | 12.00(4.57) | 2.58(1.62) | <0.001a |
- Note: Measures of polysomnography and self-reported sleep quality measures in the NM and CTL groups at baseline. Bold indicates significant differences at the Šidák-corrected significance level.
- Abbreviations: CTL, control group; MA, movement arousals; NM, nightmare group; PSQI, Pittsburgh Sleep Quality Index; REM, rapid eye movement; SE, sleep efficiency; SL, sleep latency; TST, total sleep time; WASO, wake after sleep onset.
- a p < 0.002 (Šidák adjusted p).
- b p < 0.05.
3.1.2 Differences in spectral power: NREM and REM during the whole night
To examine differences between the NM group and controls, we first compared absolute spectral power between groups using 2 (group) × 3 (location) ANOVAs for each frequency band. There were no significant effects of Group from the delta to the sigma band (all p ≥ 0.057). The NM group as compared to the controls showed higher EEG oscillatory activity in the beta frequency band (16.25–31 Hz) in both NREM and REM sleep, as indicated by a significant main effect of Group (NREM: F[1,46] = 6.877, p = 0.012, = 0.130; REM: F[1,46] = 7.332, p = 0.009, = 0.137, Figure 1). The significant effect of Group was also present in the low gamma band (31.25–35 Hz; NREM: F[1,44] = 8.222, p = 0.006, = 0.157; REM: F[1,46] = 5.931, p = 0.019, = 0.114, Figure 1). This effect was not modulated by the electrode position (all p ≥ 0.121 for interaction group × location).
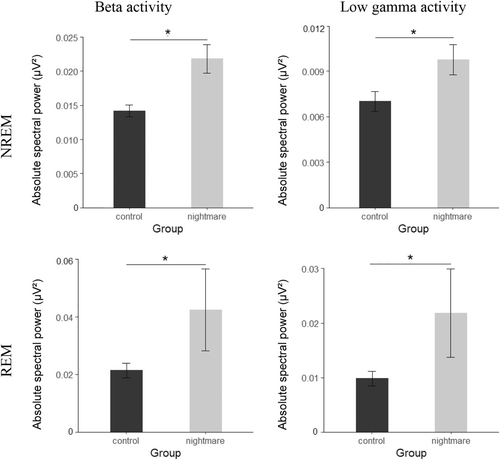
3.1.3 Differences in spectral power: pre- and post-REM spectral power
Previous findings (Blaskovich et al., 2020) indicate that the transitional period from NREM to REM sleep is especially important for the study of cortical hyperarousal. Therefore, we compared the 10-min intervals before and after REM separately in a 2 (group) × 2 (time: pre-/post-REM) × (Location) for the beta and gamma band. Beta activity did not differ between the pre- and post-REM periods (p ≥ 0.064 for Time × Group, Location × Time interactions and Time × Group × Location interactions).
3.1.4 Heart rate variability
There was a trend for heart rate when comparing the NM group and the control group (F[41,1] = 2.125, p = 0.094, η2 = 0.067) with the NM group showing increased heart rate in the last 5 min of REM. The NM group and controls did not differ on any of the other HRV parameters when comparing the last 5 min of REM sleep (p = 0.808).
3.2 Comparison between nightmare and no nightmare nights within the NM group
3.2.1 Sleep architecture and subjective sleep quality
There were more movement arousals and a lower rating of concentration in nightmare nights compared to no nightmare nights. However, these differences did not survive correction for multiple testing (Table 3).
| Nightmare, n = 19 | No nightmare, n = 19 | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Polysomnography data | |||
| SE | 88.23(6.65) | 88.34(8.12) | 0.752 |
| TST | 413.74(42.63) | 411.38(42.64) | 0.909 |
| WASO | 33.53(26.64) | 30.18(28.73) | 0.800 |
| SL | 21.03(12.16) | 21.31(12.93) | 0.588 |
| MA total | 59.42(24.57) | 57.00(23.6) | 0.023a |
| MA REM | 12.74(7.92) | 13.53(9.07) | 0.066 |
| REM duration | 83.14(31.18) | 75.93(23.67) | 0.580 |
| N1 duration | 30.35(13.34) | 27.24(13.35) | 0.315 |
| N2 duration | 224.45(29.67) | 226.33(30.7) | 0.904 |
| N3 duration | 74.52(32.73) | 81.79(38.54) | 0.090 |
| REM density | 14.21(6.32) | 13.26(6.65) | 0.471 |
| Subjective sleep quality | |||
| Tiredness during daytime | 4.21(1.58) | 3.47(1.71) | 0.080 |
| Concentration | 4.00(1.49) | 3.53(1.35) | 0.042a |
| Mood | 3.95(1.22) | 3.63(1.46) | 0.739 |
| Sleep quality | 3.05(1.18) | 3.37(1.12) | 0.124 |
| Relaxation | 4.00(1.15) | 3.74(1.28) | 0.213 |
- Note: Measures of polysomnography and self-reported sleep quality measures in the nights with nightmare occurrence and no nightmare occurrence within subjects in the nightmare group.
- Abbreviations: MA, movement arousals; REM, rapid eye movement; SE, sleep efficiency; SL, sleep latency; TST, total sleep time; WASO, wake after sleep onset.
- a p < 0.05.
3.2.2 Differences in spectral power: NREM and REM during the whole night
To compare spectral activity between nightmare and no nightmare nights, we first compared absolute spectral power between nightmare and no nightmare nights in 2 (nightmare occurrence) × 3 (location) ANOVAs for each frequency band and for REM and NREM periods separately. Nights in which a nightmare occurred did not differ from nights without nightmares in any of the frequency bands (all main effects and interactions for REM and NREM p ≥ 0.084).
3.2.3 Heart rate variability
There were no significant differences between the last 5 min of REM sleep when comparing nightmare and no nightmare nights in any of the analyses (all p ≥ 0.451).
3.3 Comparison within the NM group before and after therapy
3.3.1 Psychometric characteristics, sleep architecture and subjective sleep quality
Over the course of therapy, impairment by nightmare symptoms was reduced significantly in both the participants' (F[8,3] = 15.942, p = 0.001, = 0.857) and clinicians' ratings (F[8,3] = 17.243, p = 0.001, = 0.866). The participants' (F[9,2] = 7.610, p = 0.012, = 0.628) and clinicians' rating (F[9,2] = 6.033, p = 0.022, = 0.573) of the improvement of nightmare symptoms was also significant (Figure 2). In the NM group, neither sleep architecture nor subjective sleep quality changed from before to after therapy (all p ≥ 0.069, Table 4). There was a trend towards reduced anxiety as indicated by the BAI (t[12] = −2.80; p = 0.015), which did not survive correction for multiple testing (Table 5).
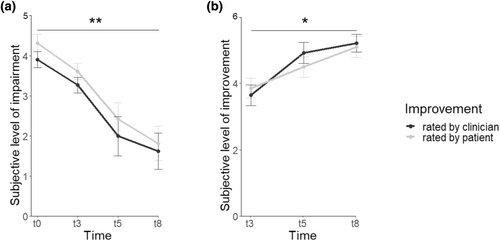
| Before, n = 17 | After, n = 17 | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Polysomnography data | |||
| SE | 88.06(9.62) | 89.95(8.65) | 0.490 |
| TST | 445.99(29.37) | 441.03(24.14) | 0.602 |
| WASO | 3.57(8.24) | 4.27(12.70) | 0.856 |
| SL | 21.84(14.45) | 18.08(15.25) | 0.241 |
| MA total | 54.82(14.68) | 67.00(31.51) | 0.145 |
| MA REM | 12.70(7.85) | 15.71(8.67) | 0.249 |
| REM duration | 94.15(30.69) | 91.16(15.99) | 0.694 |
| N1 duration | 24.43(11.74) | 23.62(12.41) | 0.763 |
| N2 duration | 222.09(35.82) | 221.71(35.64) | 0.980 |
| N3 duration | 75.03(53.00) | 79.79(41.01) | 0.578 |
| REM density | 15.06(6.58) | 13.53(6.98) | 0.416 |
| Subjective sleep quality | |||
| Tiredness during daytime | 4.06(0.73) | 3.71(1.05) | 0.371 |
| Concentration | 3.33(0.85) | 3.24(1.25) | 0.779 |
| Sleep quality | 3.41(0.61) | 3.73(1.40) | 0.412 |
| Restedness | 3.58(1.01) | 3.75(1.22) | 0.635 |
| Retrospective subjective sleep quality | |||
| PSQI | 10.38(4.74) | 7.54(4.70) | 0.069 |
- Note: Measures of polysomnography and self-reported sleep quality measures in the nightmare group (NM) before and after completing six sessions of imagery rehearsal therapy.
- Abbreviations: MA, movement arousals; PSQI, Pittsburgh Sleep Quality Index; REM, rapid eye movement; SE, sleep efficiency; SL, sleep latency; TST, total sleep time; WASO, wake after sleep onset.
| Before, n = 17 | After, n = 17 | p | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Psychological symptom severity | |||
| BDI-II | 19.77(11.98) | 15.73(12.77) | 0.078 |
| BAI | 18.79(11.22) | 14.11(10.60) | 0.015a |
| SCL-90-S total | 59.75(16.16) | 59.38(12.39) | 0.861 |
| Emotion Regulation | |||
| EMOCheck | |||
| Positive affect | 2.03(0.80) | 2.00(1.11) | 0.936 |
| Negative affect | 1.38(0.99) | 1.14(0.73) | 0.265 |
| Emotional competence | 2.45(0.84) | 2.53(0.97) | 0.737 |
| ERQ | |||
| Reappraisal | 3.51(1.38) | 4.03(1.57) | 0.112 |
| Suppression | 4.07(1.29) | 3.85(1.16) | 0.232 |
- Note: Psychological symptoms and measures of emotion regulation in the nightmare group (NM) before and after completing six sessions of imagery rehearsal therapy.
- Abbreviations: BAI, Beck's Anxiety Inventory; BDI-II, Beck's Depression Inventory; CTL, control group; ERQ, Emotion Regulation Questionnaire; IES-R, Impact of Event Scale–revised; NM, nightmare group; SCL-90-S, Symptom-Checklist-90-Standard.
- a p < 0.05.
3.3.2 Differences in spectral power: NREM and REM during the whole night
To assess changes in spectral power before and after therapy, we calculated 2 (Time) × 3 (Location) ANOVAs for each frequency band in NREM and REM sleep. In NREM sleep, spectral power did not change when comparing before and after 6 weeks of psychotherapy (all p ≥ 0.189). However, in REM sleep there was a significant effect of time with regard to low gamma activity (F[15,1] = 6.529, p = 0.022, = 0.303) with gamma activity being higher before than after therapy (Figure 3). There were no significant interactions between time and electrode position (all p ≥ 0.103).
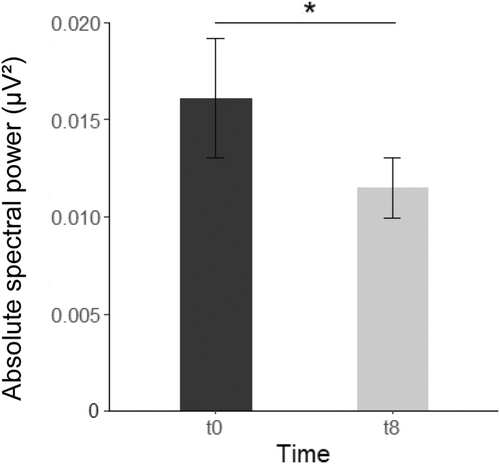
3.3.3 Heart rate variability
There was no significant effect of time on any HRV parameters in the last 5 min of REM sleep (all p > 0.361).
3.4 The role of comorbidities in the NM group
As the NM group was heterogeneous concerning symptom load and comorbidities, we calculated additional correlations between symptom scores (BDI-II, BAI, IES-R and CTQ) and beta and low gamma activity in the NM group. In NREM sleep, there were significant correlations of BAI scores with beta activity over all locations (all p < 0.029), as well as frontal (r = 0.507, p = 0.016) and central low gamma activity (r = 0.519, p = 0.013). In REM sleep, the IES-R was correlated with frontal gamma activity (r = 0.609, p = 0.047). However, all these correlations did not survive the correction for multiple testing (Šidák corrected p = 0.0021). As the BAI also was the only symptom measure that showed a trend towards changing after therapy, we correlated the change scores of low gamma in REM sleep with changes on the BAI. There was a significant correlation of changes in frontal gamma with changes in BAI (r = 0.607, p = 0.021) that also did not survive correction for multiple testing (Šidák corrected p = 0.016).
We also analysed whether participants in the NM group having a psychiatric comorbidity had an influence on their beta and low gamma activity in between-subject univariate ANOVAs. There were no significant differences between the group with and without any comorbidities in any of the frequency bands for both NREM and REM sleep (all p > 0.265). Moreover, we repeated the group comparison between the NM group and controls including only the participants from the NM group without psychiatric comorbidities. The differences in beta activity in both NREM (F[24,1] = 10.991, p = 0.003, η2 = 0.314) and REM (F[32,1] = 7.936, p = 0.008, η2 = 0.199) remained significant. For low gamma activity, the difference between groups remained significant in REM sleep (F[32,1] = 4.821, p = 0.035, η2 = 0.131).
4 DISCUSSION
In this study, we investigated which nightmare characteristics, especially cortical and autonomous hyperarousal, are more indicative of the nightmare state itself, which are rather a trait of people with frequent nightmares, and whether any of these measures change after successful treatment with IRT. We found significantly higher beta and low gamma activity in REM and NREM during the whole night in the NM group as compared to the control group, but not when comparing nightmare with no nightmare nights in the NM group. During IRT, impairment by nightmare symptoms significantly declined in both the participants' and clinicians' ratings. Moreover, low gamma activity in REM in the NM group was significantly lower after IRT compared to before the intervention.
The results of increased EEG activity in the beta and gamma band in participants in the NM group as compared to controls is in line with the idea that cortical hyperarousal is also central to nightmares, especially in NREM sleep (Blaskovich et al., 2020; van der Wijk et al., 2020). A potential source of cortical hyperarousal has already been identified with previous research pointing to increased left anterior cingulate cortex and right inferior parietal lobule activity, which has also been associated with other measures of physical and psychological arousal in people with nightmares (Shen et al., 2016).
Besides the increased EEG activity in the beta and gamma range in the NM group as compared to controls, we found no difference in these frequency bands between nights with and without nightmares within the NM group. This suggests that the differences probably tend to be more the result of a trait rather than a state. In previous research on nightmare biomarkers in general (Simor et al., 2012), and on cortical hyperarousal in nightmares in particular (Blaskovich et al.; van der Wijk et al., 2020), study designs favoured either group comparisons or online nightmare recordings without group comparisons (Phelps et al., 2018). To date, only Paul et al. (2019) chose a design that allowed for state versus trait comparison, albeit with HRV measurements (see following paragraph). Another difficulty in earlier studies was that nightmares tend to occur less often in the sleep laboratory, making this study with 19 pairs of nightmares and no nightmare nights a good opportunity for discerning trait versus state.
With cortical hyperarousal skewing more towards the trait side in our dataset and the presence of anxiety and other symptoms in our sample that are associated with cortical hyperarousal, more questions are opened towards the origins of this trait and potential commonalities and interactions with other disorders. Network approaches, as have been recently proposed by Sheaves et al. (2022), seem to be a promising new avenue of research in that direction. Cortical hyperarousal, potentially together with impaired fear extinction (Gieselmann et al., 2019), might be a crucial part of a network connecting nightmares, negative affect, and anxiety. Future studies should investigate both long-term symptom development in symptom clusters and the effect of symptom-specific interventions to uncover potential causalities and networks. This could further strengthen the transdiagnostic quality of cortical hyperarousal, having already been found in PTSD (Wang et al., 2020), social phobia (Sachs et al., 2004), and in comorbid (Kwan et al., 2018) and idiopathic insomnia (Zhao et al., 2021).
Broadening the view from the role of cortical hyperarousal as a transdiagnostic factor, this process might be related to difficulties in memory consolidation during sleep (Puetz et al., 2011) but also with increased dream (Moyne et al., 2022) and nightmare recall (Marquis et al., 2017). Moreover, beta (Moyne et al., 2022; Scarpelli et al., 2017) and gamma activity (Scarpelli et al., 2022) have been associated with processing of emotional information and a transcranial direct current stimulation (tDCS) induced excess of gamma activity during sleep has been associated with worse mood during the next morning (Marshall et al., 2011). Tying these findings together, increased beta and gamma activity seems to be present in non-pathological processes like dream recall and emotion processing but could potentially go wrong in nightmares by causing persisting negative affect and arousal and facilitating nightmare recall.
It is important to note that the degree of suffering caused by nightmares points to potential daytime impairment in the NM group, which is potentially more severely impacted than in other studies and suggests a diagnosis of nightmare disorder in a substantial number of the participants. However, nightmare symptom severity (measured with the item degree of suffering on the nightmare questionnaire) and presence of comorbidities were not related to high-frequency EEG activity in the NM group. Additionally, the degree of suffering might be the more clinically relevant criterion in assessing and diagnosing nightmares (Gieselmann et al., 2019) and a moderate to high degree of suffering is to be expected in this more naturalistic help provide a more realistic sample of nightmare sufferers willing to seek treatment for their nightmares.
When looking at the 10-min intervals pre- and post-REM, cortical hyperarousal persisted in the post-REM phase as well. This is in contrast to the findings of Blaskovich et al. (2020) who reported that the difference in cortical hyperarousal between groups disappeared after REM sleep. As the transition to REM sleep has not been broadly studied yet in the nightmare context, these results are difficult to interpret. A possible explanation is that this difference might be due to higher psychological symptom severity in our sample, especially concerning higher depression and anxiety levels (Table 1), thus potentially leading to higher levels of cortical hyperarousal that is not alleviated after REM sleep.
In our present study, we found no differences in any measures of autonomous hyperarousal, especially in measures of HRV. In previous studies changes in autonomous arousal seemed to be more closely related to nightmare occurrence (Paul et al., 2019; Phelps et al., 2018) but no significant effects could be detected in this study. This might be mainly due to the way nightmare occurrence was sampled in our study, where it was only reported the morning after, compared to Paul et al. (2019) who made participants record nightmare experience as soon as they woke up from a nightmare or a non-nightmare dream. Future studies should therefore utilise online recording of nightmares and measures of both cortical and autonomous arousal to further investigate the notion, that cortical arousal is indeed more of a trait in people with nightmares while autonomous hyperarousal seems to be more indicative of the nightmare state.
The intervention that the NM group received led to changes both on the symptom and the physiological level when comparing before and after therapy. The changes on the nightmare symptoms are in line with what could be expected from an IRT intervention, given that it is the ‘gold standard’ for nightmare interventions (Morgenthaler et al., 2018). However, the reduction in low gamma activity after the intervention is a more novel finding, as studies on IRT usually do not include data from spectral analysis. This is an especially interesting finding as it points to cortical hyperarousal being malleable by intervention despite being a trait-like phenomenon in people with frequent nightmares. As these changes were, however, more of a by-product of the successful treatment with IRT, an important next step to better understand the role of cortical hyperarousal in nightmares, and potentially in other disorders, would be studies that directly experimentally manipulate cortical hyperarousal. Using tDCS in the theta band has already been shown to increase gamma activity in healthy participants (Marshall et al., 2011), but as this experimental manipulation also led to worse mood, it might not be that suitable for therapeutic purposes. Delta activity has already been targeted with open-loop audiovisual stimulation and improved subjective sleep quality in patients with insomnia-related cortical hyperarousal (Fries et al., 2008) but that stimulation did neither target nor affect high-frequency activity. Thus, studies that experimentally manipulate cortical hyperarousal in people with frequent nightmares might not only help with better understanding of underlying processes but also with the development of treatment options.
5 LIMITATIONS
As briefly mentioned, when discussing the results on HRV, a major limitation of the study design was that nightmare occurrence was only recorded retrospectively in the morning and not whenever the participants awoke at night directly after a nightmare, as was done by Paul et al. (2019). Even though this allowed for less disturbed sleep for the participants, it also meant that physiological data, especially spectral power, and measures of autonomous hyperarousal, could not be analysed more closely around an actual nightmare occurrence. Moreover, not every awaking, particularly from REM, could be traced back to a nightmare occurrence with certainty, further complicating a more fine-grained analysis. This especially impedes the interpretation of differences between nightmare and no nightmare nights, as transient phenomena around actual nightmare occurrence might not have been detected when comparing data of the entire night.
Furthermore, in contrast to previous studies, we did not differentiate between idiopathic and traumatic nightmares. As there is evidence for cortical hyperarousal during sleep both in people with frequent idiopathic nightmares (Blaskovich et al., 2020) and in patients with PTSD with nightmares (Wang et al., 2020), possible differences between idiopathic and traumatic nightmares regarding cortical hyperarousal should be quantitative rather than qualitative.
Another limitation to this study is the measurement of gamma activity, which was limited to 35 Hz due to an in-built filter in the recording device so not all effects in the gamma band might have been detected. However, as problems with artefacts in high-frequency bands are very likely (Muthukumaraswamy, 2013) using data from frequency bands >30 or 35 Hz might not be advisable anyway.
A major limitation when interpreting the effects of IRT on EEG activity is the lack of a randomised control group that also had nightmares but did not receive an intervention. This only allows for a comparison of symptoms and EEG activity before and after the intervention within the intervention group. While a reduction in subjective parameters such as anxiety could also be explained by expectancy effects (Hjorth et al., 2021), the before–after difference on an objective measure such as gamma activity is more likely an effect of the intervention. The correlation of changes in anxiety symptoms and frontal gamma activity further supports this assumption. Nevertheless, future studies need to include a control group of participants with nightmares who do ideally receive a minimal intervention that does not target nightmares or related symptoms. Another consideration in that regard is that our design does not allow us to discern which component of the intervention, that is, dream rescripting specifically, relaxation, psychoeducation etc., contributed to the changes in symptoms. Future research using randomised controlled designs should also evaluate the effective components of IRT on EEG activity.
6 CONCLUSION
Cortical hyperarousal seems to be the central parameter identified in this study and is more likely to be a trait occurring in people with nightmares than an indicator of the nightmare state. Additionally, it is possibly malleable by successful therapy. More research is needed as to which mechanisms are behind cortical hyperarousal in nightmares, its potential role in broader symptom networks, and how it might be used in the development of new treatment options.
AUTHOR CONTRIBUTIONS
Clara Sayk, Klaus Junghanns, Nicole Koch and Ines Wilhelm contributed to the design of the study. Sophia Saftien and Nicole Koch carried out recruitment and data collection and provided treatment delivery. Clara Sayk and Hong-Viet V. Ngo performed the statistical analysis. Clara Sayk and Ines Wilhelm drafted the manuscript. All authors contributed to and approved the paper.
ACKNOWLEDGEMENTS
The study was funded by the Swiss National Science Foundation, grant: 10001C_179241 awarded to Dr Ines Wilhelm. Open Access funding enabled and organized by Projekt DEAL.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing financial interests in relation to the reported work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




